Mapping continuous neuronal activation without an ON–OFF paradigm: initial results of BOLD ceiling fMRI
Abstract
Standard functional magnetic resonance imaging (fMRI) requires alternations between activation (ON) and baseline (OFF) periods to map the haemodynamic response to neuronal activation. Consequently, standard fMRI cannot map continuous activations in conditions like tinnitus without an ON–OFF paradigm. We present a novel approach to fMRI that allows mapping of continuous neuronal activation. Compared with standard fMRI, we introduced the application of CO2 as potent vasodilator. CO2 induces a ‘global’ blood oxygenation level-dependent (BOLD) response. The neurovascular coupling in conjunction with the limited cerebral vasodilation implies a limitation or ceiling of the BOLD response. We hypothesize that active areas exhibit a reduced CO2-induced ΔBOLD due to pre-existing ‘local’ task-induced BOLD response. This putative reduction in ΔBOLD might be exploited for mapping of continuous neuronal activation. BOLD ceiling fMRI was tested in the auditory system. Six healthy subjects performed three runs: only continuous monaural auditory; only 10% CO2; simultaneous auditory and CO2 stimulation. First, we demonstrated the ceiling of ΔBOLD during continuous auditory activation. According to the known predominantly contralateral auditory processing, monaural auditory stimulation reduced predominantly contralateral (0.41 ± 0.13%; P < 0.00001) and significantly less (P < 0.0001) ipsilateral ΔBOLD (0.33 ± 0.17%; P < 0.00001). The non-auditory area was not affected. Second, this BOLD ceiling was exploited to generate an initial activation map of continuous auditory activation (ON period). In contrast to standard fMRI, an OFF period without neuronal activation was not required. BOLD ceiling fMRI is proposed as a complement to standard fMRI for those conditions where ON–OFF paradigms are impossible.
Introduction
Functional magnetic resonance imaging (fMRI) maps brain activations due to a local vascular response to neuronal activation and the resulting blood oxygenation level-dependent (BOLD) contrast (Ogawa et al., 1990, 1992; Belliveau et al., 1991; Kwong et al., 1992; Villringer & Dirnagl, 1995; Logothetis, 2002). Ever since the introduction of fMRI in the early 1990s, alternations between activation (ON) and baseline (OFF) periods have remained the basic principle of standard fMRI (Ogawa et al., 1990; Belliveau et al., 1991; Kwong et al., 1992; Ogawa et al., 1992). Brain activation maps can be generated from ‘local’ task-induced BOLD response differences using this ON–OFF paradigm design (Kwong et al., 1992; Friston et al., 1995). Standard fMRI cannot (directly) map continuous neuronal activations in physiological or pathological conditions like tinnitus, continuous hallucinations or chronic pain, where ON–OFF paradigms are unachievable.
We present a novel approach to fMRI as a complement to standard fMRI that allows mapping of continuous neuronal activations. Compared with standard fMRI, we introduced the application of a potent vasodilator. In contrast to local task-induced BOLD activations, vasodilatative compounds like CO2 (Cohen et al., 2002) or acetazolamide (Vorstrup et al., 1984; Bruhn et al., 1994; Kleinschmidt et al., 1995; Carusone et al., 2002; Brown et al., 2003) evoke a ‘global’ BOLD response in the entire brain. The neurovascular coupling (Villringer & Dirnagl, 1995; Buxton & Frank, 1997) links the fractional changes in BOLD response proportionally to the fractional changes in cerebral blood flow (Hoge et al., 1999). The capability of cerebral vessel dilation is limited (Bayliss, 1902; Furchgott, 1983; Palmer et al., 1987). Consequently, there is a ceiling of the cerebral perfusion, which was noted already in 1900 (Biedl & Reiner, 1900). The conjunction of neurovascular coupling and the limited cerebral perfusion implies also a limitation or ceiling effect of the BOLD response (Bruhn et al., 1994; Buxton & Frank, 1997). Previous investigations documented a reduction in the task-induced BOLD response during simultaneous CO2 inhalation for high CO2 concentrations (Corfield et al., 2001; Cohen et al., 2002; Sicard & Duong, 2005). The proposed BOLD ceiling fMRI, however, requires the ‘inverse’ effect, notably the reduction of CO2-induced ΔBOLD during pre-existing and continuous neuronal activation (Fig. 1). We aim to exploit this putative BOLD ceiling for mapping of continuous neuronal activations. We hypothesize that inactive brain areas are in the BOLD baseline state and present the full drug-induced ΔBOLD amplitude. In contrast, we postulate a reduction of ΔBOLD in continuously active brain areas due to the pre-existing task-induced BOLD response. This putative reduction in ΔBOLD might be exploited for mapping of continuous neuronal activations.
