Axonal transport of BDNF precursor in primary sensory neurons
H.W. and L.L-y.W contributed equally to this work.
Abstract
Recent studies have shown that the precursor of brain-derived neurotrophic factor (pro-BDNF) activates p75NTR with high affinity to induce apoptosis. Here we show that pro-BDNF is transported anterogradely and retrogradely in sensory neurons of adult rats. After a crush injury of sciatic nerves, dorsal roots or dorsal column in adult Sprague–Dawley rats, the immunoreactivity for pro-BDNF accumulated at both the proximal and distal segments. The accumulation reached a maximum at 24 h after injury. Western blot analysis also revealed pro-BDNF in sciatic nerve segments proximal and distal to the ligature and in the spinal cord. Biotinylated or Alexa-488-labelled pro-BDNF injected into sciatic nerve was internalized and transported both retrogradely and anterogradely within sensory neurons. These results demonstrate that pro-BDNF is anterogradely and retrogradely transported in sensory neurons, suggesting that endogenous pro-BDNF may be released and play important functions.
Introduction
Neurotrophins play important roles in the proliferation, differentiation and survival of neurons during development and in the maintenance of normal functions of the mature nervous system (Chao, 1992; Hefti, 1994; Poo, 2001; Lu, 2003). Biologically active mature neurotrophins are formed by proteolytic cleavage of their precursors (proneurotrophins) (Suter et al., 1991; Seidah et al., 1996a; Seidah et al., 1996b; Mowla et al., 2001). Proneurotrophins can be cleaved intracellularly by furin (Hefti, 1994; Fahnestock et al., 2001; Mowla et al., 2001), and extracellularly by several proteases including prohormone convertases, tissue-activated plasminogen/plasmin, MMP-3 and MMP-7 in vitro (Heymach & Shooter, 1995; Fahnestock et al., 2001; Mowla et al., 2001). Recently, it has been shown that unprocessed pro-NGF preferentially activates p75NTR with high affinity and that binding of pro-NGF to p75NTR and sortilin leads to apoptosis of cells that express both p75NTR and sortilin (Friedmann et al., 2001; Nykjaer et al., 2004), whereas the mature NGF activates TrkA to promote cell survival (Lee et al., 2001). Pro-NGF is also involved in the elimination of oligodendrocytes by activating the apoptotic machinery via p75NTR after spinal cord injury (Beattie et al., 2002). Pro-BDNF has biological activities as demonstrated by its ability to mediate TrkB phosphorylation in vitro (Mowla et al., 2001) and bind sortilin to induce apoptosis (Teng et al., 2005). Sortilin is important in intracellular sorting of pro-BDNF to the regulated secretory pathway (Chen et al., 2005).
While the retrograde neurotrophic hypothesis is well recognized, accumulating evidence indicates that neurotrophins such as brain-derived neurotrophic factor (BDNF) and NT-3 are trafficked anterogradely, released in an activity-dependent manner and taken up by second- or third-order target neurons (Zhou & Rush, 1996; Conner et al., 1998; Tonra et al., 1998; Kohara et al., 2001; von Bartheld et al., 2001; von Bartheld, 2004). The anterogradely transported and released BDNF regulates neuronal survival, differentiation and dendritic morphology, synaptic plasticity, neurotransmitter expression and in addition exerts functions as an excitatory neurotransmitter (Altar et al., 1997; Kohara et al., 2003; Wang & Zhou, 2002; Wirth et al., 2005). A single nucleotide polymorphism in the BDNF gene (BDNFmet) at codon 66 in the prodomain results in the reduction of BDNF transport and activity-dependent secretion (Egan et al., 2003). This mutation in humans is associated with reduction of hippocampal volume, impairment of episodic learning (Hariri et al., 2003; Pezawas et al., 2004) and a number of neurological diseases (Momose et al., 2002; Ventriglia et al., 2002). The polyQ expansion in the huntingtin gene reduces BDNF transport and leads to the degeneration of striatal neurons (Egan et al., 2003; Gauthier et al., 2004). These studies suggest that the intraneuronal transport of BDNF is critical for normal brain function. In the present study, we have examined whether pro-BDNF, like the mature molecule, is transported in neurons. We used primary sensory neurons as a model system as these cells express not only BDNF and their receptors trkB and p75NTR (Schecterson & Bothwell, 1992; Farinas et al., 1998), but also transport mature BDNF (Zhou & Rush, 1996; Tonra et al., 1998).
