A potent non-monoaminergic paradoxical sleep inhibitory system: a reverse microdialysis and single-unit recording study
Abstract
Using reverse microdialysis and polygraphic recordings in freely moving cats, we investigated the effects on sleep–waking states of application of excitatory and inhibitory amino acid agonists, cholinergic agonist and monoamines to the periaqueductal grey and adjacent mesopontine tegmentum. Single-unit recordings during behavioural states were further used to determine the neuronal characteristics of these structures. We found that muscimol, a GABAA receptor agonist, induced a significant increase in paradoxical sleep (PS) only when applied to a dorsocaudal central tegmental field (dcFTC) located just beneath the ventrolateral periaqueductal grey. In this structure, both kainic and N-methyl-aspartic acids caused a dose-dependent increase in wakefulness (W) and decrease in both slow-wave sleep (SWS) and PS. Norepinephrine and epinephrine, and to a lesser extent histamine, also increased W and decreased SWS and PS, whereas serotonin, dopamine and carbachol, a cholinergic agonist, had no effect. Two types of neurones were recorded in this structure, those exhibiting a higher rate of tonic discharge during both W and PS compared with during SWS, and those showing a phasic increase in firing rate during both active W and PS. Both types of neurones showed a gradual increase in unit activity during PS. Our study demonstrated for the first time that the ventrolateral periaqueductal grey and dcFTC play different roles in behavioural state control, that the dcFTC neurones are critically involved in the inhibitory mechanisms of PS generation, playing a central part in its maintenance, and that these neurones are under the control of GABAergic, glutamatergic, adrenergic and histaminergic systems.
Introduction
Paradoxical sleep (PS), also known as rapid eye movement sleep, is thought to be controlled by two opposite mechanisms, i.e. PS-generating (or PS-executive) and PS-inhibiting (or PS-permissive) mechanisms (Sakai et al., 2001; Pace-Schott & Hobson, 2002; Jones, 2004; McCarley, 2004). PS appears to be generated as a result of a combination of tonic excitation of cholinergic and non-cholinergic (presumably glutamatergic) PS-on neurones serving an executive function, and the cessation of activity of monoaminergic and non-monoaminergic (possibly GABAergic) PS-off neurones playing an inhibitory role in PS generation by inhibiting PS-on neurones during wakefulness (W) and slow-wave (or non-rapid eye movement) sleep (SWS) (Sakai et al., 2001). In the pons, PS-on neurones, responsible for the initiation and maintenance of PS, are found almost exclusively in the peri-locus coeruleus alpha (peri-LCα) [equivalent to the sublaterodorsal nucleus (Swanson, 1998) or the dorsal subcoeruleus nucleus (Paxinos & Watson, 1997) of the rat brain], located ventromedial to the locus coeruleus alpha which contains tightly packed noradrenergic neurones. The rostral part of the peri-LCα contains a dense population of cholinergic neurones that send axons to the thalamus and/or hypothalamus, whereas the caudal peri-LCα contains mainly non-cholinergic and non-monoaminergic descending neurones (Sakai, 1991). Pressure or microdialysis application to the caudal peri-LCα of carbachol, a potent cholinergic agonist (Vanni-Mercier et al., 1989; Yamamoto et al., 1990; Sakai & Onoe, 1997), or kainate, an excitatory amino acid agonist (Onoe & Sakai, 1995; Crochet & Sakai, 1999; Boissard et al., 2002), results in a paramount increase in PS, whereas muscimol, a GABAA receptor agonist, applied to the same structure leads to the suppression of PS (Sakai et al., 2001; Xi et al., 2001). PS-off neurones are found in both serotonergic dorsal raphe and noradrenergic locus coeruleus nuclei (for review see Steriade & McCarley, 1990). Although some previous studies with brain lesion, cooling or local application of GABA reported their possible permissive, disinhibitory role in PS generation (Cespuglio et al., 1982; Portas et al., 1996; Mallick et al., 2001), their role in the inhibitory mechanisms of PS generation is still unclear, particularly as previous studies and our recent studies in the cat showed that inactivation of either of these monoaminergic neurones does not result in a significant increase in PS (Jones et al., 1977; Petitjean et al., 1978; Sakai & Crochet, 2001, 2004). Therefore, the exact localization of brain structures that play a critical role in the inhibitory mechanisms of PS generation still remains to be determined. In this respect, early lesion experiments showed that bilateral lesioning of a small region overlapping the ventrolateral region of the periaqueductal grey (PAG) and adjacent mesopontine tegmentum produces a marked (up to 56% of 24-h recording time) and long-lasting (4–5 days) increase in PS (Petitjean et al., 1975). The results indicate that this tegmental region plays a pivotal role in the inhibitory mechanisms of PS generation. Indeed, such a pronounced increase in PS has never been produced by any other brain lesions (Jouvet, 1972; Moruzzi, 1972; Steriade & McCarley, 1990). A dramatic increase in PS has recently been reported following bilateral microinjections of muscimol into the ventrolateral PAG (vlPAG) and its immediate vicinity of the mesopontine reticular core (Sastre et al., 1996), referred to as the ‘central tegmental field (FTC)’ by Berman (1968). These studies indicate that the PS-enhancing effects observed are mainly due to inactivation of neurones but not destruction of fibres of passage. The exact mesopontine tegmental zone and neurones critically implicated in this PS-inhibiting mechanism, however, remain to be determined. Recent studies have suggested that GABAergic inhibitory input from the vlPAG may be important for the inactivation of monoaminergic (noradrenergic and serotonergic) PS-off neurones and the control of PS (Nitz & Siegel, 1997a,b; Gervasoni et al., 1998, 2000). Here, we report, using reverse microdialysis and extracellular single-unit recordings across behavioural states in freely behaving animals, that the dorsocaudal FTC (dcFTC), but not the vlPAG, is critically implicated in the inhibitory mechanisms of PS generation, playing a central part in the maintenance of PS, and that these neurones are under the control of GABAergic, glutamatergic, adrenergic and histaminergic systems.