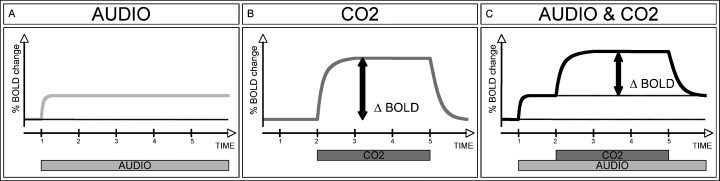
The principle of the hypothesized BOLD ceiling fMRI in the context of the actual investigation. Continuous monaural auditory stimulation should evoke a prolonged task-induced BOLD response predominantly on the contralateral side, which begins after 1 min and then remains constant (A, AUDIO). The application of only CO2 should evoke a drug-induced BOLD response, which begins after 2 min and ends after 5 min (B, CO2). Under the assumption of a BOLD ceiling, i.e. a limitation of the maximum possible BOLD response, we expect that the drug-induced ΔBOLD response is reduced in simultaneously active areas compared to inactive areas, which are at BOLD baseline state (C, AUDIO & CO2). Continuously active areas can thus be identified based on a reduced drug-induced ΔBOLD.
In this study, the principle of BOLD ceiling fMRI was tested in the human auditory system because acoustic sine tone simulation is well investigated and yields robust activations (Belin et al., 1999; Di Salle et al., 2003). Furthermore, it allows an internal interhemispheric comparison, because it is known from anatomy (Rubel & Dobie, 1989) and neuroimaging studies (Woldorff et al., 1999) that monaural acoustic stimulation evokes predominantly contralateral brain activation. We chose CO2 inhalation as a vasodilatative agent because of its good safety profile, its fast on- and offset, and the ease of use (Kety & Schmidt, 1948; Symon et al., 1973; Forster, 1987; Klocke, 1987). We tested the hypothesis that the global drug-induced ΔBOLD response to inhalation of 10% CO2 declines during pre-existing, continuous monaural auditory stimulation predominantly in the contralateral auditory cortex and, to a lesser extent, in the ipsilateral auditory cortex. We postulate no effect in non-auditory areas. Additionally we tested whether this putative ceiling of the BOLD response may be exploited for mapping of continuous acoustic activation during a single neuronal activation ON period. In contrast to standard fMRI, an OFF period with little or no neuronal activation is not required.
Materials and methods
Subjects
Six healthy volunteers (two females, mean age 31.5 years, 4.1 years standard deviation) gave their written informed consent prior to inclusion in the study, which was approved by the local ethics committee. Subjects were non-smokers with normal hearing and without current medication, had no history of medical, neurological or psychiatric disorders, and were strongly right-handed (four), moderately right-handed (one) and strongly left-handed (one) according to the Edinburgh Handedness Inventory (Oldfield, 1971).
Experimental tasks
First, a standard fMRI auditory block-design paradigm was performed to identify individual primary auditory cortices with six repetitions of 20 s ON, 20 s OFF, bilateral auditory stimulation. This auditory paradigm was used to define auditory volumes-of-interest. The auditory stimulus was a binaural sine tone of 1000 Hz pulsating at 6 Hz. We chose this stimulus because pulsating sounds induce a strong and long-lasting BOLD response (Seifritz et al., 2002). Then three experimental conditions were performed in random order and counterbalanced across volunteers, as illustrated in Fig. 1. The first minute served as low-level baseline in all conditions.