Materials and methods
Animals
All experiments on rats were approved by the Animal Welfare Committee of Flinders University, and performed under the guidelines of the NHMRC Ethics Committee. A total of 35 adult Sprague–Dawley rats and three BDNF–/–, BDNF+/– and BDNF+/+ neonatal mice, male or female, were used in this study. All animals were anaesthetized by inhalation of Halothane and surgery was performed under aseptic conditions. Rodents were killed by an overdose of pentobarbital and perfused through the left ventricle of the heart with 4% paraformaldehyde. All tissues were fixed in the same fixative for two hours and soaked in 30% sucrose over night before cryosectioning.
Sciatic nerve ligation
The left sciatic nerves in rats were ligated or double ligated at the mid-thigh level with 5–0 silk. These animals were killed 24 h after the nerve ligation.
Dorsal root crush
To see whether pro-BDNF synthesized in sensory neurons is transported to the spinal cord, the L5 dorsal root, 5 mm distance from the ganglion, was crushed for 30 s with a pair of fine forceps. This was performed after a semilaminectomy over the L5 dorsal root of dorsal root ganglia (DRG). The rats were killed 24 h after crush and the injured dorsal roots were sectioned. To determine whether the immunoreactivity for pro-BDNF in the dorsal horn was derived from DRG, the L4, 5, 6 dorsal roots of DRG were cut 5 mm distance from spinal cords. Animals were allowed to survive for 3 or 5 days and the corresponding spinal cords were dissected and sectioned.
Spinal cord injury
To examine whether endogenous pro-BDNF in the spinal cord is transported, the dorsal column of the spinal cord was cut with a pair of microscissors as previously described (Song et al., 2004). Laminectomy was carried out over the 10th thoracic (T10) spinal cord segment. The depth of the lesion was approximately 0.5 mm. Animals were allowed to survive for 24 h. After perfusion, the lesioned spinal cord was dissected and prepared for longitudinal sections.
Pro-BDNF immunohistochemistry
Antibodies to pro-BDNF were generated by immunization of rabbits with synthetic peptide, corresponding to the 14 amino acids of the preregion sequence of pro-BDNF, which was conjugated to haemocyanin (KLH). The specificity of the antibodies was verified after affinity purification by reaction with the immunizing antigen, and characterized by Western blot. The antibodies specifically recognized endogenous pro-BDNF and recombinant pro-BDNF and were well characterized in our previous studies (Zhou et al., 2004). To further characterize the antibodies, we performed pro-BDNF immunohistochemistry on the brain sections from BDNF–/–, BDNF+/– and BDNF+/+ neonatal mice. The results showed that the antibodies stained cortical neurons and hippocampal neurons and nerve terminals in the brain sections of wild-type and the staining intensity was reduced in BDNF+/– mice but completely abolished in BDNF knockout mice (data not shown). The data further support that the immunostaining in our present and previous studies is specific for pro-BDNF.
After immersion in 30% sucrose in PBS overnight at 4 °C, the dissected tissues were serially cryosectioned at 20 µm. The sections were washed and treated with 50% ethanol and 0.3% H2O2 for 45 min, then the sections were blocked in 20% normal horse serum (NHS) and incubated overnight in affinity-purified polyclonal antibodies to pro-BDNF (1 µg/mL) or normal rabbit IgG (1 µg/mL) diluted in PBS containing 1% NHS and 0.3% Triton X-100 at room temperature (RT). After extensive washes, the sections were incubated in biotin-conjugated antibody to rabbit IgG (raised in sheep, 1 : 250 diluted with 1% NHS in PBS,Vector Laboratory) for 2 h at RT, followed by ABC reagent (1 : 100, Vector Laboratories) for 1 h at RT. The sections were washed in PBS and incubated with glucose oxidase-DAB-nickel for 5 min. To demonstrate the specificity of the staining, some sections were processed with the antibodies following preabsorption with the immunizing peptide (12.5 µg/mL).