Materials and methods
Animals and surgery
The experiments were carried out on 10 adult cats which were born and bred in our laboratory at Claude Bernard University Lyon 1. All procedures were carried out in conformity with the EEC Council Directive 86/609, and was approved by the department of human biology from the University of Lyon 1, and every effort was made to minimize the number of animals used. The surgery was performed under pentobarbital (Nembutal) anaesthesia (25 mg/kg, i.v.). Polygraphic measurements included the neocortical and dorsal hippocampal electroencephalograms (EEGs), electro-oculogram, neck muscle electromyogram, and ponto-geniculo-occipital (PGO) waves recorded from the dorsal lateral geniculate nucleus. In addition to the conventional fronto-occipital leads for EEG recordings, the leads from the sensorimotor cortices were used to detect sleep spindles and gamma waves. For microdialysis application of drugs, a total of four guide cannulae (23 gauge) with in-dwelling stylets were implanted bilaterally in the brain under stereotaxic guidance, with their tips lying 5 mm above the PAG. For extracellular single-unit recording, two bundles of six flexible, Formvar-coated, stainless steel wires (32 µm in diameter) were implanted in the PAG of three animals through the guide cannulae (24 gauge) of a mechanical drive assembly, fixed to the skull. In addition, in two of three animals, bipolar stimulating electrodes consisting of two stainless steel wires (200 µm diameter, 1.0 mm apart, bared 0.5 mm at the tip), were placed in the ventral tegmental area (A, 4.0 mm; L, 2.0 mm; DV, −4.0 mm) and lateral posterior hypothalamus (A, 9.0 mm; L, 3.0 mm; DV, −4.0 mm) at a 90° angle, and the ventrolateral medulla (P, 8.5 mm; L, 2.5 mm; DV, −9.0 mm) and peri-LCα (P, 1.0 mm; L, 2.0 mm; DV, −3.5 mm) at angles of, respectively, 52° and 60° to the horizontal. After recovery from surgery, the cats were housed individually in a sound-attenuated and electrically shielded recording chamber at 24–26 °C under dim illumination from 06:00 to 24:00 h. Food and water were available ad libitum. The animals were habituated to the experimental conditions until they displayed normal sleep–waking cycles (Crochet & Sakai, 1999).
Polygraphic and unit recordings and analysis
The animals were connected to a polygraph (Model 78B, Grass Instrument, Quincy, MA, USA) through a slip-ring. Polygraphic signals were amplified and band-pass filtered at either 0.5–120 Hz for the electro-oculogram and the neocortical (fronto-occipital leads) and hippocampal EEGs or 5–120 Hz for the neck muscle electromyogram, lateral geniculate and sensorimotor cortex EEGs. Unit recordings were made differentially between the active and reference microelectrodes within the same bundle. The unit activities were amplified with low- and high-cut filters of 100 Hz and 10 kHz, respectively (model P15 amplifier, Grass Instrument), and were monitored on a storage oscilloscope (5103N, Tectronix, Les Ulis, France) and on a digital memory oscilloscope (DRO 1604, Gould Electronique, Longjumeau, France). The polygraphic and unit data were digitized using a 1401 data processor (Cambridge Electronic Design, Cambridge, UK) at a sampling rate of 128 Hz and 20 kHz, respectively. The behaviour of the animal was monitored and noted during experiments through a digital video camera attached to the inside of the recording chamber. Behavioural states were determined by both automated and visual analysis of the electromyogram, electro-oculogram, and fronto-occipital, hippocampal and lateral geniculate nucleus (PGO waves) EEG recordings. States of vigilance were scored minute by minute and classified according to standard criteria into the four phases of W, light SWS (S1), deep SWS (S2) and PS. If drowsiness was accompanied by more than 10 large (> 100 µV) rapid eye movements per minute, it was scored as W and if not, it was scored as S1. Power spectra were computed for consecutive 4-s epochs of the EEG data using a fast Fourier transform routine and the spike2 analysing program (Cambridge Electronic Design). Mean spectra were calculated for 1-min epochs by averaging 15 consecutive 4-s epochs (Crochet & Sakai, 1999).
Analysis of unitary activity was performed using a 1401 data processor and spike2 software (both Cambridge Electronic Design). The mean firing rates were calculated from either continuous 60-s recordings or over 50-s recordings using 5- or 10-s bins for each of the following states: (1) active W, characterized by the presence of gross body movements; (2) quiet W, characterized by the absence of gross motor activity; (3) S2; (4) SWS with PGO waves; (5) PS without PGO waves and rapid eye movements; and (6) PS with PGO and rapid eye movement bursts. The firing rate of FTC neurones in the different states was analysed using one-way anova and Tukey-Kramer HSD (alpha = 0.05) to compare the mean values (data not shown).
Reverse microdialysis
The microdialysis technique has previously been described in detail (Crochet & Sakai, 1999). Briefly, a microdialysis probe (A-L-50-01, EICOM, Kyoto, Japan) was inserted through a guide cannula on the day before each experimental session in order to avoid any effect of acute tissue damage on the EEG and behavioural states. Each probe was left in place for 5–6 days. The probe membrane was 1 mm in length and had an external diameter of 0.22 mm. To keep the experimental factors constant, drug application was begun at least 1 h after a control recording session consisting of at least one complete sleep–wake cycle, defined as an initial period of waking, a series of slow-wave (S1 and S2) sleep epochs and subsequent PS episodes lasting more than 3 min. After the control recording (within 10–15 min after the last PS episode), the perfusate was switched from Ringer's solution to drug solution for 2 consecutive hours and then the perfusate was switched back to Ringer's solution in order to assess recovery. At the end of each experimental session, the probe was removed from the brain and the cat was allowed to recover for at least 2 days before the next experimental session. For all experiments, the order of drug application was random and successive microdialysis probe insertions through the same cannulae to more ventral sites were separated by at least 2 weeks. One or a maximum of three insertions were made through the same guide cannula. Only in six cases out of 32 were more than two trials (same drug, same concentration) performed in the same cat. In any case, multiple trials from the same animal came from different sites, often the contralateral side.