- 1
AUDIO: continuous, monaural auditory stimulation starting after 1 min and lasting for 5 min.
- 2
CO2: 10% CO2 applied via a nasal cannula starting after 2 min and lasting for 3 min.
- 3
AUDIO & CO2: both stimuli AUDIO and CO2 simultaneously with identical timing as above.
The auditory stimulus was always applied monaurally to the right ear and consisted of the same 1000 Hz sine tone pulsating at 6 Hz as above. CO2 was applied in a concentration of 10% mixed in synthetic air via a nasal cannula at a flow of 8 L per minute.
Image acquisition
Imaging was performed on a 1.5 Tesla routine whole-body MR scanner (AVANTO, Siemens Medical Solution, Erlangen, Germany). A continuous-sound EPI was used to avoid interference between pulsating auditory stimulus and pulsating MR gradient noise, and at the same time to enhance the auditory BOLD response. Details of sequence design are described elsewhere (Seifritz et al., 2006). The matrix size was 64 × 64 (FOV 192 mm × 192 mm), and 27 slices were acquired (4 mm slice thickness, 1 mm gap) covering the whole brain. The resulting resolution was 3 × 3 × 5 mm. Repetition time (TR) was 2.97 s, flip angle (FA) 90 ° and echo time (TE) 61 ms. Each run of AUDIO, CO2, and AUDIO & CO2 consisted of 101 volumes. The first two volumes were discarded from further analysis to avoid non-steady-state saturation effects. After functional scanning, high-resolution data were acquired (1 mm Iso-Voxel T1w MPRage, Matrix 256 × 256, 176 slices), and used for co-registration and spatial normalization.
Image analysis
Anatomical and functional images were analysed using BrainVoyager QX (Brain Innovation, Maastricht, the Netherlands). Pre-processing of functional time series consisted of three-dimensional motion correction, interscan slice time correction, Gaussian spatial filtering [full-width half-maximum (FWHM) 4 mm], and transformation into standard space (Talairach & Tournoux, 1988). Temporal filtering was not performed. The primary auditory cortex was determined using a fixed-effects general linear model (GLM) group analysis with statistical threshold corrected for multiple comparisons based on the false discovery rate (FDR) (Genovese et al., 2002) at a false-positive probability of q(FDR) < 0.05 for single subject analysis and q(FDR) < 0.001 for the group analysis, which corresponds to P(uncorrected) < 0.0003 or t > 3.75 and P(uncorrected) < 1.3 × 10−10 or t > 6.5, respectively. A spatial filter was applied to the activation maps (FWHM 4 mm). The spatial extent threshold was 500 mm3. Additionally we defined a reference volume of interest (VOI) outside the auditory cortex but in the territory of the middle cerebral artery (MCA) centre [Talairach & Tournoux (1988) coordinates: x, −42; y, −2; z, 8; 13 × 13 × 13 mm3].
BOLD response time-course analysis
The mean event-related average BOLD responses for all subjects were calculated in these defined VOIs. Three periods were defined: a low-level baseline period in the steady state of the BOLD response of the first minute (volumes 9–18), an auditory period during the steady state of the second minute (volumes 29–38), and a CO2 period during the steady state of CO2 application (volumes 60–95). The CO2-induced ΔBOLD was assessed as the CO2 period minus the auditory period in those conditions where CO2 was applied (conditions CO2, and AUDIO & CO2). The task-induced BOLD response to auditory stimulation was assessed as the auditory period minus the baseline period in the condition without CO2 stimulation (AUDIO). Two-tailed t-tests were used for statistical comparison. The contralaterality index (Woldorff et al., 1999) was calculated as the relative ΔBOLD reduction in the right auditory area divided by the sum of the relative ΔBOLD reduction in the left and right auditory areas.