Western blot
Previous studies showed that proneurotrophin could be processed by several proteases (Heymach & Shooter, 1995; Fahnestock et al., 2001; Mowla et al., 2001). To confirm the immunohistochemical data that endogenous pro-BDNF is present around the ligature, we performed Western blot analysis in rat tissues. The sciatic nerves were ligated at mid-thigh level with 5–0 silk 24 h after ligation, the proximal and distal segments of ligated sciatic nerve and a segment of normal sciatic nerve were homogenized in homogenization buffer (5 mM Tris, 10 mm EDTA, 1% Triton X-100) in the presence of protease inhibitors (Roche). Then the supernatants (30 µg protein in 10 µL) and 50 ng of recombinant pro-BDNF isolated from Escherichia coli were electrophoresed by SDS-PAGE and transferred onto a nitrocellulose membrane (Amersham Pharmacia biotech). The membrane was blocked with 5% skim milk in PBST at room temperature for 1 h and then incubated with pro-BDNF polyclonal antibodies (1 µg/mL) at RT for 1 h. After incubation with HRP-conjugated anti-rabbit IgG (Sigma, 1 : 5000; RT, 1 h), a signal was detected using ECL detection system. The molecular weight was determined by comparison with prestained standard markers from Invitrogen.
Transport of biotinylated pro-BDNF and Alexa-488 labelled pro-BDNF in the sciatic nerve
Pro-BDNF was cloned and expressed as described (Zhou et al., 2004). Soluble refolded recombinant pro-BDNF or BSA was labelled with biotin using Easy-link biotin according to the protocol provided by the manufacturer (Pierce) and with the Alexa-488 kit (Invitrogen). Biotinylated pro-BDNF or biotinylated BSA (2 µg/2 µL PBS) was injected into the sciatic nerve in adult rats (n = 3 each). Six hours after injection, the rats were perfused with 4% paraformaldehyde. The sciatic nerves were dissected and sectioned with a cryostat. The sciatic nerve sections were incubated in ABC kit (Vector Laboratories) and developed as described above.
To observe the transport of Alexa-488 conjugates, labelled pro-BDNF or biotinylated BSA (2 µg/2 µL) was injected into the sciatic nerve of adult rats (n = 3 each). Sixteen hours after injection, the rats were perfused as above. The entire length of sciatic nerve and ipsilateral and contralateral L4 and L5 DRG were dissected and sectioned as above. The sections were mounted directly on glass slides and observed under an AX50 Olympus microscope.
Results
Anterograde and retrograde transport of endogenous pro-BDNF in the sciatic nerve
Sciatic nerve ligation resulted in the accumulation of pro-BDNF-immunoreactivity (IR) on both sides of the ligature (Fig. 1A). On the proximal side, pro-BDNF-IR was present in varicose and nonvaricose fine nerve fibres (Fig. 1B), most abundant in the nerve adjacent to the ligature and reduced to background levels over a distance of 3 mm. In contrast, on the distal side of ligature pro-BDNF-IR was present in a few varicose and coarse nerve fibres. The staining was most intense in the nerve at the distance of 0.5 mm from ligature and extended for approximately 1.5 mm (Fig. 1C). In the double-ligated nerves, pro-BDNF-IR accumulated both distal and proximal to the ligatures, but little in the segment between ligatures, suggesting that the accumulation of pro-BDNF-IR was due to the result of blockade of its transport rather than from production at the site of the lesion (Fig. 1A). No immunoreactivity was found in control sections incubated with normal rabbit IgG or antibody preabsorbed with immunized peptide (Fig. 1F) or in the absence of antibodies.