Statistical analysis
Amounts of the different wake–sleep states were expressed in minutes and the means ± SEM calculated for the 2 h of drug application. The baseline values for each behavioural state of each experimental session were obtained by averaging the values of control recordings with Ringer's solution alone (once or twice a week). Data were first subjected to one-way anova and Tukey-Kramer HSD (alpha = 0.05) was used to compare the mean values within each experimental group (data not shown). Control values were obtained using one to three control recordings from each cat. For a more specific comparison between each experimental condition and the control condition, a two-tailed Student's t-test was used; the effects were analysed by comparing the experimental values with those of paired controls obtained from the same animal, site and experimental period (Crochet & Sakai, 1999). In all instances P < 0.05 was considered statistically significant. Site dependency of the effect of muscimol was analysed by comparing the amounts of PS during the 2-h drug application period after local application of 0.5 mm muscimol with those after application of Ringer's solution alone (baseline). A change greater than two SDs from the mean baseline value was considered significant.
Drugs
The drugs used were carbamylcholine chloride (carbachol), arterenol (norepinephrine) bitartrate salt (NE), epinephrine hydrochloride (E), 3-hydroxytyramine (dopamine) hydrochloride (DA), 5-hydroxytryptamine (serotonin) hydrochloride (5-HT), histamine dihydrochloride (HA), kainic acid (KA), N-methyl-d-aspartic acid (NMDA) and muscimol (all from Sigma RBI, Natick, MA, USA). All drugs were dissolved in Ringer's solution (pH ca. 6.0) just before use and protected from exposure to light and heat during the experiment. Drug solutions were adjusted to pH 5.0–6.0, if necessary, with 0.5 n NaOH.
Histology
At the end of the experiments, all animals were deeply anaesthetized by i.v. injection of pentobarbital and perfused through the ascending aorta with 1000 mL of Ringer's lactate solution, followed by 2500 mL of an ice-cold fixative containing 4% paraformaldehyde, 0.05% glutaraldehyde and 0.2% picric acid in 0.1 m phosphate buffer (pH 7.4). The positions of the dialysis probes were determined on neutral-red-stained sections (Fig. 1A; for more details see Crochet & Sakai, 1999).
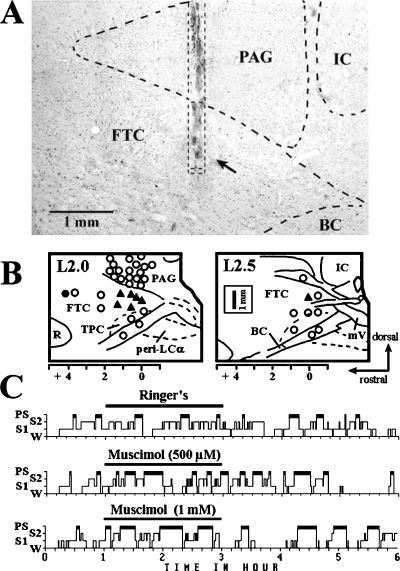
(A) Photomicrograph showing location of microdialysis probe in a sagittal section. An arrow indicates the probe membrane located in the dorsocaudal central tegmental field (dcFTC). When applied to this site, 500 µm muscimol induced a two-fold increase in paradoxical sleep (PS), whereas muscimol had no effect when applied to the periaqueductal grey (PAG) at 1 mm above the dcFTC. BC, brachium conjunctivum; IC, inferior colliculus. (B) Drawings of sagittal sections at L2.0 and L2.5 of the cat brainstem illustrating drug infusion sites (tip of the microdialysis probe membrane). Arrows on the right show the orientation (dorsal and rostral) for the drawings and also for the photomicrograph in A. ○, site at which muscimol had no significant effect on PS; , site at which the amount of PS during 2-h application of 0.5 mm muscimol was increased to greater than 2 SDs from the mean baseline value; •, site at which the amount of PS was reduced to less than 2 SDs from the mean baseline value and the amount of wakefulness (W) was increased to greater than 2 SDs from the mean baseline value. FTC, central tegmental field; mV, mesencephalic tract of trigeminus; peri-LCα, peri-locus coeruleus alpha; R, red nucleus; TPC, cholinergic component of the nucleus tegmenti pedunculopontinus, pars compacta. (C) Hypnograms showing the effect on the sleep–wake states of the application of Ringer's (control), 500 µm or 1 mm muscimol. S1, light slow-wave sleep; S2, deep slow-wave sleep.
Results
Effects of application of muscimol on wake–sleep states
The effects of the microdialysis application of 0.5 mm muscimol on behavioural states were first examined in the PAG and adjacent dorsal mesopontine tegmentum. As shown in Fig. 1B, muscimol had no significant effect on behavioural states when applied either dorsally to the PAG or ventrally to the cholinergic component of the pedunculopontine tegmental nucleus and adjacent brachium conjunctivum. In contrast, muscimol caused a significant increase in PS without affecting other behavioural states when applied to the dcFTC just ventral to the vlPAG at (AP) planes A0 to A1.5 (Fig. 1A). When applied to the rostrodorsal FTC at A4.0 plane, it produced a significant decrease in PS, in parallel to an increase in W. The PS-enhancing effect of muscimol was dose dependent (Fig. 2) and resulted from a significant increase in both the mean duration and number of PS episodes. The latency to onset of PS decreased but the difference was not significant (Fig. 1C, Table 1). Figure 2A depicts the effects of application of muscimol to the vlPAG just dorsal to the dcFTC. As seen in this figure, muscimol had no effect on PS at concentrations of 50–1000 µm, whereas at the highest concentration, it induced a significant decrease in S2 (see Discussion).