ΔBOLD spatial mapping
ΔBOLD parameter maps were calculated for the AUDIO & CO2 runs only. Two predictors/regressors of 10 volumes each (approximately 30 s) were defined in the steady states of only auditory stimulation (A, volumes 29–38), and CO2 and auditory stimulation (ACO2, volumes 69–78). Single-subject ΔBOLD parameter maps were calculated by estimating a GLM (Friston et al., 1995) with the predictors ACO2 minus A. The same approach was used for the group analysis in a fixed-effects GLM of all subjects. These activation maps represent the CO2-induced ΔBOLD response during simultaneous and continuous auditory stimulation. The low-level baseline during the first minute is not considered in the evaluation.
Results
Event-related average ΔBOLD
Based on the auditory paradigm with bilateral auditory stimulation, VOI were defined in left and right auditory cortex using a fixed-effects group analysis. The resulting VOIs are ‘left A1’ (centre: x, −40; y, −28; z, 10; size 2158 mm3) and ‘right A1’ (centre: x, −48; y, −20; z, 8.8; size 1665 mm3). Additionally, we used a non-auditory reference VOI in the territory of the MCA.
The BOLD response to continuous auditory stimulation (condition AUDIO) was 0.94 ± 0.11% in the contralateral left A1, 0.52 ± 0.21% in the ipsilateral right A1, 0.10 ± 0.17% in the MCA, and remained constant during the continuous stimulation (Fig. 2).
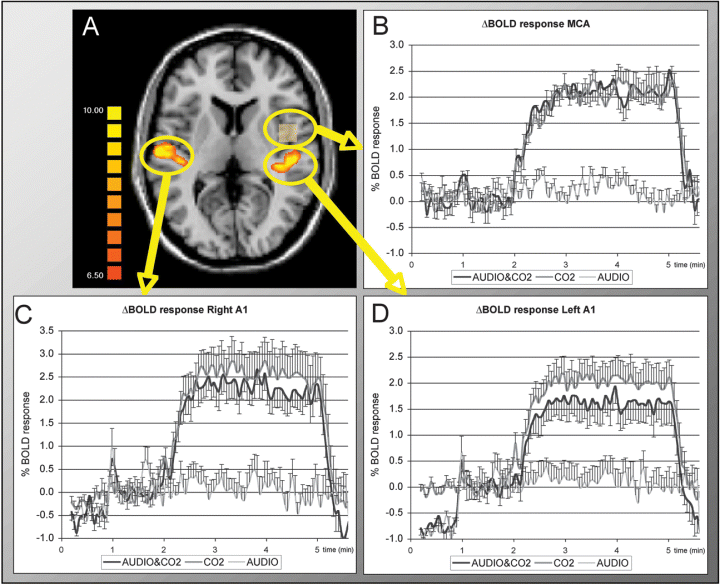
The primary auditory cortex was defined based on a standard fMRI ON-OFF auditory paradigm with bilateral auditory stimulation (A). The BOLD response to monaural, rightsided continuous auditory activation (AUDIO), 10% CO2 only and simultaneous AUDIO & CO2 stimulation is illustrated for the ipsilateral right A1 (C), contralateral left A1 (D) and non-auditory reference area in the middle cerebral artery territory (B). The figures are illustrated relative to the second minute. This period is prior to CO2 application in condition AUDIO & CO2, but continuous auditory stimulation was already present in this period. Simultaneous, continuous monaural auditory stimulation reduced CO2-induced ΔBOLD in contralateral (0.41% ± 0.13%; P < 0.00001) and significantly less (P < 0.0001) in ipsilateral auditory cortex (0.33% ± 0.17%; P < 0.00001), while no difference was present in the non-auditory reference area. Error bars indicate standard deviation.
In the contralateral left A1, the CO2-induced ΔBOLD response was 2.04 ± 0.09% without auditory stimulation (condition CO2) and 1.63 ± 0.10% with auditory stimulation (condition AUDIO & CO2). This corresponds to a reduction of 0.41 ± 0.13%ΔBOLD (P < 0.00001) or a relative reduction of 20.3% due to simultaneous, monaural, contralateral auditory stimulation. In the ipsilateral right A1, CO2-induced ΔBOLD was 2.61 ± 0.12% without and 2.28 ± 0.16% with auditory stimulation, which corresponds to a reduction of 0.33 ± 0.17%ΔBOLD (P < 0.00001) or a relative reduction of 12.7%. This corresponds to a contralaterality index of 62%. The reduction in ΔBOLD due to monaural stimulation was significantly stronger in the contralateral left A1 compared with the ipsilateral right A1 (P < 0.0001).
