Paradoxical effects of NPY in the suprachiasmatic nucleus
Abstract
The circadian clock in the suprachiasmatic nucleus (SCN) is synchronized by the 24 h, light : dark cycle, and is reset by photic and non-photic cues. The acute effects of light in the SCN include the increase of mRNA levels of the circadian clock gene Per1 and a dramatic reduction of pineal melatonin. Neuropeptide Y (NPY), which appears to mediate the phase-resetting effects of non-photic stimuli, prevents the ability of light, and stimuli that mimic light, to phase shift the circadian clock when injected into the SCN. The purpose of the present study was to determine if NPY inhibits the ability of light to suppress pineal melatonin. Surprisingly, NPY injected into the SCN of hamsters mimicked the effects of light by suppressing pineal melatonin levels. To confirm that NPY inhibited the effects of light on the induction of Per1 mRNA levels, Per1 mRNA levels in the SCN were measured in these same animals. NPY significantly reduced Per1 mRNA levels induced by the light pulse. The suppression of melatonin by NPY appears to be mediated by the same subtype of NPY receptors in the SCN that mediate the modulation of phase shifts. Injection of Y5 receptor agonists mimicked the effects of NPY on pineal melatonin, while injection of a Y2 agonist did not. Thus, these data are the first to demonstrate the paradoxical effects of NPY within the SCN. NPY mimics the effects of light on pineal melatonin and inhibits the effects of light on the induction of Per1 mRNA.
Introduction
In mammals, circadian rhythms are endogenously generated by a primary circadian clock in the suprachiasmatic nucleus of the hypothalamus (SCN) and are entrained to the 24 h light : dark cycle. Light serves to entrain the circadian clock in the SCN by resetting the phase of the clock on a daily basis (Moore-Ede et al., 1982). The SCN also mediates the dramatic reduction in pineal melatonin that occurs when light is provided at night (Klein & Weller, 1970; Tamarkin et al., 1979). Environmental lighting information is detected by intrinsically photosensitive retinal ganglion cells, which directly project to the SCN via the retinohypothalamic tract (RHT) (Pickard, 1985; Berson et al., 2002). Glutamate released from RHT terminals mediates at least some of the effects of light in the SCN, and activates N-methyl-d-aspartate (NMDA) and non-NMDA glutamate receptors (Colwell et al., 1991; Colwell & Menaker, 1992; Ebling, 1996; Mintz & Albers, 1997; Mintz et al., 1999; Paul et al., 2003).
Another projection that plays an important role in mediating the resetting of the circadian clock in the SCN is the geniculohypothalamic tract (GHT). The GHT is a direct projection from the intergeniculate leaflet of the thalamus (IGL) to the SCN (Card & Moore, 1989). A number of stimuli other than light can reset the circadian clock. These non-photic stimuli typically produce large phase advances when presented during the day (Turek & Losee-Olson, 1986; Reebs & Mrosovsky, 1989; Hastings et al., 1992). The phase shifts produced by non-photic stimuli may be mediated by neuropeptide Y (NPY) released into the SCN from terminals of the GHT (Morin et al., 1992; Biello et al., 1994). Injection of NPY into the SCN mimics the phase shifts produced by non-photic stimuli, and injection of antisera to NPY inhibits the phase shifts produced by non-photic stimuli (Albers et al., 1984; Biello et al., 1994; Huhman & Albers, 1994). During the day, NPY is thought to act on Y2-like NPY receptors (Golombek et al., 1996; Huhman et al., 1996), although there have been differing reports as to whether these receptors are located in the SCN (Parker & Herzog, 1999; Fetissov et al., 2004).
Another action of NPY in the SCN is the ability to inhibit the circadian effects of light. Injection of NPY into the SCN significantly reduces the phase advances produced by light during the late night (Weber & Rea, 1997; Lall & Biello, 2003), but does not reduce light-induced phase delays during the early night (Weber & Rea, 1997; Gamble et al., 2005). However, NPY applied to the SCN in vitro during the early night inhibits light-induced increases in one of the genes thought to be essential in the molecular mechanism underlying circadian timing and entrainment (i.e. Per1 mRNA levels) (Brewer et al., 2002). During the night, NPY is thought to act on NPY Y5 receptors because a Y5 receptor agonist inhibits phase delays (in the early night), and a Y5 receptor antagonist blocks the ability of NPY to inhibit phase delays in vitro (Yannielli & Harrington, 2001). In addition, both a Y1/Y5 agonist and a more selective Y5 agonist inhibit light-induced phase advances (in the late night) in vivo (Lall & Biello, 2003; Gamble et al., 2005).
