CaV3 T-type calcium channel isoforms differentially distribute to somatic and dendritic compartments in rat central neurons
Abstract
Spike output in many neuronal cell types is affected by low-voltage-activated T-type calcium currents arising from the Cav3.1, Cav3.2 and Cav3.3 channel subtypes and their splice isoforms. The contributions of T-type current to cell output is often proposed to reflect a differential distribution of channels to somatic and dendritic compartments, but the subcellular distribution of the various rat T-type channel isoforms has not been fully determined. We used subtype-specific Cav3 polyclonal antibodies to determine their distribution in key regions of adult Sprague–Dawley rat brain thought to exhibit T-type channel expression, and in particular, dendritic low-voltage-activated responses. We found a selective subcellular distribution of Cav3 channel proteins in cell types of the neocortex and hippocampus, thalamus, and cerebellar input and output neurons. In general, the Cav3.1 T-type channel immunolabel is prominent in the soma/proximal dendritic region and Cav3.2 immunolabel in the soma and proximal-mid dendrites. Cav3.3 channels are distinct in distributing to the soma and over extended lengths of the dendritic arbor of particular cell types. Cav3 distribution overlaps with cell types previously established to exhibit rebound burst discharge as well as those not recognized for this activity. Additional immunolabel in the region of the nucleus in particular cell types was verified as corresponding to Cav3 antigen through analysis of isolated protein fractions. These results provide evidence that different Cav3 channel isoforms may contribute to low-voltage-activated calcium-dependent responses at the somatic and dendritic level, and the potential for T-type calcium channels to contribute to multiple aspects of neuronal activity.
Introduction
Low-voltage-activated (LVA) calcium currents provide an important contribution to spike output patterns of neurons. T-type channels are recognized as key determinants of LVA calcium-dependent responses, including low-threshold calcium spikes (LTS), bistable behaviour, rebound depolarizations and augmentation of synaptic responses (Huguenard, 1996; Perez-Reyes, 2003). As such, T-type channels are important to cell and circuit functions that range from sensory and pain transmission through thalamocortical sleep-wake cycles.
Molecular analyses have identified three isoforms of the T-type calcium channel, α1G/Cav3.1, α1H/Cav3.2, and α1I/Cav3.3 (Lee et al., 1999; McRory et al., 2001; Perez-Reyes, 2003). Expression studies indicate that these isoforms can differ in their voltage-dependent and kinetic properties (McRory et al., 2001; Chemin et al., 2002; Perez-Reyes, 2003), suggesting the potential to differentially affect spike output. In situ hybridization has established a widespread cellular expression of the three isoforms, with distinct differences in mRNA signal intensity between neighbouring but functionally distinct brain nuclei (Craig et al., 1999; Kase et al., 1999; Talley et al., 1999). By comparison, the distribution of Cav3 channel proteins has been described over only a limited subset of nuclei and focused on the Cav3.1 and Cav3.3 T-type channels (Craig et al., 1999; Yunker & McEnery, 2003; Molineux et al., 2006).
It is important to identify the subcellular distribution of all three Cav3 calcium channel isoforms, as the contribution of T-type current to cell output has been attributed in many cases to its activation over specific regions of the soma-dendritic axis (Huguenard, 1996). In hippocampal pyramidal cells single channel T-type activity has been recorded at somatic and dendritic levels, where LVA calcium spikes can be initiated in response to synaptic activation (Magee et al., 1995; Golding et al., 1999). In thalamus, differences in the distribution and kinetic properties of T-type currents have been shown capable of influencing the nature of oscillatory output of principal cells and inhibitory interneurons involved in the sleep-wake cycle (Huguenard et al., 1993; Pape et al., 2004; Joksovic et al., 2006). These differences can even extend to kinetic properties of inactivation between the somatic and dendritic compartments, suggesting a selective distribution or modulation of Cav3 channel isoforms over discrete regions of the cell axis (Joksovic et al., 2005a). In cerebellar Purkinje cells T-type current has been detected in somatic and dendritic regions and contributes to both burst and interburst depolarizations (Cavelier & Bossu, 2003; Swensen & Bean, 2003). In each case the distribution of Cav3 channel isoforms over the soma-dendritic axis may be a primary determinant of LVA responses, but their pattern of distribution has not been established.
In the present study we compare the distribution of immunolabel for Cav3 channel isoforms in four regions reported to exhibit LVA calcium current or spike responses, i.e. cortex, hippocampus, thalamus and cerebellum. We report a widespread and distinct expression of Cav3 isoforms that may underlie LVA responses in somatic and dendritic regions of particular cell types. We further identify Cav3 expression in nuclear protein fractions from the hippocampus, revealing a previously unrecognized potential for these channels to contribute to nuclear activity.
Materials and methods
Animal care
Male Sprague–Dawley rats (n = 38; Charles River, Canada) were maintained and prepared for histology according to guidelines approved by the Canadian Council for Animal Care.
Immunocytochemistry
All chemicals were obtained from Sigma (St. Louis, MO) unless otherwise indicated. General procedures for Cav3.x immunocytochemistry followed the procedures detailed in Molineux et al. (2006). Briefly, male rats (P18–P40) were anaesthetized with sodium pentobarbital (65 mg/kg; MTC Pharmaceuticals, Cambridge, ON, Canada) and perfused intracardially with 250 mL of 0.1 m phosphate-buffer (PB, pH 7.4), followed by 100 mL of 4% paraformaldehyde (Para, pH 7.4) at room temperature. Brains were postfixed in 4% Para at room temperature for 1 h and overnight at 4 °C. Brains used for glutamic acid decarboxylase (GAD) 67 staining were fixed with 4% paraformaldehyde and 0.3% gluteraldehyde, postfixed 1 h on ice, and overnight at 4 °C. All tissue was cut as free-floating 30–40 µm parasagittal or coronal sections by vibratome in ice-cold PB, and reacted in a working solution of 3% normal donkey, horse or goat serum (Jackson Immuno-Research, West Grove, PA), 0.1% Tween, and 1% dimethylsulphoxide (DMSO) in PB, with gentle agitation throughout all reactions. Primary antibodies were added to the working solution and reacted at 4 °C for 24–48 h and washed 3 × 15 min in working solution at room temperature. Secondary antibodies were incubated for at least 4 h at room temperature and washed in PB. Sections were then mounted on gel-coated slides and cover-slipped with antifade medium (90% glycerol/PBS/0.1%p-phenylenediamine; pH 10), sealed with nail polish and stored at −20 °C. Controls consisted of omitting the primary antibodies and preabsorbing primary antibodies with an excess of purified protein.
Antibodies directed to the Cav3.1 calcium channel were produced using the Cav3.1 I–II linker sequence (ELRKSLLPPLIIHTAATPMS), which corresponds to amino acids #1010–1027 (accession number # AF290212). The channel peptide was cross-linked to KLH, injected into rabbits, and the serum purified for use. The epitopes used for the Cav3.2 and 3.3 antibodies were produced using the Caulobactor expression system (Invitrogen) and corresponded to amino acids 1195–1273 (accession number # AF290213) and 1013–1115 (accession # AF290214) for the Cav3.2 and Cav3.3 calcium channel II–III linkers, respectively. Purified fusion proteins were injected into rabbits and the polyclonal antibodies were IgG purified before use. The specificity of each of the Cav3 antibodies has been thoroughly established (Molineux et al., 2006). All reactions were carried out using flourophore-conjugated secondary antibodies to avoid spurious labelling of endogenous biotin (McKay et al., 2004). Cav3.x antibodies were used at concentrations of Cav3.1 (1 : 250–1000), Cav3.2 (1 : 1000–10 000), Cav3.3 (1 : 2000–10 000) and revealed using a secondary Cy3-donkey anti-rabbit IgG (1 : 1000; Molecular Probes, Eugene, OR). Monoclonal or polyclonal antibodies used to counterlabel cell specific proteins were localized using secondary (1 : 1000) Alexa Fluor 488-goat anti-mouse IgG or -donkey anti-mouse IgG (Molecular Probes), or Alexa Fluor 555-goat anti-rabbit IgG or -goat anti-chicken IgG. Primary antibodies used for colabelling studies were monoclonal anti-microtubule-associated protein (MAP-2, 1 : 500), calbindin-28DK (1 : 1000, Swant, Bellinzona, CH), parvalbumin (1 : 25 000), or GAD67 (1 : 1000), Chemicon, Temecula CA), and polyclonal chicken anti-lamin A (1 : 1000, Cedarlane, Hornby ON Canada) and rabbit anti-annexin A3 (1 : 500, Abcam, Cambridge UK). Immunolabel was imaged using an Olympus FV300 BX50 confocal microscope (Carsen Group, Markham ON) equipped for isolating Cy3 and FITC-like fluorolabels. All image adjustments were confined to brightness/contrast and intensity levels using Photoshop 7 and Illustrator 9.