The non-auditory control region MCA showed no significant difference in ΔBOLD response without (2.11 ± 0.14%) and with (2.11 ± 0.11%) auditory stimulation.
The amplitude of the task-induced BOLD response in the contralateral auditory cortex was 0.94 ± 0.11% in standard fMRI, which was substantially higher compared with the 0.41 ± 0.13%ΔBOLD reduction in BOLD ceiling fMRI.
ΔBOLD parameter maps
The ΔBOLD parameter maps were calculated for the AUDIO & CO2 runs. The resulting parameter maps are illustrated for a single individual (Fig. 3A) and for the group analysis (Fig. 3B). CO2-induced ΔBOLD response is predominantly reduced within the left temporal region, which is apparent in the form of a minimum in the ΔBOLD parameter maps. These results of the BOLD ceiling fMRI are closely co-located and partly overlap with the standard fMRI auditory paradigm. By way of comparison, the standard fMRI bilateral auditory paradigm is superimposed in Fig. 3. The region of overlap is bright red.

The BOLD ceiling fMRI (red-blue) and the standard fMRI (orange-yellow) activation maps for a single subject (A) and the group analysis (B). The first row displays the results of the binaural standard fMRI ON-OFF paradigm auditory localizer, used to functionally determine bilateral auditory cortices [q(FDR), corrected for multiple comparisons < 0.05 for the single subject and < 0.001 for the group analysis]. The second row illustrates initial ΔBOLD activation maps for the BOLD ceiling fMRI that is magnified in the third row. Due to the BOLD ceiling effect and the preexisting task-induced BOLD response, continuously active auditory areas can be identified based on the reduced ΔBOLD response to inhalation of CO2 during one single ON-period without neuronal activation baseline. BOLD ceiling fMRI demonstrates the continuously active auditory cortex as minima in the CO2-induced global ΔBOLD activation maps (white arrow). In contrast, standard fMRI demonstrates activations as maxima. Note that in contrast to the standard fMRI auditory experiment, we used monaural stimulation in BOLD ceiling fMRI to benefit from the expected predominantly contralateral activation as internal validation. In accordance with monaural, right-sided auditory stimulation, ΔBOLD is reduced dominantly in contralateral left auditory cortex. The contours of the standard fMRI activation clusters are superimposed for comparison (white contour). Radiologic convention, left hemisphere on right-hand side. TAL Z indicates Talairach Z-coordinates, scale indicates t-values.
Discussion
We present a novel approach to fMRI that allows mapping of continuous neuronal activations. In contrast, standard fMRI requires alternations between neuronal activation (ON) and baseline (OFF) periods, and consequently cannot be applied to conditions with continuous neuronal activations like tinnitus or continuous hallucinations, where ON–OFF paradigms are impossible. Compared with standard fMRI, we introduced the application of CO2 as a potent vasodilator, which induces a global ΔBOLD response. We exploit the limitation or ceiling of the maximum BOLD response. Continuously active brain areas with pre-existing local task-induced BOLD can be identified due to a diminished CO2-induced ΔBOLD. This principle of BOLD ceiling fMRI was successfully tested in the auditory system and allowed an initial activation map of continuous auditory activation. Compared with standard fMRI, an OFF period without or reduced neuronal activation was not required. In contrast, a CO2-OFF period is mandatory. Note that for the investigation of continuous neuronal activations, neuronal OFF periods are impossible, while CO2-OFF periods are unproblematic. BOLD ceiling fMRI is proposed as a complement to standard fMRI for those conditions where neuronal activation ON–OFF paradigms are impossible. The identification of continuous neuronal activation during a single activation period via application of a vasoactive agent represents a fundamental distinction to other neuroimaging methods like standard fMRI, event-related potentials in electroencephalography or magnetoencephalography, which are all based on the task-induced modification in neuronal activation relative to a neuronal activation baseline in the sense of ON–OFF paradigms.