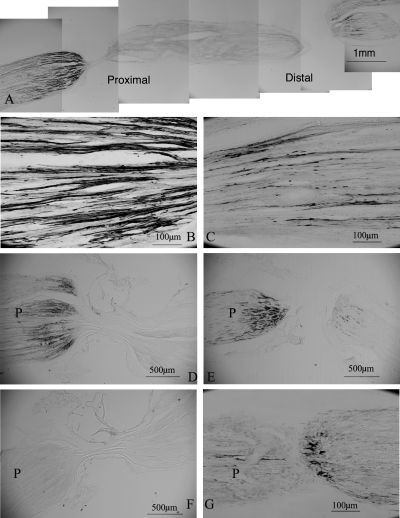
Transport of endogenous pro-BDNF in the sciatic nerve and dorsal root. P, proximal to ligatures or crush site. (A) A montage of micrographs showing accumulation of pro-BDNF-IR on both sides of ligatures 24 h after ligation. (B) A high magnification micrograph of sciatic nerve proximal to the ligature in A. (C) A high magnification micrograph of sciatic nerve distal to the ligature in A. (D) A micrograph of the ligated sciatic nerve 3 days after ligation. (E) Pro-BDNF-IR in the dorsal root 3 days after a crush lesion. (F) A micrograph of the ligated sciatic nerve incubated with antibody preabsorbed with the immunized peptide. (G) A high magnification micrograph of the crushed dorsal root immunoreactive for pro-BDNF.
Transport of pro-BDNF in the crushed dorsal roots and spinal cord
Pro-BDNF-IR was also accumulated on both sides of a crushed dorsal root (Fig. 1G). In contrast to the sciatic nerve where stronger staining of pro-BDNF was present on the proximal side of ligature, the accumulation of pro-BDNF-IR was more obvious on the distal side than on the proximal side of the crush in the dorsal roots (Fig. 1E and G). The staining pattern in the injured dorsal roots (Fig. 1G) was similar to those in the sciatic nerve. In the normal spinal cord, pro-BDNF-IR was mainly detected in varicose nerve terminals in laminae I and II (Fig. 2G and H). Scattered pro-BDNF-IR nerve terminals were also found in the deeper layers and the intermediolateral column of the spinal cord (Fig. 2G and H). In the spinal cord of rats with cut L4–L6 dorsal roots, pro-BDNF-IR was significantly reduced in the dorsal horn on the ipsilateral side (Fig. 2A and C).
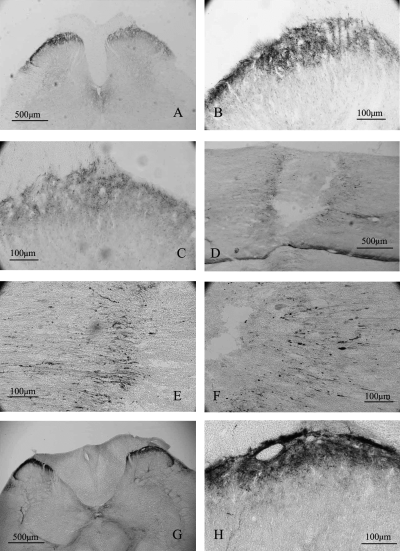
Effects of lesions to the dorsal root and dorsal column on pro-BDNF-IR in the spinal cord. (A) A low-magnification micrograph of spinal cord 3 days after dorsal root cut. (B) A high magnification micrograph of the contralateral dorsal horn to the lesion in A. (C) A high magnification micrograph of ipsilateral dorsal horn to the lesion in A. (D) A low-magnification micrograph of lesioned dorsal column. (E) A high magnification micrograph of the same section as in D showing pro-BDNF-IR accumulation caudal to the dorsal column lesion. (F) A high magnification micrograph of the same section as in D showing pro-BDNF accumulation rostral to the dorsal column lesion. (G) A cross section of normal spinal cord immunoreactive for pro-BDNF. (H) A high magnification micrograph of the same section in G showing pro-BDNF-IR in presumptive nerve terminals of the dorsal horn.