Western blotting
Male Sprague–Dawley rats were anaesthetized with sodium pentobarbital and the hippocampus removed and placed on ice. Cell compartment components from the hippocampus were isolated as to the protocol detailed in the Qiagen Qproteome Cell Compartment Kit (Qiagen, Mississauga ON). For Western blots 5 µg of whole protein from each cell compartment sample was prepared in 2× SDS loading buffer enriched with 0.1 m DTT, separated by a 10% SDS-PAGE gel, and transferred to PVDF membrane (Millipore, Billerica, MA). Membranes were blocked in PBS, 0.1% Tween 20 and 5% (w/v) powdered milk prior to overnight incubation with polyclonal antibodies against annexin A3 (1 : 1000), lamin (1 : 1000), Cav3.1 (1 : 5000), Cav3.2 (1 : 15 000) or Cav3.3 (1 : 15 000). Secondary antibodies used were horseradish peroxidase (HRP)-conjugated rabbit anti-chicken (1 : 5000, Abcam, Cambridge UK) or goat anti-rabbit IgG (1 : 5000) and detected using the Amersham's HCL detection kit as to the manufacturer's instructions.
Results
Subcellular distribution of Cav3.1, Cav3.2 and Cav3.3 T-type calcium channels
The current study examined Cav3 T-type calcium channel isoform distribution in key regions of rat brain in which T-type currents have been suggested to have important roles in modulating cell output. We were particularly interested in Cav3 channel distribution between the soma and dendritic structures of individual cell types in neurons of the cortex, hippocampus, thalamus, and cerebellar input and output cells.
The polyclonal antibodies used to localize Cav3 channel isoforms have previously been shown to be specific to each of the rat Cav3 isoforms, and brain sections are negative if preabsorbed with purified Cav3 fusion protein or if the primary antibody is omitted (Molineux et al., 2006). We expect the antibodies to label all known alternatively spliced isoforms of Cav3.1, 3.2 and 3.3 given the conserved regions used to generate the antibodies. We observed consistent penetration of Cav3 antibodies to ∼10–15-µm depth under our conditions, and always compared the pattern of Cav3 label to a counterlabel against internal or structural proteins to improve identification of positive or negative cell structures. The use of cell-specific immunolabels (i.e. parvalbumin, calbindin-28K, GAD) allowed both analysis of Cav3 expression in identified cell classes and the detailed examination of dendritic structures (MAP-2).
Interpretation of immunolabel distributions
The localization of immunofluorescent signals in relation to functional ion channels in situ requires careful consideration of the limitations inherent to resolving low-level signals in light microscopy. For instance, identifying plasma membrane labelling of channels in neurons is more demanding than that encountered in expression systems, where overexpression of an ion channel can readily support visual identification of membrane-associated label. As often found in localization studies of native channels, a distinct membrane-associated Cav3 label that can be interpreted as channel insertion in the plasma membrane was detected in only specific cell types. It is important to recognize that a lack of apparent membrane labelling does not rule out insertion of these channels, as LVA T-type calcium currents are recorded in many cell types that did not present with distinct Cav3 immunofluorescence at the membrane level. Rather, in most cells Cav3 immunolabel was seen as a diffuse label in the cell interior, a pattern expected for channels localized to at least the Golgi complex or in the process of being transported to the membrane (referred to here as ‘cytoplasmic’, which includes organelles). In some cell types Cav3 immunolabel could also be detected in the region of the nucleus, a pattern not typically expected but readily visualized in particular cell types (i.e. Cav3.3 in dentate gyrus granule cells, Fig. 3J). We previously performed exhaustive control studies to establish that these antibodies are specific to Cav3 proteins in rat CNS, ruling out non-specific labelling (Molineux et al., 2006). There is ample precedence for the expression of ion channels in the nuclear envelope, including voltage-gated potassium, chloride and cation channels, and calcium-activated potassium channels, with additional expression of Na-K ATPase (Maruyama et al., 1995; Draguhn et al., 1997; Valenzuela et al., 1997; Franco-Obregon et al., 2000; Mazzanti et al., 2001; Garner, 2002; Marchenko et al., 2005). Moreover, a resting transmembrane potential can be recorded across the nuclear envelope at a level more negative than the plasma membrane potential, and extensive systems exist to regulate calcium concentration in the nucleoplasm (Draguhn et al., 1997; Mazzanti et al., 2001; Marchenko et al., 2005; Marchenko & Thomas, 2006). Therefore, to understand better the source of Cav3 channel immunolabel we first carried out a series of protein separation/Western blot and colocalization studies.
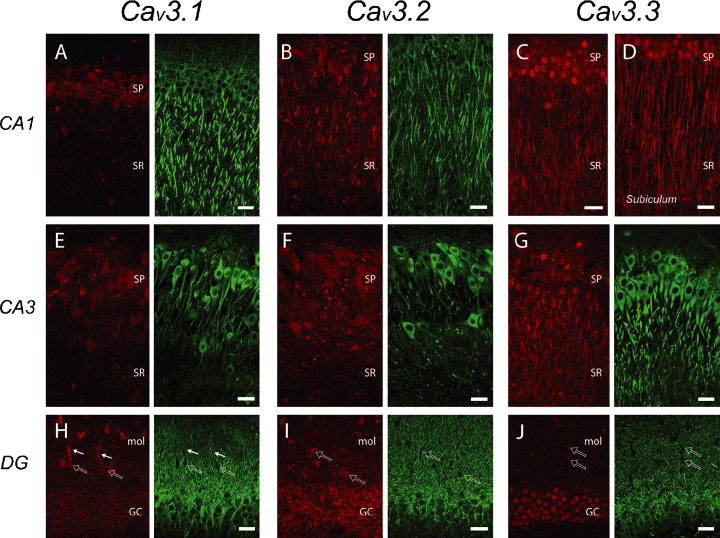
Differential expression and distribution of Cav3.x isoforms in hippocampus. Columns indicate immunolabel for Cav3.x isoforms (red) with corresponding MAP-2-labelled (green) cell structures. Rows correspond to regions of labelling. A low level of expression for Cav3.1 is detected primarily in the somatic region of CA1 (A) and CA3 pyramidal cells (E) and dentate gyrus granule cells (H). Cav3.2 immunolabel extends at a light density over at least the proximal regions of pyramidal cell apical dendrites (B and F) but only the soma of dentate granule cells (I). Cav3.3 immunolabel is expressed at higher levels in all regions of hippocampus, with clear labelling of CA1 and CA3 pyramidal cell apical dendrites (C and G), but only somatic labelling of dentate granule cells (J). The highest intensity and greatest extent of apical dendritic label is present in pyramidal cells of the subiculum (D). (H–J) A class of interneuron positioned in the molecular layer of dentate gyrus is positive for all three Cav3 isoforms at the soma (open arrows), with additional dendritic label for Cav3.1 (H; solid arrows). GC, granule cell layer; mol, molecular layer; SP, stratum pyramidale; SR, stratum radiatum. Scale bars, 20 µm.
To test for the subcellular distribution of T-type calcium channel isoforms we separated plasma membrane, cytoplasmic, nuclear and cytoskeletal proteins from hippocampus using a commercially available protein separation kit. We first verified the specificity of the extraction by establishing that an antibody against annexin A3, a member of an extensive family of membrane and cytoplasmic proteins, labelled the appropriate fractions on a Western blot (Fig. 1A; Gerke et al., 2005). Conversely, the nuclear-specific protein lamin was detected on Western blots only in the nuclear protein fraction (Fig. 1A; Gruenbaum et al., 2005). A nuclear localization of lamin but exclusion of annexin A3 from the nucleus was verified further through double-label immunocytochemistry of CA3 hippocampal pyramidal cells (Fig. 1B). Finally, Western blot analysis of isolated protein fractions from hippocampus established that antibodies to the three Cav3 channel isoforms detected bands of the correct molecular weight in all three fractions of plasma membrane, cytoplasmic and nuclear proteins, but not cytoskeletal proteins (Fig. 1C). These data indicate that each of the Cav3 calcium channel isoforms are expressed at the plasma membrane at levels at least as high as that found in the cytoplasm. We cannot fully distinguish whether labelling in the nuclear region corresponds to channel expression in the nuclear envelope, internal nuclear membranes or even potential inclusion of membranes of the smooth ER, which is continuous with the outer membrane of the nuclear envelope. However, these data confirm that labelling in the nuclear region, when observed, is specific to Cav3 calcium channel isoforms.