The focus of the present investigation is to illustrate and validate the concept of BOLD ceiling fMRI. The fundamental precondition of the presented BOLD ceiling fMRI evidently is the existence of a ceiling, i.e. limitation, of the maximum BOLD response, and consequently a diminished drug-induced global BOLD response during simultaneous local task-induced BOLD response. Previous investigations assessed the effect of CO2 application on the magnitude of the task-induced BOLD response, and the results are discussed controversially. Corfield et al. (2001) demonstrated no detectable change on visual BOLD response under CO2 administration. Posse et al. (2001) showed an increase in T2* contrast to visual activity at moderate CO2 levels and then a decrease at higher levels. It was demonstrated in humans that the task-induced BOLD response linearly decreases as a function of end tidal expired CO2 concentration from hyperventilation-induced hypocapnia to 5% CO2-induced hypercapnia (Cohen et al., 2002), and the CO2-induced BOLD response increases with increasing CO2 concentration from 5% to 7% (Ziyeh et al., 2005). Additionally, Sicard & Duong (2005) demonstrated in an animal model that 10% CO2 significantly reduced stimulus-evoked BOLD response, while no significant reduction was present at 5%. In summary, there seems to be a consensus for a reduction of the task-induced BOLD response at least for high CO2 concentrations. Lower CO2 concentrations might induce insufficient vessel dilatation for a BOLD ceiling response (Corfield et al., 2001; Sicard & Duong, 2005). In contrast to these investigations, we introduced a fundamental modification and assessed the effect of neuronal activation on the magnitude of the CO2-induced ΔBOLD response. Only this ‘inverse’ approach might allow spatial mapping of neuronal activation in the proposed BOLD ceiling fMRI − if a BOLD ceiling in this ‘inverse’ approach is indeed present.
In a first step, we successfully demonstrated the presence of the BOLD ceiling effect to CO2 inhalation during pre-existing and continuous auditory activation at a high level of significance in the region of interest time series analysis. Simultaneous CO2 and auditory stimulation (condition AUDIO & CO2) were compared with only CO2 stimulation (condition CO2). Due to the pre-existing local task-induced BOLD, active brain areas showed a reduced CO2-induced ΔBOLD signal response. In accordance with monaural auditory stimulation, ΔBOLD decreased predominantly in the contralateral auditory cortex. The non-auditory area was not affected. The ΔBOLD decreased by 20.3% in contralateral and by 12.7% in ipsilateral auditory area. The resulting contralaterality index of 62% is in good agreement with the reported 67% in standard fMRI (Woldorff et al., 1999).