Transport of pro-BDNF in the lesioned spinal cord
To test whether the transport of pro-BDNF also occurs in other neuronal systems, we injured the spinal cord at a site containing descending neuronal axons from the brain. The injured spinal cord was longitudinally sectioned and reconstructed from lateral to medial, as shown in the Supplementary material (Fig. S1). One day after spinal cord injury, pro-BDNF-IR had accumulated in both stumps of the lateral column (Figs 2D–F, and Supplementary material, Figs S1, and S2, A and B), dorsal horn (supplementary Figs S1, and S2, C and D), ventral column (supplementary Figs S1, and S2, E and F), dorsal column (supplementary Figs S1, and S2, H) and corticospinal tract (supplementary Figs S1, and Fig. S2, G). Pro-BDNF was present in injured nerve fibres close to the transection site. Pro-BDNF-IR was mainly present in varicose fine nerve fibres and in a few coarse nerve fibres (supplementary Fig. S2). No significant difference in the staining intensity and staining pattern was found in the rostral and caudal stumps.
Intact pro-BDNF was transported in vivo
Our immunohistochemistry data could not distinguish the cleaved N-terminal region of the precursor molecule and uncleaved full-length precursor. To determine whether pro-BDNF could be transported in unprocessed forms, we performed the Western blot analysis in rat tissues. The pro-BDNF polyclonal antibodies recognized 50 ng of the pro-BDNF molecule expressed in E. coli (Fig. 3, lane E), and endogenous 35-kDa pro-BDNF in the spinal cord (Zhou et al., 2004) (Fig. 3, lane C), sciatic nerve segment proximal (Fig. 3, lane A) and distal (Fig. 3, lane B) to the ligature. In contrast, no pro-BDNF band was detected in the normal unligated sciatic nerve. We were not able to detect any species of the cleaved prodomain fragment by the Western blot analysis. These results suggest that pro-BDNF was transported as an intact molecule in the rat sciatic nerve. Alternatively, these antibodies might preferentially recognize intact molecules only.

Western blot assay of pro-BDNF in rat tissues 24 h after sciatic nerve ligation. (A) Sciatic nerve segment proximal to the ligature. (B) Sciatic nerve segment distal to the ligature. (C) Normal spinal cord. (D) Normal sciatic nerve. (E) Recombinant pro-BDNF protein expressed from E. coli.
Transport of labelled recombinant pro-BDNF
To test whether exogenous pro-BDNF is also transported in both directions, we labelled pro-BDNF with either biotin or with Alexa-488 using labelled BSA as controls. Six hours after injecting 2 µg of biotinylated pro-BDNF into the sciatic nerve of adult rats, pro-BDNF was detected in axons along the sciatic nerve (Fig. 4). HRP reaction product was present in varicosities moving away from the injection sites distally and proximally (Fig. 4). Six hours after injection, pro-BDNF was present in varicose vesicles 3–4 cm away from the injection site (Fig. 4). No biotin-labelled BSA was detected in axons after injection into the sciatic nerve. The HRP reaction product of BSA remained confined to the injection site and appeared diffuse without structural localization (data not shown), suggesting no axonal transport.
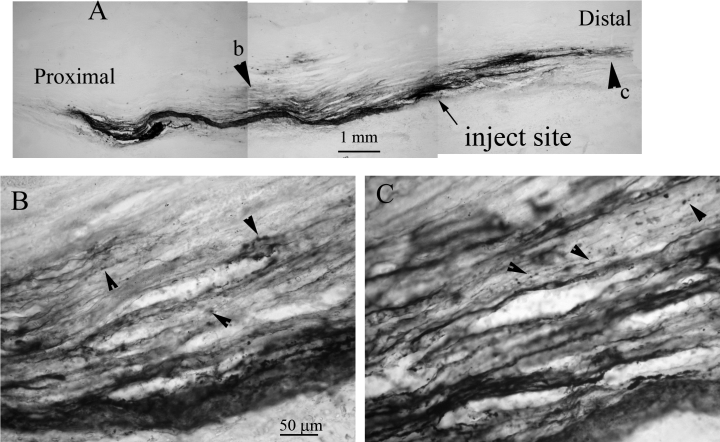
Transport of biotinylated pro-BDNF in the sciatic nerve. (A) A montage of lower magnification micrographs shows the transport of exogenous pro-BDNF away from the injection site 6 h after injection. Arrowheads indicate the same areas of high magnification micrographs shown in panels B and C, respectively. Arrowheads in panels B and C indicate varicose axons and varicosities. The scale bar in B also applies to C.
