Hippocampal CA3 pyramidal cells selectively innervate aspiny interneurons
Abstract
The specific connectivity among principal cells and interneurons determines the flow of activity in neuronal networks. To elucidate the connections between hippocampal principal cells and various classes of interneurons, CA3 pyramidal cells were intracellularly labelled with biocytin in anaesthetized rats and the three-dimensional distribution of their axon collaterals was reconstructed. The sections were double-stained for substance P receptor (SPR)- or metabotropic glutamate receptor 1α (mGluR-1α)-immunoreactivity to investigate interneuron targets of the CA3 pyramidal cells. SPR-containing interneurons represent a large portion of the GABAergic population, including spiny and aspiny classes. Axon terminals of CA3 pyramidal cells contacted SPR-positive interneuron dendrites in the hilus and in all hippocampal strata in both CA3 and CA1 regions (7.16% of all boutons). The majority of axons formed single contacts (87.5%), but multiple contacts (up to six) on single target neurons were also found. CA3 pyramidal cell axon collaterals innervated several types of morphologically different aspiny SPR-positive interneurons. In contrast, spiny SPR-interneurons or mGluR-1α-positive interneurons in the hilus, CA3 and CA1 regions were rarely contacted by the filled pyramidal cells. These findings indicate a strong target selection of CA3 pyramidal cells favouring the activation of aspiny classes of interneurons.
Introduction
Inhibitory interneurons are crucially involved in various cortical oscillations (Buzsáki & Eidelberg, 1983; Fox et al., 1986; Buzsáki & Chrobak, 1995; Klausberger et al., 2003; Whittington & Traub, 2003; Klausberger et al., 2004; Somogyi & Klausberger, 2005). Beside their intrinsic electrophysiological characteristics, their input–output connections and, potentially, neurochemical properties (calcium binding protein and other neurochemical marker content, and expression of various neurotransmitter receptors) are important in biasing and timing network firing patterns (reviewed in Freund & Buzsáki, 1996; Freund, 2003). The different functional types of interneurons are known to play crucial roles in the regulation of behaviour-dependent network activity in the hippocampus (Miles et al., 1996; Csicsvári et al., 1999; Klausberger et al., 2003). The CA3 field is an important region in the generation and maintenance of various behaviour-dependent hippocampal network firing patterns, including gamma oscillations (Csicsvári et al., 2003), initiation of sharp waves and interictal spikes (Buzsáki et al., 1983; Miles & Wong, 1983; Wong & Traub, 1983; Buzsáki, 1986). Through their Schaffer collaterals to the CA1 region, they provide a critical input for theta oscillations and ‘ripples’ (O'Keefe & Nadel, 1978; Buzsáki et al., 1992; Buzsáki, 2002). Because the various interneuron classes have distinct functions and are involved in the various oscillations in specific ways (Freund & Buzsáki, 1996; Somogyi & Klausberger, 2005), understanding their connectivity with each other and the principal cell populations is important. Previous studies have provided relatively detailed information about the axon collateral distribution and termination patterns of CA3 pyramidal cells (Ishizuka et al., 1990; Pokorny & Schwartzkroin, 1991; Li et al., 1994) and offered preliminary evidence for their bias for interneuron targets (Sík et al., 1993; Blasco-Ibanez & Freund, 1995).
To examine the postulated target selection of CA3 pyramidal cells, we investigated their subtance P receptor (SPR)- and metabotropic glutamate receptor 1α (mGluR-1α)-immunoreactive interneuron targets in all subregions of the dorsal hippocampus of the rat. The choice of antibody was based on the observations that the SPR antibody stains most interneurons in a Golgi-like fashion but no axon collaterals. All three major functional interneuron types, i.e. perisomatic, dendritic inhibitory cells and interneuron selective interneurons were found among SPR-positive cells (Acsády et al., 1997; Sloviter et al., 2001). Because our observations with SPR-immunostained neurons suggested that spiny interneurons are only exceptionally contacted by axon collaterals of CA3 pyramidal cells, we also examined mGluR-1α-stained sections, as this antibody preferentially reacts with several types of spiny interneurons (Martin et al., 1992; Baude et al., 1993; Hampson et al., 1994) and because the SPR antibody does not label all known spiny interneurons in the stratum oriens. mGluR-1α-immunoreactive interneurons are known to colocalize the neuropeptide somatostatin (Baude et al., 1993; Hampson et al., 1994). Axon collaterals of in vivo labelled CA3 pyramidal cells were fully reconstructed from sections double-labelled for SPR and mGluR-1α, and putative synaptic contacts with the interneurons were quantified at the light microscopic level, followed by electron microscopic verification. We find that CA3 axon collaterals preferentially innervate aspiny (vs. spiny) interneurons in all hippocampal regions.
Materials and methods
Intracellular recording and labelling of CA3 pyramidal cells
Sprague–Dawley rats (Hilltop Laboratories, Scottsdale, PA; n = 9) were anaesthetized with urethane (1.5 g/kg; Sigma) and placed in a stereotaxic apparatus. The body temperature of the rat was kept constant by a small animal thermoregulation device. The scalp was removed and a small (1.2 × 1.2 mm) bone window was drilled above the hippocampus (centred at AP −3.5 and L 2.5 mm from bregma) for extra- and intracellular recordings. Once the intracellular electrode tips was placed in the brain, the bone window was covered by a mixture of paraffin (50%) and paraffin oil (50%) to prevent drying of the brain and decrease pulsation. The intracellular micropipette was then advanced into the CA3 pyramidal layer and a cell was impaled. After the completion of the intracellular physiological data collection for another experiment, biocytin was injected through a bridge circuit using 500 ms depolarizing pulses at 0.6–2 nA at 1 Hz for 10–60 min. Neuronal activity was followed throughout the procedure and the current was reduced if the electrode was blocked and/or the condition of the neuron deteriorated. Experiments were carried out following the guidelines laid down by the NIH in the US. The protocol was approved by the Institutional Animal Care and Use Committee of Rutgers University.