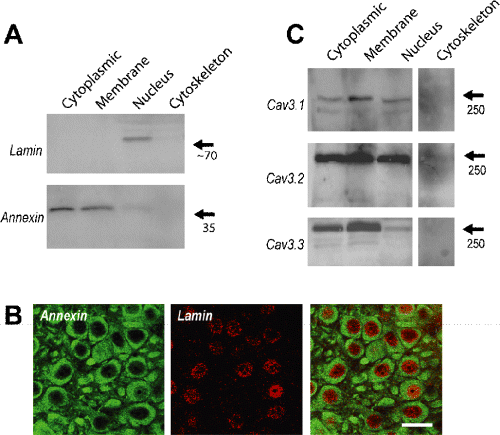
T-type calcium channel isoforms are localized to multiple cell compartments. (A) Western blots establish the ability to isolate select protein fractions from the adult rat hippocampus, as shown by labelling with an antibody against the nuclear-specific protein lamin, and a cytoplasmic and membrane expression for annexin A3. Molecular weights are indicated on the right. (B) Double-label immunocytochemistry of CA3 pyramidal cells to confirm the exclusion of annexin A3 immunolabel from the nuclear region (left panel) and restricted nuclear immunolabel for lamin (centre panel). (C) Bands at the correct molecular weight are detected by each of the Cav3 isoform antibodies in a Western blot of hippocampal cytoplasmic, membrane and nuclear protein fractions, but not in the cytoskeletal fraction.
It also became clear that Cav3 immunolabel could differ in terms of relative fluorescent intensity between nuclei or in its distribution over the soma-dendritic axis of individual cells. Differences in the intensity of immunolabel were also apparent between Cav3 isoform labels processed in parallel from the same animal. The extent to which this could reflect actual differences in membrane channel expression or density of membrane-inserted calcium channels is unknown. We did not attempt to quantify these differences. Ultimately the level of channel expression in the membrane needs to be verified with direct electrophysiological measurement. Yet, relative differences in the perceived fluorescent intensity that were apparent between Cav3 isoforms when compared at the same confocal laser intensity were prominent enough to warrant a descriptive comparison (Table 1). Comparisons between Cav3 intensity levels made here are thus necessarily subjective and expressed in terms of relative intensity levels, with no implied relation to protein level or density of membrane-associated and functional channel isoforms.
| Cell class and Cav3 isoform | Soma | Proximal dendrites | Mid dendrites | Distal dendrites |
|---|---|---|---|---|
| Cortex | ||||
| Layer V pyramidal | ||||
| Cav3.1 | ++ | + | – | – |
| Cav3.2 | ++ | ++ | – | – |
| Cav3.3 | +++ | +++ | +++ | ++ |
| Layers 1–4 pyramidal | ||||
| Cav3.1 | ++ | + | – | – |
| Cav3.2 | +++ | ++ | – | – |
| Cav3.3 | +++ | ++ | – | – |
| Hippocampal formation | ||||
| CA3 pyramidal | ||||
| Cav3.1 | +/++ | + | – | – |
| Cav3.2 | ++ | ++ | +/++ | – |
| Cav3.3 | +++ | +++ | +++ | ++ |
| CA1 pyramidal | ||||
| Cav3.1 | ++ | + | – | – |
| Cav3.2 | ++ | ++ | +/++ | – |
| Cav3.3 | +++ | +++ | ++ | –/+ |
| Subicular pyramidal | ||||
| Cav3.1 | ++ | + | – | – |
| Cav3.2 | ++ | ++ | +/++ | – |
| Cav3.3 | +++ | +++ | +++ | +++ |
| Dentate gyrus granule cells | ||||
| Cav3.1 | + | – | – | – |
| Cav3.2 | ++ | – | – | – |
| Cav3.3 | + | – | – | – |
| Midline thalamic nuclei | ||||
| MHb | ||||
| Cav3.1 | +/++ | – | – | nd |
| Cav3.2 | + | – | – | nd |
| Cav3.3 | + | – | – | nd |
| LHb | ||||
| Cav3.1 | +++ | – | – | nd |
| Cav3.2 | ++ | –/++ | – | nd |
| Cav3.3 | + | – | – | nd |
| PV | ||||
| Cav3.1 | +++ | – | – | nd |
| Cav3.2 | ++ | – | – | nd |
| Cav3.3 | + | – | – | nd |
| Thalamus | ||||
| Relay nuclei | ||||
| Cav3.1 | ++ | –/++ | – | nd |
| Cav3.2 | ++ | –/++ | –/+ | nd |
| Cav3.3 | + | –/+ | –/++ | nd |
| nRT | ||||
| Cav3.1 | ++ | –/++ | –/++ | nd |
| Cav3.2 | ++ | – | – | nd |
| Cav3.3 | –/+ | –/+ | –/++ | nd |
| Local circuit cells | ||||
| Cav3.1 | + | –/+ | –/+ | nd |
| Cav3.2 | ++ | –/+ | – | nd |
| Cav3.3 | ++ | – | – | nd |
| Cerebellum | ||||
| Purkinje | ||||
| Cav3.1 | + | –/+ | –/+ | – |
| Cav3.2 | ++ | –/+ | –/+ | – |
| Cav3.3 | +++ | +++ | +++ | +++ |
| Deep cerebellum | ||||
| Cav3.1 | –/+++ | –/+++ | –/+++ | –/+++ |
| Cav3.2 | –/+ | – | – | – |
| Cav3.3 | –/+++ | –/+++ | –/+++ | –/+++ |
| Inferior olive | ||||
| Cav3.1 | ++ | – | – | nd |
| Cav3.2 | – | – | – | nd |
| Cav3.3 | + | – | – | nd |
- Ratings for intensity refer to that most commonly observed in relation to other regions within a tissue section or between isoforms at a set laser intensity. Definitions: proximal dendrites, <30 µm; mid-dendrites, 30–100 µm; distal dendrites, >100 µm; +, light; ++, moderate; +++, intense; –, below threshold for light microscopic detection (−/++ and −/+++ refer to cells that were either negative or positive for a given isoform); nd, not determined. Label distribution for cortical and hippocampal pyramidal cells refers only to apical dendrites, and in the DCN to the large diameter cell class.
Cav3 neuronal expression
Cav3 channel isoforms were widely expressed, with most cell types labelling for one or more isoforms. As a general rule, Cav3.1 subtype immunolabel was primarily restricted to the somatic and/or proximal dendritic region and exhibited the lightest labelling of all three isoforms. Cav3.2 channel immunolabel was often more intense than Cav3.1, with a distribution at the soma and over at least the proximal to mid portions of dendrites of a number of cell classes. Cav3.3 channels typically exhibited the most intense immunolabel, and could be expressed at the soma as well as extended regions of dendrites. It is important to note that this represents only a general pattern to which several exceptions could be found. The extent of Cav3 isoform distribution between somata and dendrites was also highly cell-specific and is detailed for each of the regions indicated below. In each case we first summarize the available physiological data implicating T-type channels in cell output to help place the immunolabel results in context with known electrophysiological patterns.
Cortex
Pyramidal neurons within layer V of the neocortex express robust LVA calcium currents (Sayer et al., 1990; de la Pena & Geijo-Barrientos, 1996). Synaptic or intracellular stimulation triggers a low threshold calcium spike (LTS) that drives a burst of somatic Na+ spikes (Markram & Sakmann, 1994; Larkum & Zhu, 2002). Patch-clamp recordings have identified a dendritic origin for LTS that is greatest in the region of the distal dendritic tuft (Larkum & Zhu, 2002). LTS behaviour has been identified further in layer V interneurons where it enables dendritic-initiated signalling to activate the soma (Goldberg et al., 2004). Cav3.1 immunolabel has been reported in cortical pyramidal cell bodies and proximal apical dendrites (Craig et al., 1999).