Sixteen hours after injection of Alexa-488-labelled pro-BDNF into the sciatic nerve, the fluorescent labelled vesicles were detected along the entire length of sciatic nerve with more present close to the injection sites. The fluorescence was also present in many sensory neuronal somata (Fig. 5), suggesting retrograde transport of pro-BDNF by these neurons. Fluorescence-labelled vesicles were also detected in the sciatic nerve segment several centimeters away and distal to the injection sites (Fig. 5). In contrast, no fluorescent vesicle was detected in the sciatic nerve after injection of labelled BSA (Fig. 5D).
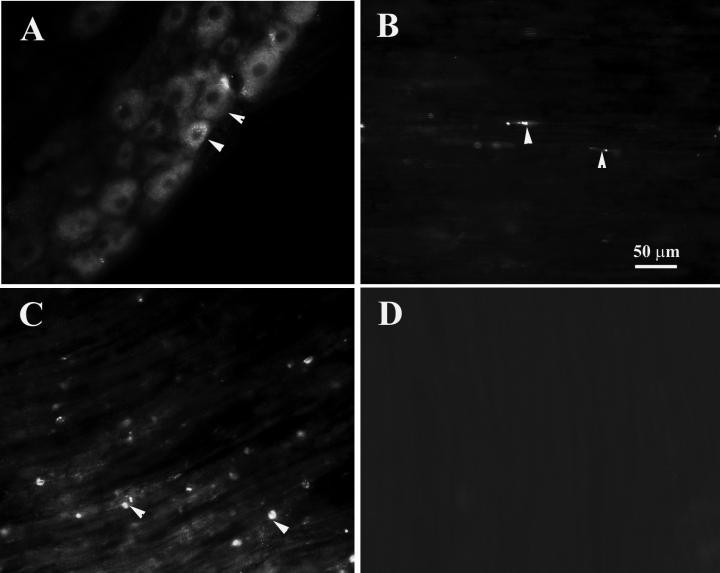
The anterograde and retrograde transport of Alexa Fluor-488 labelled pro-BDNF in the sciatic nerve and L4 DRG 16 h after sciatic nerve injection. (A) A DRG section shows neurons with green fluorescence. Arrowheads indicate neurons accumulating Alexa-488 labelled pro-BDNF. (B) A sciatic nerve section 4 cm proximal to the injection site. Arrowheads indicate fluorescent vesicles transported retrogradely. (C) A sciatic nerve section taken 2 cm distal to the injection site. Arrowheads indicate Alexa-488-labelled pro-BDNF fluorescent vesicles transported anterogradely along the sciatic nerve. (D) A control section showing no fluorescent labelled vesicles in the sciatic nerve 2 cm distal to the injection site with Alexa-488-labelled BSA. The scale bar in B also applies to other panels.
Discussion
Although the function of mature neurotrophins is well studied, the function of their precursors just started to be revealed. Surprisingly, recent studies showed that pro-BDNF, via p75NTR, has an opposite role to mature BDNF in neuron survival, resulting in apoptosis (Teng et al., 2005) and in its synaptic plasticity, resulting in long-term depression (Woo et al., 2005). To understand the function of pro-BDNF, it is essential to examine its distribution, cellular and subcellular localization and its intracellular trafficking. In this study, we examined the transport of endogenous and exogenous pro-BDNF primarily in the rat sciatic nerve. Pro-BDNF immunoreactivity accumulated in the segments proximal and distal to the lesion on the sciatic nerve, dorsal roots and spinal cord. Consistent with these observations, there was a significant reduction of labelling in the ipsilateral dorsal horn after dorsal root crush. After injection of fluorescence-labelled or biotin-labelled exogenous recombinant pro-BDNF into the sciatic nerve, pro-BDNF containing vesicles could be detected several centimeters away from the injection sites. Labelled pro-BDNF also accumulated in the somata of sensory neurons. Together, these data provide strong evidence that pro-BDNF is transported both retrogradely and anterogradely in axons, stored in synaptic vesicles and is released and internalized by neurons.