Histological analysis
One to ten hours after the biocytin injection, still under urethane anaesthesia, the animals were perfused intracardially with physiological saline followed by 300 mL fixative containing 4% paraformaldehyde, 0.05% glutaraldehyde and 0.2% picric acid in 0.1 m phosphate buffer (PB). The brains were then removed and postfixed in the same solution overnight. Coronal sections (70-µm thick) were cut from the dorsal hippocampus on a Vibroslice, washed with 0.1 m PB, then cryoprotected in 30% sucrose in 0.1 m PB overnight, and freeze-thawed three times over liquid nitrogen. After extensive washes with 0.1 m PB sections were incubated in avidin-biotin horseradish peroxidase complex (ABC, 1 : 200, Vector Laboratories, Burlingame CA) for 1.5 h. Biocytin labelling was visualized by ammonium nickel sulphate-intensified 3,3′-diaminobenzidine-4HCl (DAB, Sigma, St Louis, MO), as a chromogene, which resulted in a black reaction product.
The sections containing CA3 pyramidal cells were immunostained against substance P receptor (in brains D261, D262, D264 and D269) or mGluR-1α (in brains D284, D288, D296 and D330). In both cases nonspecific immunostaining was blocked by 2% bovine serum albumin (1 h). A polyclonal rabbit antiserum against substance P receptor (SPR, 1 : 5000; Chemicon) or mGluR-1α (1 : 100, Görcs et al., 1993) was used for two days at 4 °C. Biotinylated anti-rabbit IgG (1 : 250, Vector) was applied as secondary serum followed by avidin-biotinylated horseradish peroxidase complex (ABC, 1 : 250, Vector). The immunoperoxidase reaction was developed by 3,3′-diamino-benzidine-tetrahydrochloride (DAB, Sigma) as a chromogene resulting in a brown reaction product. Sections were then treated with 1% OsO4 and 7% glucose in PB for 30 min, dehydrated in ethanol (1% uranyl acetate was added at the 70% ethanol stage for 30 min) and mounted in Durcupan (ACM, Fluka). After light microscopic analysis, areas of interest were re-embedded, and sectioned for electron microscopy. Ultrathin serial sections were collected on Formvar-coated single slot grids, stained with lead citrate, and examined with a Philips Tecnai electron microscope.
Axon reconstruction, measurement of interterminal intervals and statistics
The NeuroLucida 3.1 program (MicroBrightField, Inc, Williston, VT) was used for the reconstruction of in vivo intracellularly labelled CA3b and c pyramidal cell axon collaterals from 13 cells in nine brains (D256–1 cell, D261–3 cells in two hemispheres, D262–1 cell, D264–3 cells in two hemispheres, D269–1 cell, D284–1 cell, D288–1 cell, D296–1 cell and D330–1 cell). One section was chosen from the brains D261, D262, D269, D288 and D330, two sections from D284, three from D296, ten consecutive sections from D264, and 48 consecutive sections from D256. Each axon collateral segment was drawn using 100× magnification, and each putative axon terminal was marked and notated whether it was in close apposition to a dendrite, spine or soma of an immunostained neuron or not (except for D256, where there was no additional immunostaining). These appositions were regarded as potential synapses between the axon and labelled interneurons. Figure 1 illustrates the location of the filled cells, the density of axon arbors and the distribution of the putative synaptic contacts.
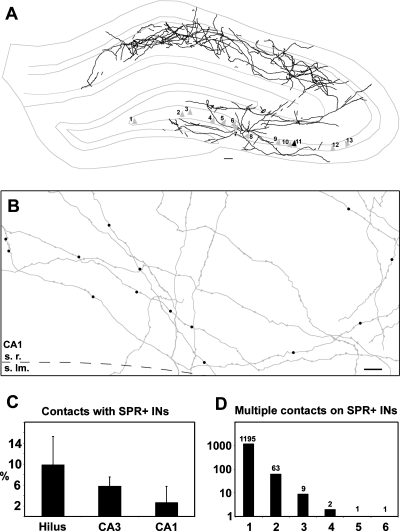
(A) NeuroLucida reconstruction shows the density of intracellularly labelled CA3 pyramidal cell axon arbor, projected from ten consecutive sections from brain D256 (black triangle, cell 11 represents the corresponding soma). Grey triangles show the location of all filled pyramidal cells examined. Cells 1, 9, 10 from brain D264, cells 2, 4, 5 from D261, cell 3 from D330, cell 6 from D296, cell 7 from D284, cell 8 from D269, cell 12 from D262, cell 13 from D288. (B) Distribution of the putative synaptic contacts on SPR-positive interneurons (black dots), reconstruction from five consecutive sections in stratum radiatum of CA1 region in D264. The CA3 pyramidal cell-SPR-positive interneuron contact probability is slightly, but not significantly different in the different regions of the hippocampus (C, from n = 4 animals, mean ± SD). The number of multiple contacts given to SPR-immunoreactive interneurons decreased exponentially (D). Scale bars, 100 µm (A), 10 µm (B).
Three consecutive sections from the brain D264 were chosen for the measurement of the interterminal intervals. Every axon collateral found in these sections was analysed, and the distance between the terminals was measured. The distance between the varicosities contacting SPR-positive interneurons was calculated, and the frequency distribution of both the interterminal intervals and the distance between varicosities contacting SPR-stained cells was determined. The axonal segments between the end of the collateral and the first/last terminal were also included in the study.