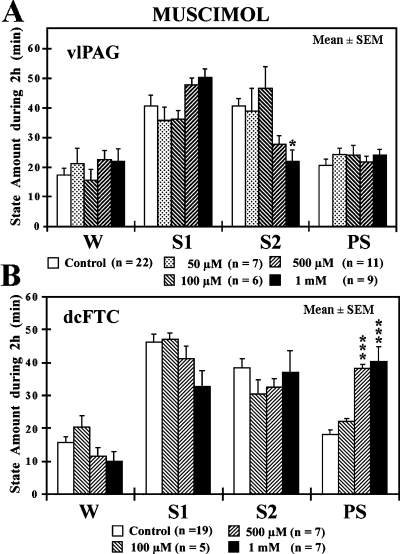
Effects of application of muscimol to the ventrolateral periaqueductal grey (vlPAG) (A) and the dorsocaudal central tegmental field (dcFTC) (B) on wake–sleep states. The values are the mean time ± SEM (min) spent in wakefulness (W), light slow-wave sleep (SWS) (S1), deep SWS (S2) or paradoxical sleep (PS) during the 2-h drug application period. During the 2-h experiment, the microdialysis probe was perfused with either Ringer's solution (control) or Ringer's solution containing 50, 100, 500 or 1000 µm muscimol. The number of trials (n) is indicated in parentheses. *P < 0.05, ***P < 0.001. Data from 11 cats.
| PS parameter | Baseline | Muscimol | ||
|---|---|---|---|---|
| Ringer's(n = 19) | 100 µm(n = 5) | 500 µm(n = 7) | 1 mm(n = 7) | |
| Latency to onset of first PS (min) | 16.7 ± 2.2 | 9.6 ± 2.8 | 11.1 ± 1.6 | 9.1 ± 2.7 |
| Duration of PS episodes (min/2 h) | 5.0 ± 0.3 | 4.5 ± 0.4 | 7.3 ± 1.0* | 8.2 ± 1.2* |
| Number of PS episodes (n/2 h) | 3.8 ± 0.2 | 5.0 ± 0.4 | 5.6 ± 0.5* | 5.1 ± 0.6* |
- The values are the mean time (min) or number (n) ± SEM observed during 2-h application to the dcFTC of Ringer's solution (baseline) and 100 µm, 500 µm or 1 mm muscimol. The number of trials is shown in parentheses. ‘Baseline’ indicates the mean values from all paired controls. PS latency is the time from the beginning of drug infusion (when the drug reaches the microdialysis membrane) to the first PS episodes. Duration and number indicate, respectively, the mean duration and number of PS episodes during 2-h drug application. *P < 0.05. Data from 10 cats.
Effects of application of excitatory amino acid agonists on wake–sleep states
We examined the effects of microdialysis application of two excitatory amino acid receptor agonists, KA and NMDA, to either the vlPAG or dcFTC. Applications of concentrations of 5–50 µm KA to the vlPAG or dcFTC resulted in a dose-dependent increase in W and decrease in both S2 and PS (3, 4). Application of 50–500 µm NMDA to the dcFTC also resulted in a significant decrease in S2 and PS and increase in S1 but had little effect on W (Fig. 4B). When applied to the vlPAG, however, NMDA caused an increase in S2 without affecting PS (Fig. 3B). As shown in Table 2, the PS-inhibiting effects of KA and NMDA applied to the dcFTC resulted from a significant decrease in both the duration and number of PS episodes. At the highest concentration, KA also induced a significant increase in the latency to onset of PS.
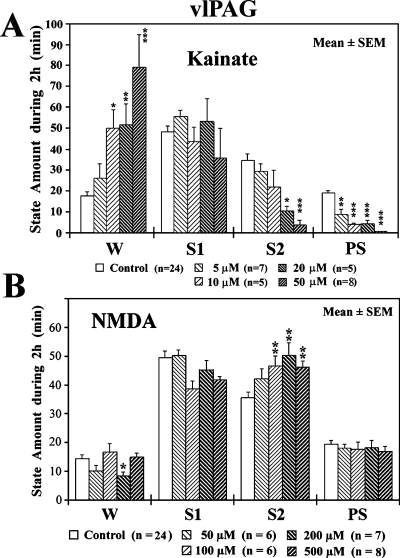
Effects of application of kainic acid (KA) (A) and N-methyl-d-aspartic acid (NMDA) (B) to the ventrolateral periaqueductal grey (vlPAG) on wake–sleep states. The values are the mean time (min) ± SEM spent in wakefulness (W), light slow-wave sleep (SWS) (S1), deep SWS (S2) or paradoxical sleep (PS) during the 2-h drug application period. During the 2-h experiment, the microdialysis probe was perfused with either Ringer's solution (control) or Ringer's solution containing 5, 10, 20 or 50 µm KA or 50, 100, 200 or 500 µm NMDA. The number of trials (n) is indicated in parentheses. *P < 0.05, **P < 0.01, ***P < 0.001. Where not indicated, the difference was not statistically significant. Data from 11 cats.