We observed immunolabel for Cav3 channel isoforms in all cortical layers and regions, with the most prominent cell type corresponding to large diameter pyramidal cells. Colabelling with MAP-2 revealed labelling of most cells for Cav3 channels, although cells negative for a given Cav3 isoform could be detected in all cases. A distinct layer-specific banding pattern previously reported for mRNA expression (Kase et al., 1999; Talley et al., 1999) was not apparent in immunostaining, with a fairly homogeneous intensity across layers for each of the Cav3 isoforms. The greatest distinction between isoform labelling was in the extent of Cav3 distribution over the soma-dendritic axis of individual cells. Cav3.1 T-type channel immunolabel was largely restricted to the soma and proximal regions of pyramidal cells as a cytoplasmic label that was sufficient to delineate the boundaries of the plasma membrane (Fig. 2A). Cav3.2 T-type immunolabel was slightly less intense than Cav3.1, but included the soma as well as more extended (<200 µm) regions of the apical dendrites (Fig. 2B). Cav3.3 T-type channel immunolabel stood out in labelling somatic regions, and in particular, extended lengths of pyramidal cell apical dendrites that emanated primarily from cells in layer V (Fig. 2C). In many cases, Cav3.3 immunolabel highlighted elongated regions of individual apical dendrites that could be tracked visually over 600 µm from cells in layer V (Fig. 2C). The distal tuft of neocortical pyramidal cell apical dendrites also expressed immunolabel but often with less apparent intensity than the primary shaft, with the thin branching dendrites readily distinguished only for Cav3.3 immunolabel (Fig. 2C). By comparison, virtually no label for any of the T-type channel isoforms was found in basilar dendrites. An additional aspect of Cav3.1 distribution was a distinctive label associated with some, but not all, local GAD-positive interneurons. A higher power image in Fig. 2D reveals that many neurons colabelled for GAD and Cav3.1 at the soma were positioned adjacent to GAD-negative and presumed pyramidal cell bodies. However, Cav3.1 label did not extend noticeably to interneuron dendritic membranes or the synaptic terminal boutons that surround pyramidal cell somata, indicating a specific subcellular distribution for this T-type calcium channel isoform in local interneurons. As found in many brain regions, Cav3 labelling in the nuclear region was primarily evident for Cav3.3 immunolabel in either pyramidal cells or interneurons (Fig. 2C).
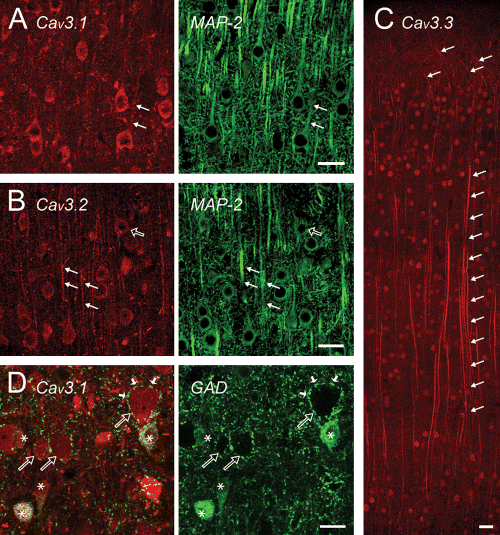
Differential subcellular distribution of Cav3.x isoforms in neocortex. Red labels indicate Cav3 channel isoforms (A–D) and green labels MAP-2-labelled structures in (A and B) and GAD-positive cells in (D). (A) Cav3.1 expression is restricted primarily to the soma and most proximal extent of apical dendrites of cortical pyramidal cells. (B) Cav3.2 labels pyramidal cell somata but extends further into apical dendrites (filled arrows). A putative interneuron is indicated by an open arrow. (C) A montage of all layers in neocortex reveals Cav3.3 label in cell bodies, extended lengths of apical dendrites (column of arrows), and in some branches of the distal tuft of pyramidal cell dendrites (top 4 arrows). Cav3.3-positive cell bodies are apparent in all layers of cortex. (D) Cav3.1 labels the cell bodies of GAD-positive neurons in cortex (asterisks), but not the GAD-positive terminal boutons (small filled arrows) that outline nearby pyramidal cell bodies (open arrows). Shown in (D) are the superimposed labels for Cav3.1 and GAD (left panel) and GAD alone (right panel). Scale bars, 20 µm.
A comparison between the cortical expression pattern of Cav3 channels to previous physiological studies indicate that at least one or more of the Cav3 isoforms are expressed in cell types exhibiting LVA calcium responses. From this pattern we can infer that LVA spikes in the somatic region of pyramidal neurons may involve all three isoforms, while dendritic T-type activity in layer V pyramidal cells likely incorporates Cav3.2 and particularly Cav3.3 in mid-distal dendritic regions.
Hippocampus
Patch-clamp recordings have shown that single T-type channels are present in the somata and apical dendrites of hippocampal pyramidal cells, with a potential increase in channel density in dendritic regions (Fisher et al., 1990; Magee & Johnston, 1995; Kavalali et al., 1997). LVA calcium currents have also been reported to contribute to burst discharge in both hippocampal and subicular pyramidal cells (Jung et al., 2001; Metz et al., 2005). Dentate gyrus granule cells express LVA calcium channels and generate LVA spike responses (Fisher et al., 1990; Zhang et al., 1993). Cav3.1 protein has further been reported in the cell body region of dentate granule cells (Craig et al., 1999). The hippocampal and dentate regions contain a multitude of interneuron subtypes (Freund & Buszaki, 1996). Although interneurons typically exhibit a fast spiking phenotype compared to the burst discharge normally associated with calcium channel expression, recordings have shown calcium conductances in dendrites (Goldberg et al., 2004).
We observed immunolabel for all three T-type calcium channel isoforms in pyramidal cells, with a gradient of relative intensity for all isoforms that could be seen in single tissue slices to increase from a low level in the CA3 field to a higher intensity in CA1, and highest in the subiculum. The general subcellular distribution for the three Cav3 isoforms was apparent in pyramidal cells, with a restricted expression of Cav3.1 in the soma/proximal dendritic region, more extended labelling by Cav3.2 in soma and apical dendrites, and finally the soma and extended lengths of apical dendrites for Cav3.3 (Fig. 3A–G). The most prominent distribution of Cav3 immunolabel was found for Cav3.3 in the soma and over the apical dendritic axis of CA1 pyramidal cells, and in subicular pyramidal cells reached an intensity and degree of dendritic extension similar to that in neocortex (Fig. 3C and D). The pattern of expression in single cells was generally a diffuse internal label, with no clearly definable puncta at the light microscopic level. Apical dendritic labelling of pyramidal cells in CA and subicular regions could only be discerned for the main dendritic shafts and not the oblique dendrites in stratum radiatum, while branches in the distal tuft of apical dendrites in stratum lacunosum-moleculare were less evident than in cortical pyramidal cells. Similarly, we could not detect a noticeable change in Cav3.3 immunolabel between mid- and distal-apical dendritic regions, and no distinct label was detected in pyramidal cell basilar dendrites in hippocampal or subicular regions. In the dentate gyrus we observed a relatively weak expression of Cav3.1 but stronger staining for Cav3.2 and Cav3.3 at the cell body level of granule cells (Fig. 3H–J). We could detect no label for any of the Cav3 channel isoforms in granule cell dendrites despite resolving these structures with a MAP-2 counterlabel (Fig. 3H–J). When present, the more prominent labelling in the nuclear region was for Cav3.3, particularly in CA1 and dentate regions (Fig. 3C and J).
We identified GAD-labelled interneurons or cells with a morphology and location consistent with interneurons that were either positive or negative for different Cav3 channel immunolabels (Fig. 4). Most interneurons were positive for Cav3.3 or in some cases Cav3.2 or Cav3.1. Figure 4A indicates a positive label for Cav3.3 at the soma and at least proximal regions of horizontally projecting processes of a parvalbumin-positive stratum oriens-alvear interneuron. GAD-positive interneurons colabelled for Cav3.3 were located within or adjacent to stratum pyramidale of the CA1 region (Fig. 4B), while many presumed multipolar and fusiform interneurons in the hilar region were positive for Cav3.3 (Fig. 4C) or Cav3.2 (Fig. 4D). Potential MOPP or VIP-containing basket cell interneurons positioned in the inner molecular layer of the dentate gyrus were positive for all three Cav3 isoforms at the soma (Fig. 3H–J; Freund & Buszaki, 1996). An additional clear extension of Cav3.1 into the dendrites of these cells that project into the molecular layer was apparent (Fig. 3H).