For the statistical analysis, the Kruskall–Wallis anova test was used with the aid of the program statistica 6.0 to determine the regional distribution of the filled CA3 pyramidal axon terminals contacting SPR-positive interneurons. The program sigmaplot 8.0 was used to fit the exponential and lognormal curves to the distribution plot of the interterminal intervals, and of the multiple contacts of filled CA3 pyramidal cell axons onto SPR-positive interneurons.
Results
Interneuron targets of CA3 pyramidal cells
Axonal segments of filled CA3 pyramidal cells were reconstructed, and putative synaptic contacts with SPR- or mGluR-1α-immunoreactive interneurons were determined (see methods). Figure 1A and B illustrates the density of axon arbors of one CA3 pyramidal cell and the distribution of the putative synaptic contacts with its SPR-positive interneuron targets. All axon collaterals and boutons of the illustrated neuron was fully reconstructed and statistical details for modelling purposes are available from the authors. The regional distribution of the axon collaterals was similar to that described earlier (Li et al., 1994). The total length of the labelled axon collaterals varied from 150 to 500 mm.
SPR-positive interneuron targets
SPR labels a large number of spiny and aspiny interneurons in the hippocampus, belonging to morphologically, neurochemically and functionally heterogeneous populations (Acsády et al., 1997).
Four brains containing labelled CA3 pyramidal cell axons were double stained for SPR, and SPR-immunoreactive interneuron targets of CA3 pyramidal cells were analysed. All biocytin filled axonal segments were drawn by camera lucida from a single representative section of each of brains D261, D262 and D269, and from ten consecutive sections of brain D264. Altogether 19 047 axon terminals were analysed in all hippocampal regions, of which 1382 (7.3%) were found to form putative synaptic contacts with SPR-positive interneurons. Statistical tests did not reveal differences in the pyramidal cell-interneuron contact probability; hilus, 9.87 ± 5.44%; CA3 region, 5.80 ± 1.79% and the CA1 region 2.65 ± 3.12% (mean ± SD, Kruskall–Wallis anova, P = 0.067, n = 4 animals, Fig. 1C), although a tendency of a decrease from the hilus to the CA1 region was observed. Virtually all putative synaptic contacts were found on dendrites and only rarely on SPR-immunoreactive somata (six out of 1382; 0.43%). Putative synapses on SPR-immunoreactive spines were never observed. The filled axon terminals formed mostly single contacts with the SPR-positive targets (87.5%) and the probability of multiple contacts decreased exponentially (P < 0.0001, coefficient of determination R2 = 0.999; Fig. 1D). Importantly, all our identified multiple synapses occurred on the same dendritic segment. It is possible that further multiple contacts occurred on multiple dendrites and from multiple axon collaterals, but because the SPR-immunoreactive interneurons could be only partially reconstructed in the three-dimensional plane, we could not routinely examine the presence of multiple contacts on the different dendrites of single interneurons. Thus, the distribution of multiple synapses, shown in Fig. 1D, is likely underestimated.
The overwhelming majority of labelled axons terminated on aspiny interneuron dendrites and only exceptionally on spiny SPR-labelled cells (Table 1). Even in these rare cases, the CA3 pyramidal cell axon established a putative contact on the dendrite but never on the spines of spiny SPR-immunoreactive interneurons. Because the distribution of synapses by CA3 pyramidal cells may depend on the relative incidence of spiny and aspiny SPR-immunoreactive neurons, we also counted the distribution of these neurons in randomly selected sections (Table 2; Fig. 2). In agreement with previous reports (Acsády et al., 1997; Sloviter et al., 2001), the incidence of spiny SPR-immunoreactive neurons in the CA1 region was very low (3.23% of all immunoreactive cells). This value was higher in the CA3 region (8.33%), likely due to the presence of a population of spiny neurons in stratum lucidum (Gulyás et al., 1992; Hsu & Buzsáki, 1993). The largest percentage of spiny SPR-immunoreactive cells were encountered in the hilar region (27.4%; 16.2% in the whole dentate gyrus). To estimate the preference of CA3 axon collaterals to aspiny vs. spiny interneurons, we divided the ratio of putative contacts on spiny and aspiny neurons by the ratio of the number of spiny and aspiny neurons in the same regions (Table 2; Fig. 3). Thus, if there is nonpreferential distribution of axon terminals, this value is 1, assuming that the dendritic arbors of spiny and aspiny interneurons are similar (Gulyás et al., 1992; Acsády et al., 1997; Gulyás et al., 1999). We found values 0.12, 0.13 and 0.32 for the hilus, CA3 and CA1 regions, respectively, indicating that CA3 pyramidal cells rarely innervated spiny interneurons compared to the more strongly innervated aspiny interneurons.
| Region(and number of CA3 pyramidal cell terminals examined) | Terminals contacting SPR-positive dendrites | SPR-positive spiny interneuron dendrites | SPR-positive aspiny interneuron dendrites | ||
|---|---|---|---|---|---|
| n | n | % | n | % | |
| Hippocampus (n = 19047) | 1382 | 18 | 0.10 | 1364 | 7.16 |
| Dentate gyrus (n = 676) | 70 | 3 | 0.44 | 67 | 9.92 |
| CA3 region (n = 11927) | 931 | 11 | 0.09 | 920 | 7.72 |
| Stratum oriens (n = 3005) | 190 | 3 | 0.10 | 187 | 6.22 |
| Stratum radiatum (n = 7201) | 592 | 8 | 0.11 | 584 | 8.11 |
| CA1 region (n = 6444) | 381 | 4 | 0.06 | 377 | 5.85 |
| Stratum oriens (n = 1581) | 72 | 0 | 0.00 | 72 | 4.55 |
| Stratum radiatum (n = 4044) | 251 | 4 | 0.10 | 247 | 6.11 |
- * These are pooled data from brains D261, D262, D264 and D269.