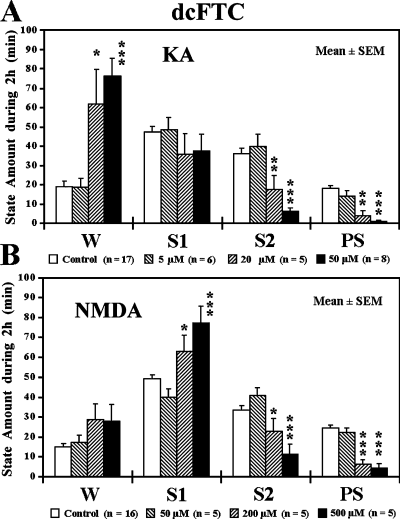
Effects of application of kainic acid (KA) (A) and N-methyl-d-aspartic acid (NMDA) (B) to the dorsocaudal central tegmental field (dcFTC) on wake–sleep states. The values are the mean time (min) ± SEM spent in wakefulness (W), light slow-wave sleep (SWS) (S1), deep SWS (S2) or paradoxical sleep (PS) during the 2-h drug application period. During the 2-h experiment, the microdialysis probe was perfused with either Ringer's solution (control) or Ringer's solution containing 5, 20 or 50 µm KA or 50, 200 or 500 µm NMDA. The number of trials (n) is indicated in parentheses. *P < 0.05, **P < 0.01, ***P < 0.001. Where not indicated, the difference was not statistically significant. Data from 13 cats.
| PS parameter | Baseline Ringer's | Kainate | NMDA | ||||
|---|---|---|---|---|---|---|---|
| 5 µm(n = 6) | 20 µm(n = 5) | 50 µm(n = 8) | 50 µm(n = 5) | 200 µm(n = 5) | 500 µm(n = 5) | ||
| Latency to onset of first PS (min) | 18.9 ± 2.9 | 20.8 ± 5.2 | 108.8 ± 63.2 | 253.5 ± 41.1*** | 14.6 ± 4.6 | 53.4 ± 19.4 | 49.4 ± 23.7 |
| Duration of PS episodes (min) | 4.7 ± 0.3 | 4.4 ± 0.7 | 1.5 ± 0.9* | 0.6 ± 0.6*** | 5.3 ± 0.9 | 1.9 ± 0.5** | 1.3 ± 0.6** |
| Number of PS episodes (N) | 4.1 ± 0.3 | 3.5 ± 0.8 | 1.2 ± 0.6** | 0.1 ± 0.1*** | 4.8 ± 1.1 | 2.6 ± 0.7* | 2.0 ± 1.3* |
- The values are the mean time (min) or number (n) ± SEM observed during 2-h application to the dorsocaudal central tegmental field of Ringer's solution (baseline) and 5, 20 or 50 µm kainate or 50, 200 or 500 µm NMDA. The number of trials is shown in parentheses. ‘Baseline’ indicates the mean values from all paired controls. PS latency is the time from the beginning of drug infusion (when the drug reaches the microdialysis membrane) to the first PS episodes. Duration and number indicate, respectively, the mean duration and number of PS episodes during 2-h drug application. *P < 0.05, **P < 0.01, ***P < 0.001; otherwise, the difference was not statistically significant. Data from 13 cats.
Effects of application of monoamines on wake–sleep states
We then examined the effects of application of monoamines at the 5-mm concentration that was found suitable for assessing the effects on the EEG and behavioural states in our previous study (Crochet & Sakai, 1999). When applied to the vlPAG, 5-HT, NE and DA had no effects, whereas HA caused a significant increase in S2 and decrease in both W and PS (Fig. 5A). Although 5-hydroxytryptamine (serotonin) hydrochloride and 3-hydroxytyramine (dopamine) hydrochloride had no effect, both NE and E, and to a lesser extent HA, produced an increase in W and decrease in both S2 and PS when applied to the dcFTC, but the effects were greater for E (Fig. 5B). The PS-inhibiting effects of NE and E were due to a significant decrease in both the number and duration of PS episodes and a significant increase in the latency to onset of PS (Table 3).
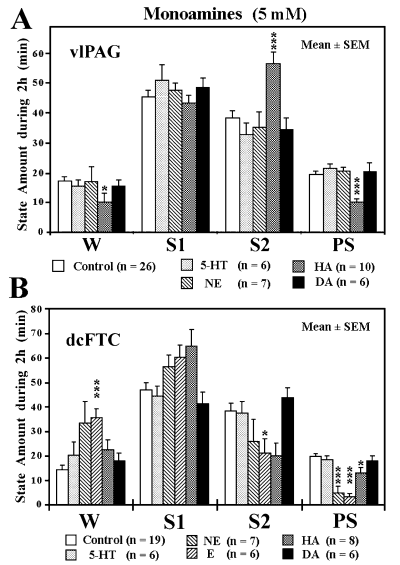
Effects of application of monoamines to the ventrolateral periaqueductal grey (vlPAG) (A) and dorsocaudal central tegmental field (dcFTC) (B) on wake–sleep states. The values are the mean time (min) ± SEM spent in wakefulness (W), light slow-wave sleep (SWS) (S1), deep SWS (S2) or paradoxical sleep (PS) during 2-h perfusion with Ringer's solution (control) or Ringer's solution containing 5 mm serotonin (5-HT), norepinephrine (NE), epinephrine (E), histamine (HA) or dopamine (DA). *P < 0.05, ***P < 0.001. Data from nine cats.
| PS parameter | Baseline | 5-HT | NE | E | HA | DA |
|---|---|---|---|---|---|---|
| Ringer's(n = 19) | 5 mm(n = 6) | 5 mm(n = 7) | 5 mm(n = 6) | 5 mm(n = 8) | 5 mm(n = 6) | |
| Latency to onset of first PS (min) | 12.5 ± 1.8 | 17.3 ± 4.8 | 80.4 ± 36.7 | 91.3 ± 37.9* | 33.6 ± 7.6* | 19.5 ± 8.8 |
| Duration of PS episodes (min) | 5.3 ± 0.4 | 4.8 ± 0.3 | 1.9 ± 0.9* | 1.0 ± 0.4*** | 5.4 ± 0.9 | 3.8 ± 0.4 |
| Number of PS episodes (N) | 4.1 ± 0.3 | 3.8 ± 0.4 | 1.4 ± 0.5*** | 1.8 ± 0.7* | 2.6 ± 0.3** | 4.7 ± 0.8 |
- The values are the mean time (min) or number (n) ± SEM observed during 2-h application to the dorsocaudal central tegmental field of Ringer's solution (baseline) and 5 mm 5-hydroxytryptamine (serotonin) hydrochloride (5-HT), arterenol (norepinephrine) bitartrate salt (NE), epinephrine hydrochloride (E), histamine dihydrochloride (HA) or 3-hydroxytyramine (dopamine) hydrochloride (DA). The number of trials is shown in parentheses. ‘Baseline’ indicates the mean values from all paired controls. PS latency is the time from the beginning of drug infusion (when the drug reaches the microdialysis membrane) to the first PS episodes. Duration and number indicate, respectively, the mean duration and number of PS episodes during 2-h drug application. *P < 0.05, **P < 0.01, ***P < 0.001; otherwise, the difference was not statistically significant. Data from nine cats.