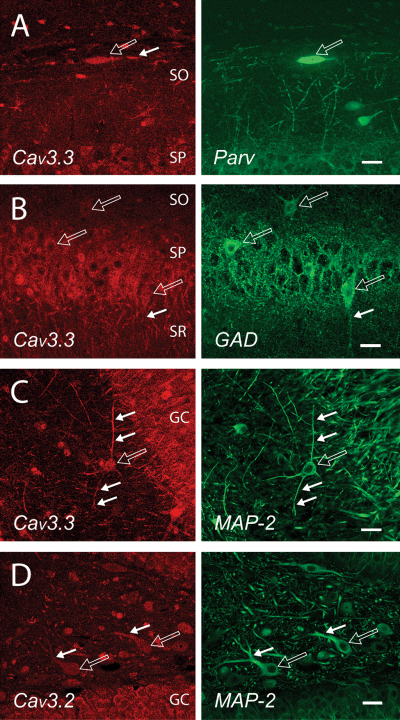
Cav3 channel expression in hippocampal interneurons. Left panels show Cav3 immunolabel and right panels the associated cell structures revealed using parvalbumin (A), GAD (B) or MAP-2 (C and D) immunolabel. Open arrows indicate cell somas and small filled arrows dendritic or axonal processes. (A and B) Immunolabel for Cav3.3 is found in the soma and proximal dendrites of a class of horizontal stratum-oriens alvear interneuron (A), and in most GAD-labelled interneurons within and next to the CA1 pyramidal cell layer (B). (C and D) The soma and MAP-2 labelled processes of many hilar interneurons are positive for Cav3.3 (C) or Cav3.2 (D). GC, granule cell layer; SO, stratum oriens; SP, stratum pyramidale; SR, stratum radiatum. Scale bars, 20 µm.
Cav3 isoforms can thus be predicted to contribute to LVA calcium-dependent responses in somatic membrane of pyramidal cells, and for at least Cav3.3 in apical dendritic regions of CA1 and particularly subicular pyramidal cells. The dendritic LVA responses reported in dentate granule cells may derive from other channel subtypes, given an apparent restriction of Cav3 channel distribution to the somatic and nuclear region. The expression of Cav3 channel isoforms in interneurons in both the hippocampal and dentate gyrus regions was also more prominent than for interneurons in neocortical regions.
Thalamus
The thalamic nuclei represent one of the most extensively studied regions for the expression and functional output of T-type calcium channels. Previous recordings provide a detailed account of the electrical properties of LVA calcium currents and their role in generating rebound discharge and membrane potential oscillations (Pape et al., 2004). Most studies have focused on three primary cell types: (i) relay cells; (ii) local GABAergic circuit neurons within principal thalamic nuclei, and (iii) neurons of the nucleus reticularis (nRT). Similar work has been carried out in the midline habenular nuclei and dorsal paraventricular (PV) thalamic nuclei (Huguenard et al., 1993; Kim & Chang, 2005; Richter et al., 2005). Together these studies provide a wealth of evidence for LVA-mediated responses associated with T-type calcium channel activity, and the importance of distributing these channels to dendritic regions in specific cell types (Huguenard, 1996). However, the extent to which the activity of thalamic subnuclei reflects a differential expression or distribution of Cav3 channel isoforms has not been fully determined. We focused attention on the regions most examined in previous electrophysiological studies, including the medial and lateral habenular nuclei (MHb, LHb), PV, dorsal thalamic nuclei [dorsal lateral geniculate (dLGN), lateral posterior (LP), and lateral dorsal (LD)], and ventrobasal (VB) nuclei [ventroposterolateral (VPl), ventroposteromedial (VPm)], and nRT.
Midline thalamic nuclei
A prominent T-type current that underlies a LVA calcium spike and rebound discharge has been distinguished in LHb neurons (Kim & Chang, 2005). T-type currents in dissociated LHb cells can be further distinguished from relay or nRT cells on the basis of kinetic properties (Huguenard et al., 1993). In contrast, cells in the adjacent MHb exhibit only tonic firing, with no evidence for rebound discharge (Kim & Chang, 2005).
We observed a difference in the intensity and number of cells labelled for Cav3 isoforms that was sufficient to clearly define habenular nuclear boundaries. Cav3.1 T-type channel label was detected in MHb and LHb nuclei as both a cytosolic and putative membrane-associated punctate label (Fig. 5A and D). Although Cav3.1 label could be detected in the MHb the relative intensity of label in individual cells was far greater in LHb neurons. In the LHb distinct puncta of Cav3.1 label outlined the circumference of individual cell somata (Fig. 5D), with little or no evidence for Cav3.1 dendritic label in either nucleus despite resolving these structures with a MAP-2 counter-label. Cav3.2 channel label was present in both MHb and LHb cells as a diffuse or a punctate membrane-associated label in individual cells (Fig. 5B and E). We further detected some extension of Cav3.2 label into proximal dendritic stumps of cells in LHb but not MHb cells, and an additional dense Cav3.2 cytosolic label in a less frequently encountered and smaller diameter cell type in the LHb (Fig. 5E). Unlike other structures examined, Cav3.3 label in the MHb and LHb was expressed in a lower percentage of cells and with a lower relative intensity (Fig. 5C and F). Similarly, we could detect no evidence for a dendritic extension of Cav3.3 T-type channels in either the MHb or LHb, with all label restricted to a punctate somatic label or in some cases an additional nuclear label.
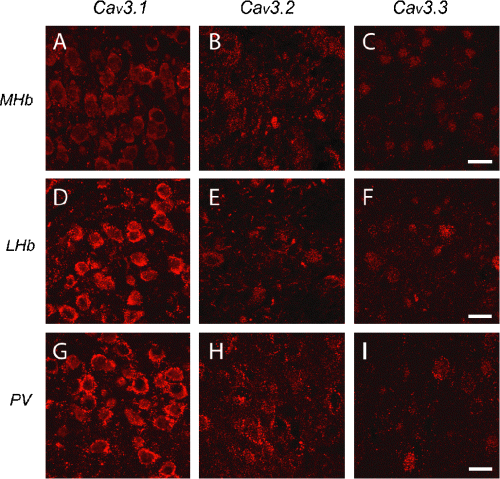
Differential expression and distribution of Cav3 isoforms in habenular and paraventricular nuclei. Columns indicate immunolabel for Cav3.x isoforms and rows correspond to regions of labelling. (A–C) MHb neurons show weak labelling for Cav3.2 and Cav3.3 and strongest labelling for Cav3.1. (D–I) LHb and PV cells show strong punctate and membrane-associated labelling for Cav3.1 that delineates nuclear and plasma membrane boundaries compared to weaker labels for Cav3.2 and Cav3.3. Scale bars, 20 µm.
Neurons of the PV were recently shown to generate LVA calcium spikes that support rebound discharge through T-type calcium channels (Richter et al., 2005). The boundaries between habenular nuclei and the PV were readily apparent in Cav3.1 immunolabelled tissue, with a decrease in the number of cells in the PV compared to MHb, but with fluorescent intensity as high as Cav3.1 in the LHb. Cav3.1 immunolabel was again apparent as an intense punctate and membrane-associated signal that surrounded PV cell somata (Fig. 5G). Cav3.2 was detected as a more sparsely distributed but intense punctate label surrounding PV cell somata (Fig. 5H). Cav3.3 labelling in the PV was light compared to Cav3.1 and 3.2, in a much lower percentage of cells and included both somatic and nuclear labelling (Fig. 5I). As found for the MHb and LHb, we could detect no Cav3 label in PV cell dendrites even with high power resolution of surrounding dendrites with MAP-2.
Cav3 labelling in the midline thalamic nuclei is consistent with previous reports of rebound discharge in LHb and PV neurons as compared to a relatively lower intensity label for Cav3 protein and only tonic spike activity in the adjacent MHb (Huguenard et al., 1993; Richter et al., 2005). These structures were also distinct in exhibiting one of the clearest examples of a punctate pattern of label that can be associated with membrane-associated channels. As compared to cortical and hippocampal neurons, labelling for Cav3.1 in the LHb and PV was among the highest intensity of all structures examined but without detectable dendritic label.