| Region(and number of cells) | Frequency of spiny SPR-positive cells (%) | Frequency of aspiny SPR-positive cells (%) | Preferential distribution |
|---|---|---|---|
| Hippocampus (n = 985) | 10.19 | 89.81 | 0.135 |
| Dentate gyrus (n = 296) | 16.22 | 83.78 | 0.23 |
| Hilus (n = 175) | 27.43 | 72.57 | 0.12 |
| CA3 region (n = 348) | 8.33 | 91.67 | 0.13 |
| Stratum oriens (n = 59) | 10.17 | 89.93 | 0.14 |
| Stratum radiatum (n = 158) | 12.03 | 87.97 | 0.10 |
| CA1 region (n = 341) | 3.23 | 96.77 | 0.32 |
| Stratum oriens (n = 65) | 1.54 | 98.46 | 0.00 |
| Stratum radiatum (n = 145) | 5.52 | 94.48 | 0.28 |
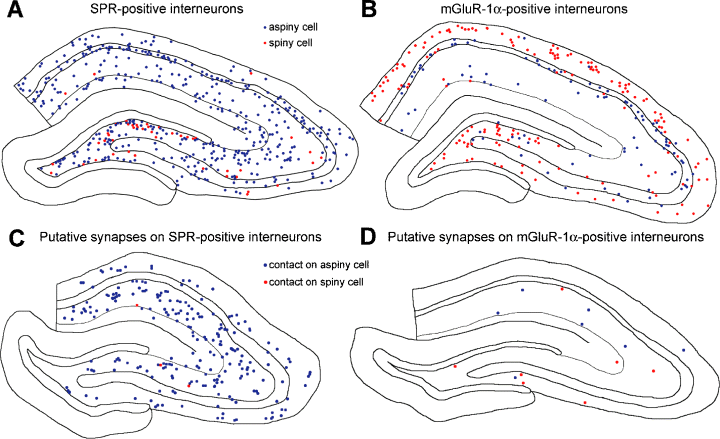
Distribution of spiny and aspiny SPR-positive (A) and mGluR-1α-positive (B) interneurons in representative 70-µm thick hippocampal sections. Blue dots, aspiny interneurons; red dots, spiny interneurons. (C and D) Distribution of synaptic contacts made by the axon collaterals of a single intracellularly filled CA3 pyramidal cell (D264) onto SPR-positive (C) and mGluR-1α-positive (D) interneurons. Contacts are from five consecutive hippocampal sections. Blue dots, putative contacts on dendrite of aspiny cell; red dots, putative synaptic contacts on dendrites of spiny interneurons. Note that CA3 pyramidal axon collaterals contact disproportionally larger numbers of aspiny interneurons compared to the available target spiny interneuron.
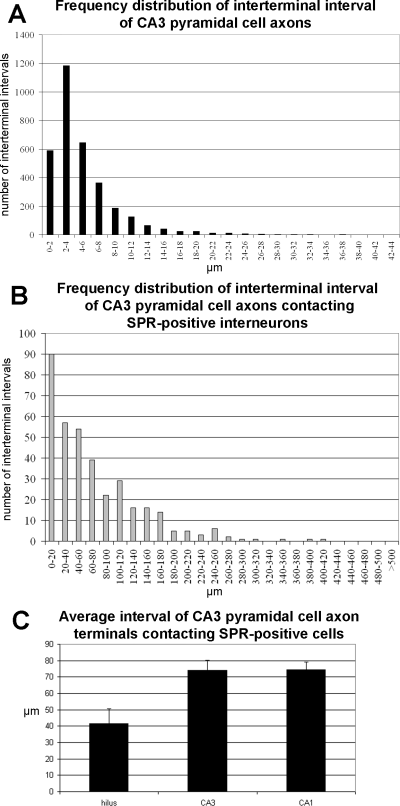
The frequency distribution of interterminal intervals of CA3 pyramidal cell axon terminals (A), and terminals contacting SPR-positive interneurons (B). The frequency distribution of interterminal intervals shows a lognormal distribution (peak at 2–4 µm), whereas the frequency distribution of terminals contacting SPR-positive interneurons shows an exponential distribution. (C) Mean interterminal interval distribution of contacts with SPR-positive interneuron targets in the hilar, CA3 and CA1 subregions.
The distance between the terminals of CA3 pyramidal cell axon collaterals was measured and the average bouton interval, as well as the distance between the boutons contacting SPR-positive interneurons were determined (Fig. 3). The frequency distribution of the interterminal intervals showed a lognormal distribution (P < 0.0001, coefficient of determination R2 = 0.993). The frequency distribution of the distances between terminals contacting SPR-positive interneurons showed an exponential distribution (P < 0.0001, coefficient of determination R2 = 0.983). The average interbouton interval was slightly but nonsignificantly different in the various hippocampal regions (hippocampus 5.6 ± 0.1 µm; hilus 4.8 ± 0.2 µm; CA3 5.8 ± 0.1 µm and CA1 5.5 ± 0.1 µm, mean ± SEM, Kruskall–Wallis anova, P = 0.335). The regional difference was more pronounced, but still not significant with regard to the terminals forming synaptic contacts with SPR-stained interneurons (Fig. 3C; hippocampus: 73.0 ± 3.6 µm; hilus 41.5 ± 9.1 µm; CA3 74.2 ± 5.8 µm and CA1 74.4 ± 4.5 µm, mean ± SEM, Kruskall–Wallis anova, P = 0.115).