Effects of application of carbachol on wake–sleep states
The application of carbachol to the dcFTC had no effect on behavioural states at a concentration of 200 µm that induces a marked increase in PS when applied to the peri-LCα (Sakai & Onoe, 1997; Crochet & Sakai, 1999). In contrast, carbachol produced a significant decrease in PS when applied to the adjacent vlPAG (Fig. 6). This PS-inhibiting effect resulted from a significant decrease in both the number and duration of PS episodes and a significant increase in the latency to onset of PS (data not shown).
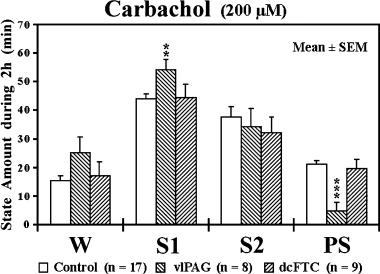
Effects of application of carbachol to the ventrolateral periaqueductal grey (vlPAG) and dorsocaudal central tegmental field (dcFTC) on wake–sleep states. The values are the mean time (min) ± SEM spent in wakefulness (W), light slow-wave sleep (SWS) (S1), deep SWS (S2) or paradoxical sleep (PS) during 2-h perfusion with Ringer's solution (control) or Ringer's solution containing 200 µm carbachol. **P < 0.01, ***P < 0.001. Data from nine cats.
Unitary characteristics of dorsocaudal central tegmental field neurones during wake–sleep states
Extracellular single-unit recordings in freely moving cats revealed two populations of neurones in the dcFTC, i.e. tonic and phasic (7, 8). Although tonic neurones exhibited a higher rate of tonic discharge during both W and PS compared with during SWS, a significant difference in discharge rate was seen between both during active W and quiet W and during PS with PGO and rapid eye movement bursts and PS without PGO waves and rapid eye movements (Fig. 8B). The tonic neurones displayed the lowest rate of discharge during SWS accompanying PGO waves, the sign of PS. Phasic neurones showed a significant phasic increase in firing rates during both active W and PS with PGO and rapid eye movement bursts compared with during quiet W, S2, SWS with PGO waves and PS without PGO waves and rapid eye movements (data not shown). As shown in Fig. 7B, four of 20 phasic dcFTC neurones exhibited a phasic discharge highly selective to PS. It should also be mentioned that a gradual increase in unit activity was seen in both tonic and phasic neurones during PS episodes (Fig. 7A and B). Two of seven tonic and one of eight phasic neurones tested showed antidromic responses to stimulation of ventrolateral medullary reticular formation (Fig. 9), whereas one of eight phasic neurones tested responded antidromically to stimulation of the ventral tegmental area (data not shown).
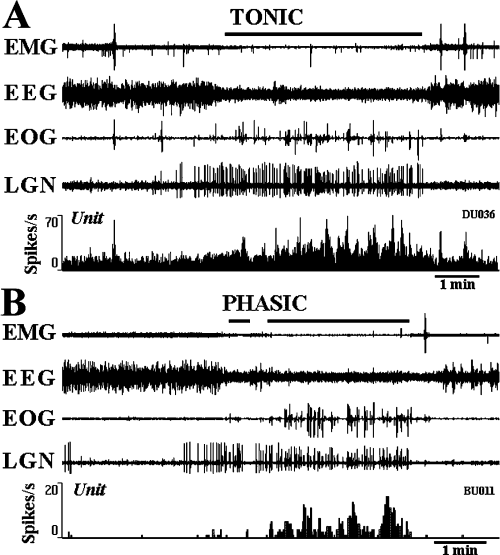
Tonically and phasically discharging dorsocaudal central tegmental field neurones during the sleep–waking cycle. (A) A tonic neurone showing a tonic increase in discharge rate during paradoxical sleep (PS) compared with during slow-wave sleep (SWS). Note a phasic increase in discharge rate during both PS with rapid eye movement and ponto-geniculo-occipital (PGO) wave bursts and wakefulness (W) with movements. Note also the gradual increase in unit activity during PS. (B) A phasic neurone showing a phasic increase in discharge rate during PS compared with during W and SWS. Note the gradual increase in phasic unit activity during PS. EMG, electromyogram; EEG, electroencephalogram; EOG, electro-oculogram; LGN, dorsal lateral geniculate EEG showing PGO waves.