In a second step, we aimed to exploit this BOLD ceiling effect to derive initial ΔBOLD activation maps of continuous neuronal activation during a single ON period without neuronal baseline. The continuous auditory stimulation represents continuous neuronal activation in conditions like, e.g. tinnitus, where ON–OFF paradigms are impossible. Because, e.g. tinnitus cannot be started and stopped voluntarily, it is impossible in potential ‘real’ applications of the proposed BOLD ceiling fMRI to achieve equivalent conditions to only CO2 and only AUDIO in the present investigation. Consequently, to be a useful tool, BOLD ceiling fMRI must be able to map continuous activations unaided in the AUDIO & CO2 condition. The only CO2 and only AUDIO conditions cannot be used for this mapping analysis, but were required above to demonstrate the ceiling of the BOLD effect during pre-existing and continuous auditory activations. Furthermore, for this mapping analysis we considered only minutes 2–6, i.e. continuous auditory stimulations was present during the entire analysed time-course. A neuronal activation baseline period was consequently not included in this analysis, corresponding to potential application of the presented BOLD ceiling fMRI to, i.e. tinnitus. Tinnitus is pre-existing and ongoing, and tinnitus baseline periods are impossible. ΔBOLD was assessed as CO2-induced BOLD response during pre-existing and continuous auditory stimulation. As predicted, monaural auditory stimulation reduced the CO2-induced ΔBOLD signal predominantly in the contralateral auditory cortex. BOLD ceiling fMRI detected continuously active auditory cortex as ‘minima’ in the global ΔBOLD maps. This is in contrast to standard fMRI, where activations are apparent as BOLD ‘maxima’. The apparent sensitivity and specificity of these initial ΔBOLD activation maps are undoubtedly less convincing in its current version than the demonstration of the BOLD ceiling effect per se in the first part of the study. Formal testing of sensitivity and specificity was not performed due to the need of a refined and advanced data analysis, as discussed below. The apparently inferior image quality in BOLD ceiling fMRI might be explained in part by the amplitude of ΔBOLD, which was only about half the amplitude of the BOLD response in standard fMRI. Presumably, this might explain why the localization of auditory areas as derived from standard ON–OFF fMRI and from the BOLD ceiling fMRI experiment were closely co-located and overlapped only partly. Additionally, some discrepancies in the spatial localization between both methods might be attributed to differences in sine tone presentation. The auditory stimulus was continuous for the BOLD ceiling fMRI but discontinuous for the ON–OFF paradigm. It is known that, for example, transient and sustained constituents influence the location of auditory activation (Seifritz et al., 2002).
Note that we implemented a basic form of data analysis for the generation of the ΔBOLD activation maps in order to reduce a priori assumptions to a minimum. The presented initial ΔBOLD activation maps should be considered with caution and regarded as an illustration of the possibility that the BOLD ceiling effect can be exploited to derive activation maps of continuous neuronal activation even without neuronal activation baseline periods. On the other hand, the observation that these ΔBOLD parameter maps are in agreement with our expectations despite the basic data analysis suggests robustness and potential of the BOLD ceiling fMRI concept. There are several possibilities for a more advanced data analysis as well as potential optimization of the vasodilator that should significantly improve the ΔBOLD activation maps in future studies. Due to putative interactions, optimization of these parameters requires a gradual process. Depending on the experimental demands, we suggest the following improvements.
Concerning data analysis, we observed differences in the absolute ΔBOLD response in bilateral auditory cortex and the non-auditory reference area during CO2 only stimulation. The bilateral auditory cortices were functionally defined and not mirror-symmetric. In the current analysis, we minimized a priori hypotheses and assume that CO2 evokes a uniform ΔBOLD response across the entire brain despite known regional differences in the neurovascular coupling (Aguirre et al., 1998; Huettel & McCarthy, 2001; Saad et al., 2001). These regional differences are the most likely origin for the paramedian frontal and occipital reduced ΔBOLD. These well-known watershed areas are the borders between the territories of anterior and middle, and between middle and posterior cerebral arteries, respectively. It appears very plausible that the perfusion reserve and consequently the ΔBOLD are reduced in these terminal arterial areas. Future improvements in the data analysis should compensate for these local differences in the neurovascular coupling by relating the local CO2-induced ΔBOLD to a normalized or standard ΔBOLD response, and consider only the resulting relative ΔBOLD response.
The current analysis of ΔBOLD activation maps compares the period with CO2 inhalation with the period without CO2 within the same subject. An additional improvement might be to calculate this within-subject ΔBOLD and relate this within-subject ΔBOLD to a normal population or another patient group.
We generated ΔBOLD activation maps from only the AUDIO & CO2 runs by simply comparing auditory and CO2 stimulation to only auditory stimulation. Although implemented as GLM analysis, this procedure is equivalent to a simple t-test. Because we consider only the steady states, we did not use a substantial proportion of the acquired data. This reduces the statistical power. In principle, it is possible to model time series regressors that include the complete time series into the data analysis. This presumably should additionally increase the statistical power. This procedure, however, requires knowledge of the exact kinetics of the CO2-induced BOLD response, which is unknown and might even show regional differences. It would of course be possible to determine the CO2 kinetic in a pilot experiment. But, we must assume that the kinetic of the CO2-induced BOLD differs between the healthy volunteers in the presented study and patients with, e.g. hypertension or cerebral artery stenosis. This might impede a direct application of BOLD ceiling fMRI to patient studies.