Electron microscopic verifications of synaptic contacts
To estimate the reliability of the statistics provided at the light microscopic level, a subset of putative synapses were examined at the electron microscopic level as well. We have chosen 39 filled CA3 pyramidal cell boutons, located on 20 axonal segments from three CA3 pyramidal cells forming putative synapses in different layers of the hippocampal regions (Table 3). Sampling was biased for putative multiple contacts, as well as for contacts with ‘horizontal’ interneurons located in the stratum oriens of the CA1 region, as these cells are known to receive excitatory synaptic input almost exclusively from CA1 pyramidal cells (Blasco-Ibanez & Freund, 1995). Of the 39 putative synapses 30 were confirmed to contact SPR-immunoreactive neurons (4-6, supplementary material Fig. S1), and the remaining nine contacted either immunonegative elements or did not form synapses. Two double boutons were verified to terminate on a spiny SPR-immunoreactive interneuron dendritic shaft, whereas the remaining boutons terminated on smooth dendrites.
| SPR-positive cell label | Region and layer | Light microscopy (single or multiple putative synapses) | Electron microscopy (verified synapses) |
|---|---|---|---|
| c1 horizontal cell | CA1 stratum oriens | 1× | – |
| c2 horizontal cell | CA1 stratum oriens | 1× | 1× (Fig. 6) |
| c3 | CA1 stratum oriens | 1× | – |
| c4 | hilus | 2× | 1× |
| c5 | hilus | 2× | – |
| c6 spiny cell | CA3 stratum lucidum | 2× | 2× (Fig. 5) |
| c7 horizontal cell | CA1 stratum oriens | 1× | 1× |
| c8 | CA3 stratum lucidum | 1× | 1× |
| c9 spiny cell | CA3 stratum lucidum | 2× | 2× |
| c10 | CA3 stratum lucidum | 3× | 2× |
| c11 | hilus | 1× | 1× (Supplementary Fig. S1) |
| c12 | CA3 stratum oriens | 2× | 1× |
| c13 horizontal cell | CA3 stratum oriens | 7× | 6× (Fig. 4) |
| c14 horizontal cell | CA1 stratum oriens | 1× | – |
| c15 | CA1 stratum radiatum | 4× | 4× |
| c16 | hilus | 1× | 1× |
| c17 | hilus | 1× | 1× |
| c18 | CA1 radiatum | 1× | 1× |
| c19 | CA3 alveus | 3× | 3× |
| c20 | CA3 stratum oriens | 2× | 2× |
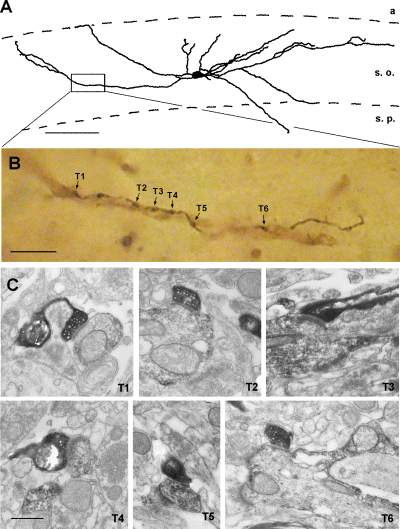
(A) CA3 axon collaterals establish multiple contacts with an aspiny interneuron (c13 horizontal cell). (B) Camera lucida drawing shows an SPR-positive interneuron in the CA3 stratum oriens, which receives six contacts to the same dendrite (T1–T6) from a single axonal segment. (C) Electron micrographs show the verified synapses (T1–T6). a, alveus; s. o. stratum oriens; s. p. stratum pyramidale. The same abbreviations are used in subsequent figures. Scale bars, 100 µm (A); 10 µm (B) and 1 µm (C).
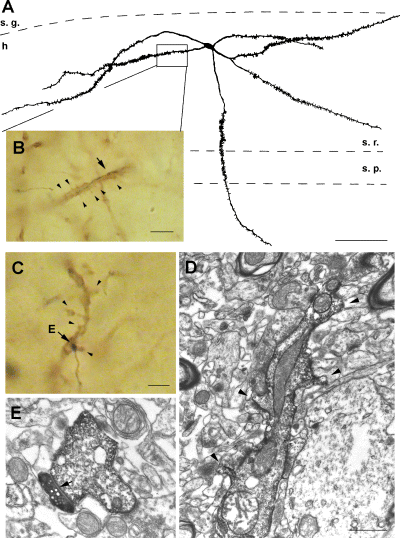
Spiny SPR-positive interneurons are rarely contacted by CA3 pyramidal cells (0.1% of total synapses). (A) A spiny SPR-positive hilar interneuron receives a putative synapse from a filled CA3 pyramidal cell axon on its dendritic shaft (arrow in B). Arrowheads, spines of the immunostained dendrite. (C) Another example of an SPR-positive spiny interneuron (c6 in CA3 stratum lucidum), innervated by a filled CA3 pyramidal cell axons (arrow, putative contact; arrowhead, spines). (D) Electron micrographs show spiny dendrite (arrowheads in D), and the synapse (E, arrow) on the shaft of the spiny SPR-positive dendrite shown on C. s. g. stratum granulosum; h, hilus; s. r. stratum radiatum. Scale bars, 100 µm (A); 10 µm (B and C) and 1 µm (D and E).