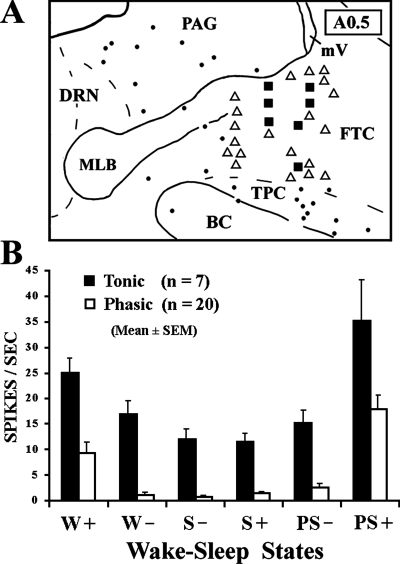
(A) Drawing of a frontal section at A0.5 illustrating unit recording sites, together with the distribution of choline acetyltransferase-immunoreactive cholinergic neurones. •, cholinergic neurones; and ▪, phasic and tonic neurones, respectively. BC, brachium conjunctivum; DRN, dorsal raphe nucleus; FTC, central tegmental field; MLB, medial longitudinal bundle; mV, mesencephalic tract of trigeminus; PAG, periaqueductal grey; TPC, cholinergic component of the nucleus tegmenti pedunculopontinus, pars compacta. (B) Discharge rates of tonic and phasic neurones across the sleep–waking cycles. W+ and W–, active and quiet wakefulness, respectively; S– and S+, slow-wave sleep without and with ponto-geniculo-occipital (PGO) waves, respectively; PS– and PS+, paradoxical sleep without or with rapid eye movements and PGO waves, respectively.
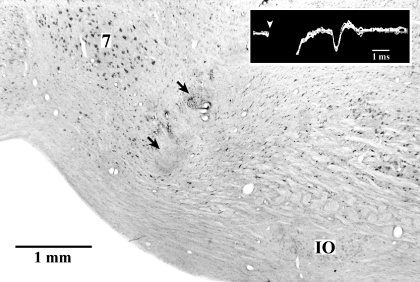
Photomicrograph of a sagittal section showing the location of the bipolar stimulation electrodes in the ventrolateral medulla and antidromic responses of a dorsocaudal central tegmental field neurone elicited by the stimulation of this stimulation site (box). The arrows indicate the tip of the electrodes and the arrowhead shows the onset of stimulation. 7, facial nucleus; IO, inferior olivary complex.
Discussion
Dorsocaudal tegmental area as a critical paradoxical sleep-inhibiting structure
Our results showed for the first time that inactivation of neurones located in the dcFTC, but not the vlPAG, results in a significant and specific increase in PS. Indeed, muscimol, a potent and long-lasting GABAA receptor agonist, dose-dependently increased PS without affecting other behavioural states when applied to the dcFTC, whereas, when the dialysis membrane was confined to the PAG, applied muscimol had no effect on PS generation at any concentration from 50 to 1000 µm. Our results are therefore at variance in part with a previous microinjection study reporting that bilateral pressure injections of muscimol (0.5 µg/0.5 µL, 8.8 mm) in either the dcFTC or vlPAG result in a large increase in PS (Sastre et al., 1996). This discrepancy seems to be due to differences in drug application technique, especially in diffusion of muscimol applied by either microinjection or microdialysis. It is well known that the microinjection technique causes acute tissue damage and that applied substances diffuse dorsally and ventrally from the tip of injection cannulae in a pear-shaped manner (see Cape & Jones, 1998). In contrast, the microdialysis application technique used minimizes the effect of acute tissue damage, substances passing in and out by diffusion (Quan & Blatteis, 1989) and diffusing mainly laterally along the membrane (Crochet & Sakai, 1999). The fact that the tip of the probe membrane is closed by an epoxy resin, the dorsal edge of the membrane being sheltered by a shaft and covered by the resin, may also explain a slight vertical diffusion of applied substances. It appears therefore that the PS-inducing effect of muscimol previously attributed to the vlPAG is due to diffusion of the drug into the dcFTC. It is worth mentioning that muscimol applied to the vlPAG produced a significant decrease in S2 without affecting PS, especially at a concentration of 1 mm (Fig. 2A). This S2-inhibiting effect may be due to diffusion of the high concentration of muscimol to the dorsal raphe nucleus located just medial to the vlPAG, as the same S2-inhibiting effect was seen following application of 100 or 500 µm muscimol to this serotonergic nucleus (Sakai & Crochet, 2001).
In the dcFTC, both KA and NMDA dose-dependently decreased PS and increased W, although NMDA had no significant W-inducing effect even at the highest concentration of 500 µm. In this structure, both applied NE and E, and to a lesser extent HA, also had W-promoting and PS-inhibiting effects. These results suggest that this tegmental structure may also be critically implicated in the mechanisms of W. The dcFTC might be a part of the midbrain EEG-activating system as reported by Moruzzi (1972) and Steriade (1981). The dcFTC, however, is clearly distinguished from the whole midbrain reticular core, as the PS-enhancing effect by muscimol is never seen with muscimol application to any other regions of the FTC.
Differential roles of the ventrolateral periaqueductal grey and dorsocaudal central tegmental field in behavioural state control
In the present study, we showed several important differences between the vlPAG and dcFTC in response to applied NMDA, HA, NE and carbachol. Both NMDA and HA induced a decrease in S2 and PS and an increase in W when applied to the dcFTC but the opposite effects were seen when applied to the vlPAG. NE had W-promoting and sleep-suppressing effects in the dcFTG but had no effect in the vlPAG. Furthermore, carbachol had a marked PS-inhibiting effect in the vlPAG but had no effect on PS generation in the dcFTC. These findings suggest a complex wake–sleep regulation mechanism involving vlPAG and dcFTC neurones. On the whole, it appears that the dcFTC may play a role in the mechanisms of W and PS, whereas the vlPAG may also play a role in the mechanisms of SWS. Ford et al. (1995) reported that, in the rat, the GABAergic neurones in the vlPAG send axons to the posterior hypothalamus, playing an important role in the maintenance of W (Moruzzi, 1972; Sakai et al., 1990; Lin, 2000). It seems likely that the sleep-enhancing effect of HA and NMDA observed in the present study may result from the excitation of these GABAergic vlPAG neurones. The present finding on the selective PS-inhibiting effect of carbachol in the vlPAG strongly suggests, however, that the vlPAG may also be an important integral component of PS generation. In line with this assumption, Thakkar et al. (2002) recently reported, in the cat, that the vlPAG contains both tonic and phasic neurones and that many of the phasic neurones showed a PS-selective discharge. The neuronal activity of the dcFTC as reported in the present study is similar to that of the PAG neurones reported by Thakkar et al. (2002), although we found a clear difference in response to PS generation between these two structures. This difference seems to be ascribed to the difference in neurotransmitter (glutamate vs. GABA), axonal trajectory (descending vs. ascending) and target (PS-off vs. wake-on) of their neurones. The PAG is involved in the control of a number of physiological functions, such as vocalization, pain transmission, processing of fear and anxiety, pressor response, respiration, and bladder reactions (Bandler & Shipley, 1994; Behbehani, 1995). The behavioural responses that we observed in the present study might result from the effects of substances used on these physiological functions. It should be mentioned, however, that we did not observe any abnormal physiological reactions or behaviours in the present study.