Standard fMRI is most frequently analysed using a GLM (Friston et al., 1995). In general, the statistical power of GLM analyses benefits from the integration of several ON–OFF repetitions. Given the fast onset and wash-out of CO2 (Kety & Schmidt, 1948; Symon et al., 1973; Forster, 1987; Klocke, 1987), this principle might be applied to BOLD ceiling fMRI by several repetitions of CO2 application.
The size of the ΔBOLD response in BOLD ceiling fMRI was smaller than the BOLD response in standard fMRI, as discussed above. This suggests that BOLD ceiling fMRI benefits in particular from MR hardware improvements like higher magnetic field strength and resulting augmented signal-to-noise ratio (Kruger et al., 2001).
Concerning the vasodilator, we assume that the potency of the vasodilatative drug has a high impact on the BOLD ceiling response. As discussed above, there is some controversy in the literature regarding the effect of CO2 application on the BOLD response. In our study, the inhalation of 10% CO2 as a vasodilatative drug was sufficient to demonstrate BOLD ceiling unequivocally. Titration of the optimum CO2 concentration and/or improvements in drug application might strengthen the BOLD ceiling and consequently improve the quality of the BOLD ceiling fMRI activation maps in future studies. Intravenous application of acetazolamide might be an alternative approach; however, this drug is less controllable compared with CO2 inhalation (Brown et al., 2003).
The presented investigation demonstrates the concept of BOLD ceiling fMRI in the model of the auditory system. The auditory system was chosen because it is easy to apply highly reproducible and robust stimuli. Additionally, we wanted unilateral stimulation to benefit from the expected predominantly contralateral activation as internal validation. Furthermore, the passive listening task largely excludes performance-related systematic confounds, which might be present in active tasks like finger-tapping in the motor system. On the other hand, the interference of auditory stimuli and MR gradient noise is a general concern for auditory fMRI, which has been addressed by numerous standard fMRI investigations (Amaro et al., 2002). We implemented a modified echoplanar imaging (EPI) sequence with almost continuous MR gradient sound, which nearly doubles the BOLD response to basic auditory stimuli (Seifritz et al., 2006). However, alternative approaches like the application of ‘silent’ MR sequences might yield better results (Loenneker et al., 2001).
BOLD ceiling fMRI is not suggested as a replacement of, but as an addition to, standard fMRI in those instances where alternations between activation (ON) and baseline (OFF) periods are not feasible. This BOLD ceiling fMRI was demonstrated only for the perception of continuous auditory stimuli. Before this concept can be generalized to other physiological and pathological conditions, like tinnitus, continuous hallucinations or chronic pain, it remains to be determined as to whether or not there is an adaptation of the local neurovascular coupling and/or cerebral perfusion. Such an adaptation might erase the BOLD ceiling effect and consequently impede the application of BOLD ceiling fMRI.
Conclusions
We demonstrated a ceiling effect of the BOLD response, i.e. selective reduction of the CO2-induced ΔBOLD response in auditory cortex during simultaneous and continuous auditory stimulation. The non-auditory area was not affected. This BOLD ceiling effect can be exploited to derive activation maps during continuous neuronal activations. A neuronal activation baseline is not required. We present an initial version of such an activation map, yet significant improvement, in particular in data analysis, is required. Eventually, BOLD ceiling fMRI is intended as a complement to standard fMRI for those conditions where ON–OFF paradigms are impossible.
Abbreviations
-
- BOLD
-
- blood oxygenation level dependency
-
- EPI
-
- echoplanar imaging
-
- FDR
-
- false discovery rate
-
- fMRI
-
- functional magnetic resonance imaging
-
- FWHM
-
- full-width half-maximum
-
- GLM
-
- general linear model
-
- MCA
-
- middle cerebral artery
-
- VOI
-
- volume of interest