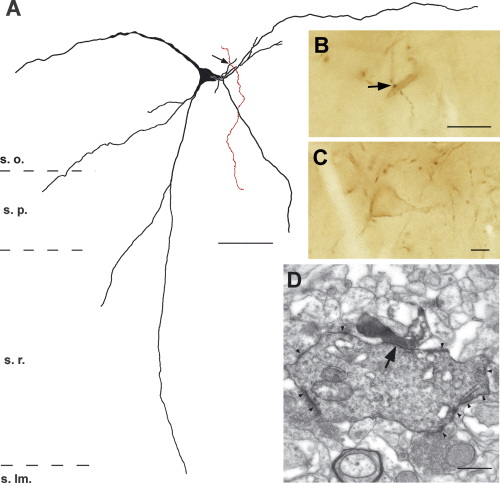
CA3 pyramidal cell axon forming a synapse with an SPR-positive aspiny interneuron (c2 horizontal cell) in CA1 stratum oriens. (A) Camera lucida drawing of an SPR-positive interneurons. Axon collateral of the filled CA3 pyramidal cell axon is shown in red. (B and C) Light micrographs show the putative synaptic contact (B, arrow) and the cell body of the interneuron (C) and the verified synapse in the electron microscope (D, arrow). Note the diffuse DAB membrane-staining (arrowheads) on (D). s. lm. stratum lacunosum-moleculare. Scale bars, 50 µm (A); 10 µm (B and C) and 1 µm (D).
In addition, a further 49 biocytin-filled axon terminals were examined in the vicinity of the verified synaptic contacts with SPR-positive dendrites. All were found to form asymmetric synapses with immunonegative elements; two of them terminated on immunonegative dendritic shafts, whereas the remaining 47 terminated on immunonegative spines, presumably belonging to pyramidal cells.
mGluR-1α-containing interneuron targets of CA3 pyramidal cells
Spiny SPR-immunoreactive interneurons were only exceptionally contacted by the axon terminals of CA3 pyramidal cells. Although we carefully followed all possible dendrites of the target interneurons, a potential source of error for the low incidence is that occasionally only the very distal dendrites of SPR-immunoreactive cells are spiny. Therefore, some of spiny interneurons could have been erroneously classified as aspiny using SPR-immunocytochemistry. To reduce the possibility of such omission error, we have examined the mGluR-1α-labelled interneuron targets of CA3 axon terminals, because most mGluR-1α-immunoreactive neurons are densely covered by long spines (Martin et al., 1992; Baude et al., 1993; Hampson et al., 1994).
Sections containing intracellularly labelled CA3 pyramidal cell axons from four brains were double stained for mGluR-1α, and the immunoreactive targets were analysed the same way as SPR-immunoreactive interneurons. Biocytin filled axonal segments were drawn by camera lucida from one section of brains D288 and D330, and two sections of brain D284, and three sections of brain D296. A total of 2972 axon terminals were examined, 13 of which (0.44%) were found to form putative synaptic contacts with mGluR-1α-positive interneurons. Six of them contacted spiny mGluR-1α-positive dendritic shafts and the remaining seven were found on thin smooth dendrites. Two of the spiny dendrites were located in the hilus, one at the hilus/CA3c dendritic region border, one in the stratum oriens, one in the stratum lacunosum-moleculare of the CA3 region and one in the stratum radiatum of the CA1 region.
We also determined the distribution of spiny and aspiny mGluR-1α-positive cells in randomly selected sections (Table 4, Fig. 2). Although the majority of mGluR-1α-immunoreactive interneurons are known to possess spiny dendrites (Baude et al., 1993; Lujan et al., 1996), a smaller population (31.7% in our sample) has smooth dendrites (Baude et al., 1993). Comparison of the distribution of spiny vs. aspiny neurons and the putative contacts of CA3 axon collaterals on these subtypes indicated preferential axon targets (ratios < 1) on the aspiny subgroup.
| Region(and number of cells) | Frequency of spiny mGluR-1α-positive cells (%) | Frequency of aspiny mGluR-1α-positive cells (%) | Preferential distribution |
|---|---|---|---|
| Hippocampus (n = 492) | 68.3 | 31.7 | 0.40 |
| Hilus (n = 85) | 91.8 | 8.2 | 0.27 |
| CA3 region (n = 193) | 66.8 | 33.2 | 0.50 |
| CA1 region (n = 214) | 60.3 | 39.7 | 0.17 |
One axon collateral segment, contacting a spiny dendrite at the hilus/CA3c border from the brain D296 (Fig. 7) was verified to be a synapse by electron microscopic examination. An additional nine axon terminals were analysed, all of which formed asymmetrical synapses. One terminal contacted an mGluR-1α-negative dendritic shaft and the remaining eight terminated on dendritic spines, presumably belonging to principal cell dendrites.