Primary sensory neurons are an excellent model for the study of axonal transport of neurotrophins as they express all neurotrophin receptors and are dependent on neurotrophins for their survival and normal function (Farinas et al., 1998). In addition sensory neuronal axons are easy to access and manipulate by ligatures for the disruption of axonal transport. Accumulation of endogenous factors around a ligature is an indicator of axonal transport (Hendry et al., 1974; Zhou & Rush, 1996). It is known that sensory neurons synthesize BDNF, which is transported anterogradely and retrogradely (Zhou & Rush, 1996). Thus in the present study, we addressed whether primary sensory neurons transport pro-BDNF. To recognize specifically the prodomain of BDNF we used the polyclonal antibodies, which were extensively characterized in our previous studies (Zhou et al., 2004).We found that pro-BDNF-IR is localized to varicose and nonvaricose fine nerve fibres on the proximal side of the ligated sciatic nerve, suggesting anterograde transport of pro-BDNF. Furthermore, pro-BDNF-IR varicose nerve terminals were also observed in the dorsal horn of the spinal cord. The immunoreactivity was lost when the dorsal root was transected. These data suggest that pro-BDNF made in sensory neurons is transported to and released from nerve terminals in the dorsal horn. Previous studies revealed that mature BDNF is retrogradely transported by both motor and sensory axons (DiStefano et al., 1992; Zhou & Rush, 1996). The pattern of pro-BDNF transport in the spinal cord was similar to that in the sciatic nerve. Internalization and transport of exogenous biotin- and fluorescence-labelled pro-BDNF in both directions by sensory neurons further support the notion of anterograde and retrograde transport of this molecule. Although we do not know how pro-BDNF is transported, mechanisms involving the anterograde transport of synthesized endogenous pro-BDNF and internalized exogenous pro-BDNF may be different. Anterograde transport may involve sorting mechanisms and kinesin molecules (Chen et al., 2005), whereas retrograde transport may involve the receptor mediated internalization and PI3 kinase-dependent endosomal sorting mechanisms (Grimes et al., 1996).
Anterograde transport of pro-BDNF in central and peripheral processes is critical for activity-dependent secretion and synaptic plasticity. The prodomain plays a role in promoting folding of the mature domain and is essential for sorting to the regulatory secretion pathway (Suter et al., 1991; Kolbeck et al., 1994; Rattenholl et al., 2001; Chen et al., 2004; Chen et al., 2005). While sortilin controls BDNF sorting to the regulatory secretion pathway via interaction with the prodomain (Chen et al., 2005), the sorting receptor carboxypeptidase E, is also important in the sorting of BDNF into the regulatory secretion pathway via interaction with a sorting motif in the mature molecule (Lou et al., 2005). The dysfunction in anterograde transport of BDNF by mutant huntingtin may be involved in the development of neurological diseases such as Huntington's disease (Egan et al., 2003; Gauthier et al., 2004). A frequent Val66Met mutation in the prodomain in humans significantly interrupts the intracellular trafficking of BDNF and affects hippocampal volume and episodic learning and memory (Egan et al., 2003; Hariri et al., 2003; Gauthier et al., 2004; Pezawas et al., 2004). These studies indicate that the intracellular trafficking of BDNF and pro-BDNF is very important for the maintenance of normal brain function.