Thalamic relay and nRT cells
Several experimental approaches have provided evidence for a distribution of Cav3 channels over extended regions of the soma-dendritic axis of thalamic relay, local circuit cells, and nRT cells. Patch recordings, calcium imaging and modelling of relay cells in the dLGN and ventrobasal (VB) nuclei indicate that T-type calcium channels are located at the soma and dendrites, with a higher peak density and activation in dendrites 18–60 µm from the soma (Munsch et al., 1997; Zhou et al., 1997; Destexhe et al., 1998; Williams & Stuart, 2000). Local GABAergic interneurons in thalamic nuclei exhibit a greater activation of LVA current in dendrites at 60 µm over that at the soma (Munsch et al., 1997) and less calcium current in dissociated cells when dendrites have been removed (Pape et al., 1994; Munsch et al., 1997; Tarasenko et al., 1997; Zhuravleva et al., 1999, 2001). Similar evidence exists for nRT cells, where models and recordings show that multiple aspects of LVA calcium-mediated responses depend on the dendritic distribution of T-type channels (Destexhe et al., 1996). Moreover, differences in the rate of inactivation of somatic and dendritic T-type currents have been measured with on-cell patch recordings in nRT cells (Joksovic et al., 2005a), directly supporting a selective distribution or modulation of Cav3 isoforms in somatic vs. dendritic regions.
Relay cells
We observed an extensive distribution of immunolabel for Cav3 isoforms in all thalamic nuclei, with Cav3 immunolabel localized to large diameter presumed relay cells, in GABAergic local circuit cells, and nRT cells (Fig. 6). There were few differences in the relative intensity of fluorescent label for Cav3 isoforms between main thalamic nuclei and the nRT. Rather, differences were apparent in the relative intensity of label for different isoforms between cells and over the soma-dendritic axis of individual cell classes. In general, Cav3.1 and Cav3.2 immunolabels were present in the soma of relay cells in both dorsal and ventral divisions, and Cav3.3 as a lighter somatic label. All Cav3 isoforms could be distinguished in individual cells to some degree in the somatic region and dendrites ≤30 µm from the soma, although no attempt was made to quantify the percentage of cells labelled in different nuclei. The most prominent dendritic distribution was apparent for Cav3.3 channels, with label detected in relay cell dendritic segments up to 50 µm in length more frequently than Cav3.1 or Cav3.2 (Fig. 6F and I). For each isoform the relative intensity and number of cells exhibiting a Cav3 label in soma or dendrites detected by MAP-2 colabelling varied, with some identified cells being negative for a given Cav3 isoform. In fact, neighbouring relay cells with dendrites of equivalent length could be negative or positive for dendritic Cav3.3 (data not shown). Labelling for any of the Cav3 isoforms could be distinguished in the nuclear region for a number of cells, but most frequently for Cav3.3 (Fig. 6A–F).
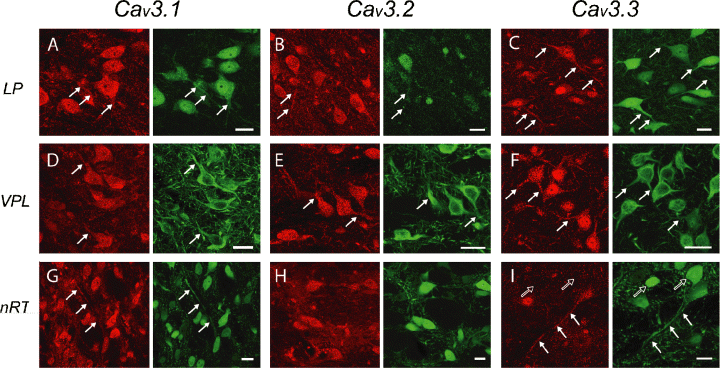
Cav3 channel expression in thalamic relay nuclei and the nRT. Columns indicate immunolabel for Cav3.x isoforms (left panels) with corresponding cell structures labelled with MAP-2 (A–F) or parvalbumin (G–I) (right panels). Rows correspond to region of labelling in the dorsal (LP) and ventral (VPL) thalamic nuclei and the nRT. (A–C) Relay cells in both LP and VPL nuclei label at the soma for Cav3 isoforms, with immunolabel in some cells at the somatic and/or proximal dendritic level (arrows). (G–I) Cells of the nRT label for all three isoforms, but with more prominent somatic label for Cav3.1 and Cav3.2. Note that some somata are negative for Cav3.3 (I) (open arrows). Extended lengths of nRT cell dendrites are positive for either Cav3.1 (G) or Cav3.3 (I) (arrows). LP, lateral posterior nucleus; nRT, nucleus reticularis; VPL, ventroposterolateral nucleus. Scale bar, 20 µm.
nRT cells
Large diameter nRT neurons and their projections were readily distinguished using a GAD or parvalbumin counterlabel (Fig. 6G–I). Immunolabel for Cav3.1 and Cav3.2 isoforms was consistently present at the somatic level of nRT cells (Fig. 6A–F). Cav3.3 label was generally of lighter relative intensity and was present in a lower percentage of nRT cells compared to Cav3.1 or Cav3.2 (Fig. 6I), suggesting that expression of this isoform was restricted to a limited number of cells. In some cases long sections of nRT cell dendrites (up to 100 µm) could be found in tissue reacted for Cav3.1 or Cav3.3 (Fig. 6G and I).
GABAergic local circuit neurons
Immunolabel for GAD revealed extensive axonal arborizations throughout all thalamic nuclei that are expected to arise from both nRT afferents and local circuit neurons (Harris & Hendrickson, 1987). A light and relatively diffuse label for Cav3 channels often found in thalamic nuclei could well correspond to labelling of this dense axonal network, but Cav3 immunolabel was not conclusively identified in axons or terminals in GAD colabelling studies. As previously reported, GABAergic cells were infrequently encountered in ventral thalamic nuclei, but were easily distinguished in the LP, lateral dorsal (LD), dLGN and medial geniculate nuclei (MGN) using a GAD counterlabel. Local circuit cells displayed a relatively light level of somatic labelling for Cav3.1, but label could be identified in some cases in dendritic regions (Fig. 7A). However, other GAD-positive processes of the same cell or nearby cells were unlabelled by Cav3.1 in the same section (Fig. 7A). Light or variable labelling for Cav3.2 could be found on somata of GAD-positive cells (Fig. 7B). Cav3.3 label was most intense in this cell class but was restricted to the nuclear and somatic region (Fig. 7C). The labelling reported here indicates that thalamic cells exhibit a distribution of Cav3 channels consistent with an important role for dendritic LVA calcium channels in determining cell output. A greater variability between MAP-2 labelled dendrites and Cav3 immunolabel compared to cortical or hippocampal regions also suggests a greater heterogeneity of Cav3 channel distribution over the soma-dendritic axis. Thus, relay cells exhibit a distribution of any of the three isoforms in at least the proximal stump of dendrites, but the extent of this distribution that could be detected at the light microscopic level varies between cells. Local circuit interneurons are somewhat unique in distributing Cav3.1 channels to extended regions of dendrites and prominent labelling for Cav3.3 in the nuclear region.
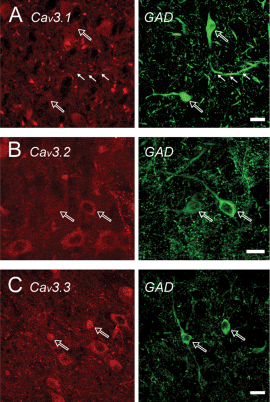
Cav3 channel expression in local circuit thalamic interneurons. Cav3 label is shown on the left and GAD67 labelling on the right. (A) Local circuit neurons in the medial geniculate nuclei (MGN) positive for GAD label lightly at the soma for Cav3.1 (open arrows). Labelling of extended lengths of dendrites of MGN local circuit cells for Cav3.1 could be found for some (filled arrows), but not all cells (see cell indicated by open arrow in lower left of A). (B) GAD-positive cells in the LP exhibit varying levels of Cav3.2 immunolabel (open arrow) with little extension into dendrites. (C) Cav3.3 labels GAD-positive cells but is concentrated in the nuclear region. Scale bars, 20 µm.