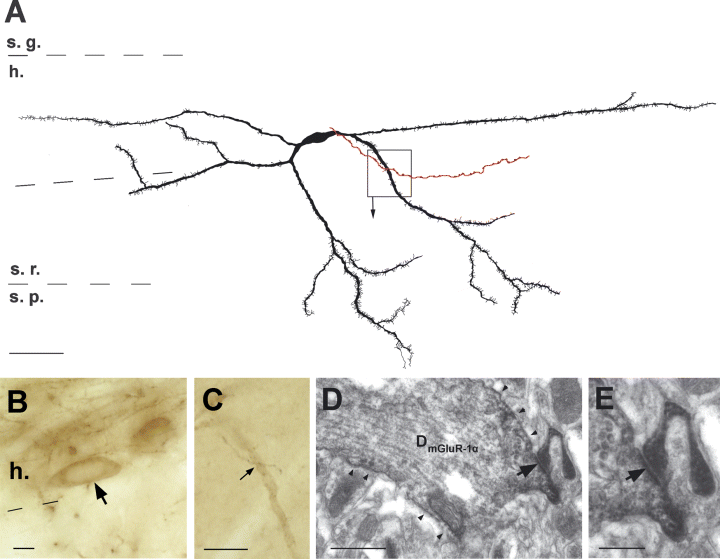
mGluR-1α-positive spiny interneurons are rarely innervated by CA3 pyramidal cell axons. (A) Camera lucida drawing shows a reconstructed mGluR-1α-positive interneurons in the hilus. The filled CA3 pyramidal cell axon is shown in red. (B and C) Light micrographs show the cell body (B, arrow) and the putative contact (C, arrow) and the verified synaptic contact on the dendritic shaft of the mGluR-1α-positive dendrite (D, arrow). Arrowheads point on the diffuse DAB-staining along the dendritic membrane. (E) shows the synaptic contact (arrow) with higher magnification. Scale bars, 50 µm (A); 10 µm (B and C); 1 µm (D) and 0.5 µm (E).
Discussion
Inhibitory interneurons of the hippocampus are heterogeneous with regard to their anatomical markers, receptor expression, afferent and efferent connections and physiological features (reviewed inFreund & Buzsáki, 1996). Although most interneurons have smooth dendrites, a significant portion of them are spiny. Our results show that the aspiny vs. spiny morphological division is functionally important because CA3 pyramidal neurons differentially innervate members of these groups. The findings suggest that CA3 pyramidal cells exert a neuron type-specific inhibition in the various hippocampal regions. The target interneurons typically receive a single bouton from the CA3 pyramidal cell but multiple boutons on the same dendritic branch were also observed.
CA3 pyramidal cells preferentially innervate interneurons with smooth dendrites
SPR-immunoreactive cells represent a heterogeneous cell population of interneurons with regard to their morphology, neurochemical content and function (Freund & Buzsáki, 1996; Acsády et al., 1997; Sloviter et al., 2001). On the basis of double-immunostaining experiments, SPR-positive interneurons with smooth dendrites in the rat hippocampus include PV- and CCK-positive perisomatic (basket and chandelier), NPY-positive dendritic inhibitory cells and CR- and VIP-positive interneuron-specific interneurons (Acsády et al., 1997; Sloviter et al., 2001). In addition, the SPR antibody also labels an unknown fraction of spiny interneurons. The largest fraction of spiny interneurons were observed in the dentate gyrus, followed by the CA3 and CA1 regions, in agreement with previous observations (Acsády et al., 1997). A similar distribution was also observed with mGluR-1α immunostaining, which preferentially labels spiny interneurons (Baude et al., 1993; Lujan et al., 1996). Independent of the antibody used, we found that CA3 pyramidal cell axons innervated a significantly higher percentage of aspiny neurons than spiny ones in all hippocampal regions than might be expected from randomly distributed contacts according to the availability of postsynaptic targets. It remains to be a functionally important question whether interneuron targets of CA3 pyramidal cells in the contralateral hippocampus also show similar target specificity.
CA3 axon terminals show a layer-specific distribution (Ishizuka et al., 1990; Li et al., 1994), but within the layers no striking clustering or specific topography is present. The lognormal distribution of the interbouton intervals of CA3 pyramidal cell axons with a mean interval of 5.6 µm is in agreement with previous studies (7.0 µm in Ishizuka et al., 1990; 4.3 µm in Sík et al., 1993 and 4.7 µm in Li et al., 1994). Assuming that every bouton is a presynaptic terminal, as supported by our electron microscopic observations (98 verified out of 98 putative terminals), CA3 pyramidal cell axon terminals are randomly distributed. Although the distribution of spiny and aspiny neurons show some laminar preference (Gulyás et al., 1992; Hsu & Buzsáki, 1993), these findings suggest that the preference of CA3 axon terminals for aspiny neurons is not explained by geometrical factors. Attractant substances may play a potential guidance mechanism. Class 3 semaphorins, ephrins, netrins and slits are molecules known to play a key role in axon guidance in the hippocampal formation during development (reviewed in Skutella & Nitsch, 2001). Different class 3 semaphorins are expressed in different cell types, and regulate axonal in- and outgrowth in the hippocampus (Pozas et al., 2001; Pascual et al., 2004; Pascual et al., 2005). Interneurons as well as principal cells are heterogeneous with regard to their semaphorin expression (Pascual et al., 2005), and thus, semaphorins are possible candidates that attract CA3 pyramidal cell axons preferentially to aspiny interneurons.
In addition to target interneuron type selectivity, CA3 pyramidal cells axon terminals are also compartment-specific. Although CA3 axon collaterals innervated a very small fraction of spiny interneurons (0.1–0.2%), even in those rare cases, the filled excitatory axon terminal was found to form a synapse with the dendritic shaft of the postsynaptic spiny interneuron (99.57%) but not on spines or rarely on the cell bodies (0.43%). This is in contrast with the interneuron innervation pattern by the granule cells as mossy fibers terminate relatively randomly on the different domains of SPR- and mGluR-1α-positive interneuron targets (Acsády et al., 1998). These observations support the view that synapse formation in the developing hippocampus depends on both postsynaptic and presynaptic factors (reviewed in Skutella & Nitsch, 2001).