Pro-BDNF may have physiological functions in vivo. We have also identified a 35-kDa intact pro-BDNF protein present in DRG, the spinal cord, and many other brain regions where mature BDNF is normally distributed (Zhou et al., 2004). In the present study, we found that pro-BDNF is present on both sides of a ligated sciatic nerve (Fig. 1). Unprocessed pro-BDNF and pro-NGF were also found on Western blotting of Schwann cells after sciatic nerve transection (Marcinkiewicz & Savaria, 1998). It is likely that intracellular pro-BDNF is largely unprocessed, and is released to act extracellular receptors. Surprisingly, we also found that pro-BDNF accumulates in the distal side of a ligature on the sciatic nerve, suggesting that pro-BDNF is internalized and retrogradely transported. Previous studies showed that a significant amount of unprocessed pro-BDNF was released into the medium by cultured AtT-20 cells and hippocampal neurons (Mowla et al., 1999). In fact, maintenance of long-term potentiation in the hippocampus by pro-BDNF requires the extracellular processing of the molecule by tPA/plasmin (Pang et al., 2004). On the other hand, pro-BDNF activates p75NTR to facilitate long-term depression in the hippocampus (Woo et al., 2005) In a manner similar to pro-NGF, which binds sortilin and activates p75NTR with high affinity to induce apoptosis (Lee et al., 2001; Beattie et al., 2002), pro-BDNF also binds to sortilin and induces apoptisis of PC12-cells and sympathetic neurons (Teng et al., 2005). These studies suggest that pro-BDNF and mature BDNF are ‘Yin and Yang’ molecules, respectively, acting differently on cell survival and synaptic plasticity via different receptors (Lu et al., 2005). As pro-BDNF is the main gene product in vivo and widely distributed in the brain of normal animals including humans (Fahnestock et al., 2002; Michalski & Fahnestock, 2003; Zhou et al., 2004; Peng et al., 2005), it is essential to investigate its functions in both physiological and pathological conditions. Our finding that pro-BDNF is transported both anterogradely and retrogradely by sensory axons strongly suggests that uncleaved pro-BDNF is released by nerve terminals and taken up and internalized by neurons. This finding is important as we provide the first indication that pro-BDNF may play a physiological role after its anterograde transport and release.
How pro-BDNF plays its role is not clear. Like the mature molecule, pro-BDNF in physiological conditions forms a dimer (Teng et al., 2005). It is possible that the mature domain of pro-BDNF activates trkB like a mature BDNF to induce trkB phosphorylation as seen in NIH3T3 cells that over-express the TrkB receptor (Mowla et al., 2001). On the other hand, its prodomain may bind sortilin, a type I transmembrane receptor functioning as a sorting molecule. It has been proposed that pro-BDNF and pro-NGF activate a sortilin/p75NTR complex, with its prodomain binding to sortilin and mature domain to p75NTR (Nykjaer et al., 2004; Teng et al., 2005). This mode of action creates higher affinity around 5 nm, and elicits apoptosis of cells expressing both of these receptors via p75NTR. However, most of these studies were carried out in the transformed cells in vitro. It remains to be established whether these actions of pro-BDNF occur in physiological or pathological conditions in vivo. Our previous and current studies showed that pro-BDNF is widely distributed in the brain and peripheral nervous system and the precursor is transported both anterogradely and retrogradely within axons. It is therefore of fundamental importance to examine whether the interacting molecular partners pro-BDNF, sortilin and p75NTR are colocalized in vivo and how they orchestrate their physiological functions during the development and how these are disrupted in pathological conditions.
Acknowledgements
This work was supported by NHMRC grants (160052, 229961). We thank Dr M Kojima from the National Institute of Advanced Industrial Science and Technology (AIST), Japan, for providing the BDNF plasmid for cloning and expressing recombinant pro-BDNF, Jin-Xian Mi for technical assistance, Professor Theo Hagg (University of Louisville, Louisville, USA) and Dr Bai Lu (NIH) for providing BDNF knockout mice. Dr Steve Johnson is thanked for critical reading and comments on the manuscript.
Abbreviations
-
- BDNF
-
- brain-derived neurotrophic factor
-
- DRG
-
- dorsal root ganglia
-
- IR
-
- immunoreactivity
-
- pro-BDNF
-
- precursor of brain-derived neurotrophic factor
-
- RT
-
- room temperature