Cerebellum and inferior olive
Electrophysiology and calcium imaging studies have established that Purkinje cells and neurons of the deep cerebellar nuclei (DCN) generate LVA-mediated responses attributed to T-type calcium current. In Purkinje cells T-type single channels can be recorded in dendritic regions (Mouginot et al., 1997) and contribute to both the burst and interburst depolarizations (Swensen & Bean, 2003; Isope & Murphy, 2005). In DCN cells Cav3 channels contribute to a rebound burst discharge following Purkinje cell inhibition (Gauck et al., 2001; Molineux et al., 2006). Inferior olivary neurons provide climbing fibre input to both DCN and Purkinje cells and were the first cell type identified as generating LVA calcium spikes (Llinas & Yarom, 1981). Unlike other major cells with prominent IT, the LTS in inferior olivary neurons was proposed to be initiated at the soma rather than dendrites (Llinas & Yarom, 1981).
The distribution of Cav3 channel proteins in cerebellar neurons was recently described, revealing a widespread expression of at least one or more Cav3 isoforms in the main cerebellar cell types, and a striking distribution of Cav3.3 to at least Purkinje cell dendrites (Molineux et al., 2006). Here we focus on the extent of dendritic Cav3 channel distribution in the three major input and output cells of cerebellum: Purkinje, DCN and inferior olivary neurons.
Purkinje cells
Cav3 channels were distributed in Purkinje cells with the same general pattern we detected in other brain regions: primarily Cav3.1 and Cav3.2 at the soma or proximal dendrites, and Cav3.3 at the soma and over extensive regions of the dendritic tree of most Purkinje cells (Molineux et al., 2006). Craig et al. (1999) found that Cav3.1 label could also extend over the dendrites of at least some Purkinje cells of mouse cerebellum. In our hands, Cav3.1 and Cav3.2 could be detected in some rat Purkinje cell dendrites, but only in a very small fraction of cells.
Figure 8A and B provides magnified images of the distribution of Cav3.3 channels in Purkinje cell dendrites. We found that Cav3.3 immunolabel was distributed as either a diffuse cytosolic label (Fig. 8A) or as a distinct punctate or patchy distribution over the primary dendritic shafts across the entire extent of the molecular layer (Fig. 8B). A lower intensity of Cav3.3 immunolabel extended into the smaller diameter secondary and tertiary dendritic branches (Fig. 8B), but no label of dendritic spines could be detected even at 100× magnification. In thin confocal sections the patchy distribution of Cav3.3 immunolabel could be visualized further as being positioned on the circumference of the dendritic shaft, as highlighted by an internal cytoplasmic core of MAP-2 counterlabel (Fig. 8B; arrows). Labelling in the region of the nucleus was also distinguished for stellate cells in the molecular layer for Cav3.3 (Fig. 8A and B).

Cav3 channel expression in principal ouput and input neurons of the cerebellum. Shown are images of Purkinje cell dendrites (A and B), deep cerebellar neurons (C and D) and inferior olivary neurons (E and F) colabelled for calbindin (A, E and F) or MAP-2 (B–D) and Cav3 channel isoforms. Images of Cav3 and colabels are shown superimposed in the right panels of A and B. (A and B) Purkinje cell dendrites exhibit an extensive distribution of Cav3.3 channels over the primary shafts (A) and secondary and tertiary dendritic branches (B). In 1 µm sections a distinct punctate or patchy distribution of Cav3.3 label can be detected surrounding the MAP-2 positive internal core (B, arrows). (C and D) Select DCN neurons label at the soma and up to 60 µm of dendrites (filled arrows) for Cav3.1 (C) and 120 µm for Cav3.3 (D). Individual dendrites of the same cell or neighbouring cells are Cav3.1 negative (C, small open arrows). (E and F) Calbindin-labelled inferior olivary neurons express Cav3.1 (E) or Cav3.3 (F) only at the soma (open arrows), with no Cav3.1 label apparent in dendritic processes (E, small open arrows) and only minimal extension of Cav3.3 into proximal dendrites (F, small filled arrow). Scale bars, 20 µm.
Deep cerebellar nucleus
We have previously shown that large diameter DCN neurons differentially label for the three Cav3 channel isoforms, with one class of bursting DCN neuron consistently labelling for Cav3.1 at the soma and a non-bursting neuron class for Cav3.3 (Molineux et al., 2006). The Cav3.1 labelled cells correspond to both excitatory and GAD-positive cells, while Cav3.3 was located only on excitatory neurons. As shown in Fig. 8C, large diameter cells occasionally exhibited Cav3.1 channel distribution up to 60 µm from the soma. More commonly, we detected a Cav3.3 channel dendritic distribution that could extend over a distance of 120 µm and beyond at least the first dendritic branch point (Fig. 8D). Labelling in the nuclear region was apparent for both Cav3.1 and Cav3.3.
Inferior olivary neurons
Inferior olivary cells identified through a calbindin counterlabel expressed detectable levels of only Cav3.1 and Cav3.3 at the soma (Fig. 8E and F) and were negative for Cav3.2 (not shown). The most intense label was found for Cav3.1 at the soma, with no apparent dendritic label for Cav3.1 (Fig. 8E). Cav3.3 label was comparatively light and associated with the somatic and nuclear regions (Fig. 8F).
Discussion
The present study establishes the distribution of Cav3.1, Cav3.2 and Cav3.3 calcium channel isoforms in key brain regions reported to express these channels and/or generate putative T-type mediated responses. Cav3 channels were detected across a large spectrum of cells, and in many cases individual cell types expressed more than one Cav3 isoform. Furthermore, Cav3 isoforms exhibit a differential distribution over somatic and dendritic regions, suggesting that select Cav3 channel isoforms differentially contribute to LVA calcium-dependent responses in distinct subcellular compartments.
Determination of Cav3 channel distribution
The function of T-type calcium channels has been investigated in depth through physiological recordings, while more recently the pattern of Cav3 mRNA expression has been detailed (Craig et al., 1999; Kase et al., 1999; Talley et al., 1999). The distribution of Cav3 protein described here agrees well with the cell types previously demonstrated to be capable of generating LVA calcium-dependent responses. The Cav3 immunostaining also largely agrees with that reported for Cav3 mRNA. However, it has become recognized that the correspondence between detectable mRNA and protein levels obtained through in situ hybridization and immunocytochemistry can differ considerably, with neither serving as a faithful predictor of the other (Gygi et al., 1999; Zhang et al., 2002). One example is the finding that ∼80% of the nRT cell T-type current is blocked by N2O (a specific blocker of Cav3.2 channels in expression systems) despite much higher apparent levels of Cav3.3 mRNA (Talley et al., 1999; Joksovic et al., 2005b). Thus, the location and relative intensity of immunolabel was similar to that reported for mRNA in some cell types (i.e. hippocampal pyramidal cells, inferior olive), while others showed less correspondence (i.e. thalamic relay nuclei vs. nRT). We previously found that the distribution of Cav3 immunolabel in cerebellar cell types was also different from the reported mRNA expression (Molineux et al., 2006). However, Cav3 protein distribution proved to entirely match the ability to generate LTS in six different cerebellar cell types when competing outward potassium currents were blocked. Thus, even when calcium channels are transported to the membrane, their activation can be masked by the coexpression of potassium channels (Pape et al., 1994; Molineux et al., 2005, 2006). Work to date then indicates that Cav3 immunostaining with these antibodies can be an accurate reflection of the cells that express T-type channels. Nevertheless, the relation between mRNA expression, immunolabel distribution, and cell activity ultimately needs to be verified through physiological recordings. For this reason we will use electrophysiological data from the literature to provide a physiological context to our results and assess the potential functional significance of our labelling patterns.
Cav3 distribution and physiological activity
Distributing select Cav3 channel isoforms with distinguishable kinetic properties to different parts of a cell would be expected to influence cell output. Indeed, one consequence of expressing multiple Cav3 isoforms may be to increase the potential range for post-translational modifications of channel function. A comparison of our immunolabel results with previous electrophysiological studies identifies some correlations between Cav3 channel expression and different T-type channel-mediated responses.