Multiple CA3 pyramidal cell-interneuron contacts are rare
A structural mechanism for increasing the reliability of synaptic transmission is to establish multiple synaptic contacts. On the basis of ‘multiple appositions’ of CA3 pyramidal cell axons terminating on CA1 pyramidal cells at the light microscopic examinations and serial electron microscopic sections, the estimated fraction of multiple contacts between these two pyramidal cell populations is relatively high (25%; Sorra & Harris, 1993). In contrast, the fraction of multiple contacts between CA3 neurons and SPR-immunoreactive interneurons (12.5%; present observations) and parvalbumin-positive interneurons (15.3%; Sík et al., 1993) is relatively low. Furthermore, most multiple synapses of CA3–CA1 pyramidal cells are distributed on spines of different dendrites of the target CA1 pyramidal cells, whereas multiple terminals on the interneurons were mainly established on the same dendrite of the interneuron. Although reconstruction of distal dendrites were not always possible, our findings (see also Sík et al., 1993) supports that serial apposition of multiple synapses are more frequent than distributed synapses on different dendrites. This distinction may be functionally important, as summation of excitatory postsynaptic potentials (EPSPs) from adjacent and nonadjacent synapses follow supralinear and linear courses, respectively (London & Hausser, 2005; Gasparini & Magee, 2006). Although pyramidal cell interneuron synapses are known to be quite reliable (Gulyás et al., 1993; Csicsvári et al., 1998), multiple synapses may further increase spike transmission probability between pyramidal cells and interneurons.
Functional considerations
A large fraction of mGluR-1α-immunoreactive spiny interneurons also express the neuropeptide, somatostatin, (Baude et al., 1993), in O-LM cells of the CA1 and CA3 regions (Léránth et al., 1990; Sík et al., 1995; Katona et al., 1999; van Hooft et al., 2000) and HIPP cells in the dentate gyrus (Han et al., 1993; Sík et al., 1997) of the rat hippocampus. These spiny interneurons mainly innervate dendrites, and mediate feedback inhibition to the principal cells (Freund & Buzsáki, 1996). A subgroup of the spiny interneurons in the hilus and stratum lucidum of the CA3 region that coexpress both mGluR-1α receptors and somatostatin also contain SPR (Acsády et al., 1997). Our results show that CA3 pyramidal cells do not innervate these spiny interneurons, extending a previous suggestion that the spiny interneurons in CA1 stratum oriens are largely under the control of CA1 pyramidal cells rather than CA3 pyramidal cells (Blasco-Ibanez & Freund, 1995). Therefore, feed-forward inhibitory communication from the CA3 region to the CA1 and dentate areas are selectively mediated by aspiny interneurons.
Interneurons are known to be critically involved in the maintenance of network oscillations at various time scales (Buzsáki & Eidelberg, 1983; Fox et al., 1986; Buzsáki & Chrobak, 1995; Klausberger et al., 2003; Whittington & Traub, 2003; Klausberger et al., 2004; Somogyi & Klausberger, 2005). In the present context, it is particularly important to notice that the CA3 pyramidal cells innervate perisomatic interneurons in both the CA3 and CA1 regions, and the synchronous drive of these neurons is critical for the tight gamma frequency oscillatory coupling between the two regions (Csicsvári et al., 2003). In contrast, the slower theta rhythm shows a large phase shift between the two regions (Dragoi & Buzsáki, 2006). The theta phase dissociation may occur because dendrite-targeting spiny interneurons are locally innervated and they play a critical role in theta-frequency oscillations (Whittington & Traub, 2003; Mann et al., 2005).
Finally, differential excitatory innervation of spiny and aspiny interneurons may bear special relevance in hippocampal pathology. The selective vulnerability of spiny somatostatin- and mGluR-1α-positive cells (Baude et al., 1993) has been shown in head trauma (Lowenstein et al., 1992), ischemia (Johansen et al., 1987; Freund et al., 1990; Arabadzisz & Freund, 1999), and various epilepsy models (Freund et al., 1992; Sperk et al., 1992; Maglóczky & Freund, 1993; Mitchell et al., 1995; Schwarzer et al., 1995; Vezzani et al., 1996; Miettinen et al., 1998; Dinocourt et al., 2003), including human epileptic tissue (de Lanerolle et al., 1989). Spiny interneurons in the CA3 stratum lucidum (Gulyás et al., 1992; Spruston et al., 1997) are particularly vulnerable in ischemia (Freund & Maglóczky, 1993; Hsu & Buzsáki, 1993) and the kainate model of epilepsy (Maglóczky & Freund, 1995). The high density of metabotropic glutamate receptors on these spiny interneurons might be related to their selective vulnerability in diverse hippocampal injuries. Metabotropic glutamate receptors can be activated by high frequency stimulation (Miles & Poncer, 1993), resulting in large Ca2+-influx (Topolnik et al., 2005), and in turn, cell death (Ogura et al., 1988; reviewed in Arundine & Tymianski, 2003). In contrast, the short-lasting membrane effects of AMPA/KA glutamate receptors are not involved in the selective vulnerability of spiny interneurons (Sanon et al., 2005). Thus, it is unlikely that even very intense spiking of the CA3 pyramidal population would induce interneuronal damage in either spiny or aspiny targets. Therefore, the selective vulnerability of the spiny interneuron population should be sought in causes other than elevated synchrony of pyramidal cells.
Acknowledgements
This research was supported by NIH (MH54671 to GB and NSO23945 to LZ). We would like to thank Alvaro Duque for support and teaching the use of NeuroLucida and Richard Miles for helpful discussions.
Abbreviations
-
- AMPA
-
- α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
-
- HIPP
-
- hilar perforant path associated interneuron
-
- KA
-
- kainate
-
- mGluR-1α
-
- metabotropic glutamate receptor subunit 1α
-
- O-LM
-
- oriens-lacunosum-moleculaire interneuron
-
- SPR
-
- substance P receptor