Role of the dorsocaudal central tegmental field in the inhibitory mechanisms of paradoxical sleep generation
The dcFTC does not contain monoaminergic (serotonergic, noradrenergic and dopaminergic) and cholinergic neurones (Crochet & Sakai, 1999) but does contain a number of glutamatergic and GABAergic neurones (Ottersen & Storm-Mathisen, 1984; Clements et al., 1987; Ford et al., 1995). A question arises as to the nature of the FTC neurones implicated in PS-inhibiting mechanisms. The inactivation and excitation of neurones located in the dcFTC produced, respectively, enhancement and inhibition of PS, indicating that dcFTC neurones are involved in the inhibitory mechanisms of PS generation either through direct inhibition of PS-on neurones or via excitation of PS-off neurones. In this respect, the present findings seem to run counter to the hypothesis that GABAergic input from the dcFTC and/or vlPAG is important for the inactivation of monoaminergic PS-off neurones, thereby enhancing PS generation. Indeed, like the vlPAG neurones (Thakkar et al., 2002), the dcFTC neurones either tonically or phasically increased their discharge rate during PS as compared with during quiet W and SWS but none of them showed a specific and tonic increase in discharge rate just prior to and during PS, as PS-on neurones do. These findings therefore do not support the existence of the hypothesized GABAergic PS-on neurones in the vlPAG and dcFTC.
On the basis of their PS-inhibiting role, one would expect the dcFTC neurones to cease firing during PS. Although non-monoaminergic PS-off neurones have been described in the medulla (Sakai & Kanamori, 1999), we could not find the existence of such neurones in the dcFTC. Our findings therefore do not support the existence, in the dcFTC, of a population of non-monoaminergic PS-off neurones controlling the activity of PS-on neurones, even though it is possible that small neurones could be difficult to record with our electrodes. Our data indicate that the activity of dcFTC neurones gradually increases during PS as compared with SWS. It is therefore unlikely that dcFTC neurones directly inhibit PS-on neurones. As an alternative hypothesis we propose that these neurones could be glutamatergic in nature and excite, via descending projections, PS-off neurones located in the pons and/or the medulla. In support of this assumption, a previous study in the cat reported the presence of descending projections from the dcFTC to the ventrolateral medulla (Luppi et al., 1988) and, in the present study, some tonic and phasic FTC neurones were activated antidromically by the stimulation of this medullary structure containing both monoaminergic and non-monoaminergic PS-off neurones (Sakai & Kanamori, 1999).
It is important to note that, as in a previous microinjection study (Sastre et al., 1996), the increase in PS by muscimol application resulted from a significant increase in both the duration and number of PS episodes but not a significant decrease in the latency to onset of PS. It is also important to mention that the PS-enhancing effect has been reported to exist even in pontine cats in which all neural tissues above the level of the rostral pons are completely removed (Sastre et al., 1996), indicating that the basic mechanisms underlying this PS enhancement by muscimol are found in the brainstem. We therefore propose that, in normal conditions, the activation of dcFTC neurones during W may excite brainstem PS-off neurones thereby inhibiting PS generation. During SWS, the reduction of their activity would decrease the excitatory drive on PS-off neurones (disfacilitation), thereby leading to their cessation of discharge, which in turn would promote PS generation. During PS, the gradual increase of dcFTC neurones would progressively drive PS-off neurones resulting in the end of the PS episode. Lesions or inactivation of the dcFTC neurones may induce a strong disfacilitation of brainstem PS-off neurones and cause a more frequent occurrence and longer duration of PS episodes that may even occur directly from S1 without passing S2 (see Fig. 1C; see also Fig. 4 of Petitjean et al., 1978; Fig. 3 of Sastre et al., 1996).
In conclusion, our study demonstrates for the first time that the vlPAG and dcFTC play different roles in behavioural state control, that the non-monoaminergic dcFTC neurones are critically involved in the inhibitory mechanisms of PS generation, playing an essential role in the maintenance of this state of sleep, and that these neurones are under the control of GABAergic, glutamatergic, adrenergic and histaminergic systems.
Abbreviations
-
- dcFTC
-
- dorsocaudal central tegmental field
-
- E
-
- epinephrine hydrochloride
-
- EEG
-
- electroencephalogram
-
- FTC
-
- central tegmental field
-
- HA
-
- histamine dihydrochloride
-
- KA
-
- kainic acid
-
- NE
-
- arterenol (norepinephrine) bitartrate salt
-
- NMDA
-
- N-methyl-d-aspartic acid
-
- PAG
-
- periaqueductal grey
-
- peri-LCα
-
- peri-locus coeruleus alpha
-
- PGO
-
- ponto-geniculo-occipital
-
- PS
-
- paradoxical sleep
-
- S1
-
- light slow-wave sleep
-
- S2
-
- deep slow-wave sleep
-
- SWS
-
- slow-wave sleep
-
- vlPAG
-
- ventrolateral periaqueductal grey
-
- W
-
- wakefulness