Calcium spike discharge
A prominent electrophysiological response associated with T-type calcium channel activation is a LVA calcium spike. Many of the cell types labelled for Cav3 isoform(s) generate somatic or dendritic calcium spike discharge. In particular, dendrites were most often associated with an extended distribution of Cav3.3 immunolabel, suggesting that at least this isoform can contribute to dendritic LVA calcium responses. LVA calcium influx in cortical pyramidal cell dendrites (Yuste et al., 1994; Jung et al., 2001; Larkum & Zhu, 2002) thus correlates with a widespread distribution of Cav3.3 channels over the apical dendritic axis. The prominent calcium spikes of cerebellar Purkinje cells can be expected to include the activation of Cav3.3 channels that are distributed over primary, secondary and tertiary dendritic branches (Swensen & Bean, 2003; Isope & Murphy, 2005). Note that although a dendritic localization of Cav3.3 immunolabel was prominent in many cell types, we could also detect a proximal to mid-dendritic distribution of Cav3.2 channels or even Cav3.1 channels, suggesting the additional involvement of these isoforms. In contrast, LVA calcium currents of inferior olivary neurons appear to derive from Cav3.1 and Cav3.3 isoforms localized to the soma.
Rebound discharge
A second output that results from the activation of T-type calcium channels is a rebound depolarization following a preceding hyperpolarization. This activity can initiate a high frequency spike output in response to inhibition or contribute to oscillatory swings in membrane potential. Recent work has indicated that rebound discharge in thalamic relay cells is blocked in a Cav3.1 knockout animal (Kim et al., 2001). An extensive analysis of mRNA through RT-PCR also reported a correlation between the expression of Cav3.1 mRNA and the ability to generate rebound bursts (Toledo-Rodriguez et al., 2004). We recently established a correlation between Cav3.1 channel expression and rebound discharge in large diameter DCN cells (Molineux et al., 2006). Cerebellar Golgi cells and Purkinje cells also label for Cav3.1 and generate rebound depolarizations, while non-bursting DCN cells and stellate cells that lack this isoform do not. A similar correlation between Cav3.1 expression and rebound burst discharge appears to exist between the habenular nuclei, where intense somatic Cav3.1 immunolabel in LHb cells correlates to rebound burst capability, whereas MHb cells that exhibit only light Cav3.1 label do not exhibit rebound bursts (Kim & Chang, 2005).
Collectively, the available data suggests that expression of Cav3.1 channels may be sufficient and potentially necessary to generate rebound discharge in a wide variety of neurons. Nevertheless, the extent to which these correlations may also reflect the relative density of Cav3.1 channel expression, varying kinetic properties of Cav3 isoforms through post-translational modulation, or the degree of interplay with other ion channels remains to be determined.
Kinetic properties
Kinetic properties of individual Cav3 isoforms have been characterized primarily in expression systems at room temperature (McRory et al., 2001; Chemin et al., 2002; Perez-Reyes, 2003). The degree of correspondence between the properties of expressed channels and those in native neurons is not fully known. As most of the cell types examined here express more than one Cav3 channel isoform, it may be difficult to predict the properties of whole-cell Cav3 current. Differences in the properties of T-type currents have been reported between thalamic regions (Huguenard et al., 1993; Joksovic et al., 2006). Our immunolabel did not reveal striking differences in the expression pattern of the Cav3 isoform protein between thalamic nuclei, but this does not rule out the selective translocation of a given isoform to the plasma membrane or post-translational modifications as a source for physiological differences. It is also difficult to relate differences in burst properties to soma-dendritic Cav3 channel distributions (i.e. relay vs. nRT cells). In general, relay cells are positive for all three Cav3 isoforms at the soma and proximal dendrites and shift to a distribution of Cav3.3 label in distal dendrites. Cav3.1 and Cav3.2 label is readily detected at the soma of nRT cells, but Cav3.1 and/or Cav3.3 immunolabel can be distributed over extensive regions of dendritic membrane in a given cell. Recent patch recordings reveal that T-type calcium channels at the soma of nRT cells exhibit a faster rate of inactivation (τ = 28 ms) than those recorded in proximal dendrites (τ = 53 ms; Joksovic et al., 2005a). These findings are at least consistent with a higher density of Cav3.3 channels in dendritic regions that may influence spike output in these cells (Destexhe et al., 1996; Destexhe et al., 1998; Williams & Stuart, 2000).
Synaptic responses
LVA calcium currents have been shown to contribute to synaptically activated calcium influx over extensive regions of the dendritic arbor (Yuste et al., 1994; Magee et al., 1995). Our data suggest that all three T-type calcium channel isoforms may contribute to this activity in proximal dendritic regions of many cell types, but that more distal involvement of T-type channels to synaptic input is likely to be mediated primarily by Cav3.3 channels. Another important potential location of T-type channel distribution is dendritic spines, where low voltage synaptic responses could be amplified by Cav3 activation. Putative T-type mediated calcium influx has been identified in the spines of Purkinje cell dendrites from younger animals (Isope & Murphy, 2005). We were unable to obtain any evidence here for Cav3 channel distribution to dendritic spines, including that of Purkinje cells, where these structures can be readily visualized using a calbindin counterlabel. A distribution to dendritic spines may then be restricted to early developmental stages or simply fall below resolution at the light microscopic level. Alternatively, calcium entry into dendritic spines may be secondary to calcium influx in the adjacent dendritic shaft or involve other LVA-like channels (Sabatini & Svoboda, 2000).
Nuclear regulation
The most unexpected labelling pattern for Cav3 protein was in the nuclear region of some cells. To our knowledge this is the first report and test for voltage-dependent calcium channels localized to the nuclear protein fraction, even though several other ion channel types have been directly recorded (Mazzanti et al., 2001). The exact location at which these channels are expressed remains to be determined. Membranes of the smooth ER are continuous with the outer membrane of the nuclear envelope, where a high density of large diameter nuclear pore complexes (NPCs) are expressed. The classical view of the NPC is of a fixed and open structure that allows free transfer of compounds between the cytoplasm and nucleoplasm. This understanding has been substantially revised with findings that the NPC is actually semipermeable, with an additional set of eight smaller diameter pores in the outer ring of the NPC that allows passage of smaller inorganic ions (Pante & Aebi, 1995). Patch-clamp recordings have measured conductances in nuclear membranes at 800–1000 nS for the unblocked NPC core, as well as smaller conductances of 2–200 nS. The source of these smaller conductances has not been determined, but could potentially correspond to the small diameter outer pores of the NPC. Altogether, the nuclear envelope acts as a high resistance and semipermeable membrane that maintains transmembrane gradients for ion species (Mazzanti et al., 2001). Nucleocytoplasmic transmembrane potentials of up to −33 mV below that of the plasma membrane potential have been measured, although this waits to be determined for neuronal nuclei. The nuclear envelope is recognized further for acting as a calcium store for the release of calcium following IP3 or ryanodine receptor activation that can react to events at the level of the plasma membrane or cytoplasm (Gerasimenko et al., 1995; Hardingham et al., 2001; Marchenko & Thomas, 2006; Zhang et al., 2006). Indeed, postsynaptic activity can regulate the extent and time-course of nuclear calcium increases, providing a means of integrating neuronal firing patterns in terms of the level of gene transcription (Hardingham et al., 2001; Power & Sah, 2002). The nucleus thus exhibits the necessary calcium concentration gradient and potentially voltage gradient to employ the LVA properties of Cav3 channels. As such, our results suggest the potential for T-type calcium channels to have previously unrecognized roles in regulating nuclear membrane activity, and add to a growing list of voltage-activated ion channels that can be localized to the nuclear region.
Acknowledgements
We gratefully acknowledge the expert technical assistance of Mirna Kruskic. Funded by the CIHR (TPS, GWZ, RWT) and Heart and Stroke Foundation (GWZ). RWT is an AHFMR Scientist, GWZ an AHFMR Senior Scholar and Canada Research Chair, and TPS a Canada Research Chair. BEM was funded by the Killam Trust, CIHR-CGS and Steinhauer Doctoral awards.
Abbreviations
-
- DCN
-
- deep cerebellar nuclei
-
- dLGN
-
- dorsal lateral geniculate
-
- GAD
-
- glutamic acid decarboxylase
-
- LTS
-
- low-threshold calcium spikes
-
- LHb
-
- lateral habenula
-
- LP
-
- lateral posterior nucleus
-
- LVA
-
- low-voltage-activated
-
- MAP-2
-
- microtubule-associated protein
-
- MHb
-
- medial habenula
-
- NPC
-
- nuclear pore complexes
-
- nRT
-
- nucleus reticularis
-
- PB
-
- phosphate-buffer
-
- PV
-
- paraventricular nucleus