The purpose of the present study was to determine whether NPY could inhibit another effect of light mediated by neurons in the SCN. Because NPY has such potent inhibitory effects on the ability of light to induce phase shifts when administered in the SCN during the late night, we hypothesized that NPY would inhibit the ability of light to suppress melatonin. In addition, we investigated the NPY receptor subtype mediating this effect.
Materials and methods
Animals and surgery
Adult male Syrian hamsters (Mesocricetus auratus, Charles River Laboratories, Wilmington, MA, USA) were group-housed (six per cage) in Plexiglas cages (20 × 40 × 20 cm) in a 14 : 10 h light : dark cycle until the time of surgery (at least 7 days after arrival). Food and water were available ad libitum. At the time of surgery, animals weighing 130–150 g were deeply anesthetized with a cocktail of ketamine (120 mg/kg), xylazine (25 mg/kg) and acepromazine (2 mg/kg; Butler, Atlanta, GA, USA) and stereotaxically implanted with 26-gage guide cannulae (11 mm, total length) aimed at the SCN. The skull was leveled before implantation using bregma and lambda as reference points (1.2 mm anterior and 1.7 mm lateral to bregma). With a 32-gage injection needle inserted into the cannula, the final depth of injection was 7.2 mm below dura. A 10 ° angle toward the midline was used for implantations. The cannula was fixed to the skull using 11-mm wound clips and cranioplastic cement.
Experimental treatments
After surgery, animals were transferred to individual Plexiglas cages (20 × 40 × 20 cm) equipped with running wheels (each with a diameter of 16 cm), housed in a constant dark room, and allowed to establish stable, free-running rhythms. To measure wheel running activity, each wheel revolution activated a microswitch on the outside of the cage that was monitored continuously by a computer using VitalView software and hardware (Minimitter, Bend, OR, USA). Animals were in constant dark for at least 10 days before treatment, which was administered at circadian time (CT) 20 (i.e. 8 h after the activity onset). Light pulses (10 min) were administered in the adjoining anteroom using white light (50 lux; measured by Sekonic Digilite model L-318B) provided by a fluorescent bulb mounted approximately 3 feet above the cage. Animals in the sham light condition were removed from their shelves for 10 min but remained in the dark room. The large number of animals used in these experiments prevented the use of the anteroom for both sham and light pulsed conditions. However, in previous experiments from this lab, animals in the sham light condition were transferred to the anteroom but were not exposed to light and did not show a change in Per1 expression, pineal melatonin levels or phase (Paul et al., 2004). Both groups of animals remained in their home cages; however, the wheel and attached lid were removed and replaced with a normal lid with no wheel. Light exposure during entry to and from the dark room was avoided by the use of two light-proof, heavy curtains on each side of the door. The animals were not exposed to light at any time other than during the 10-min, 50-lux, light pulse. After 10 min, the cage lids were replaced with the wheel-lid assemblies in both groups of animals, and the cages were transferred back to the shelves.
All microinjections were given immediately before the light presentation. Microinjections (200 nL) were given using a 16-mm, 32-gage needle attached by polyethylene tubing to a 1 µL Hamilton syringe. NPY (0.23 mm; porcine; Sigma, St. Louis, MO, USA) (Ala31, Aib32)-NPY (Y5 agonist; 0.23 mm and 1.15 mm; porcine; Bachem, Torrance, CA, USA), [Leu31, Pro34]NPY (Y1/5 agonist; 0.23 mm and 1.15 mm; porcine; Sigma) and NPY 13–36 (Y2 agonist; 2.30 mm; porcine; Sigma) were dissolved in a vehicle of 0.9% saline. The concentration of NPY 13–36 was chosen based on the relatively low in vivo potency compared with NPY [ED50 (nmol): 14.80 compared with 1.29, respectively] (Wyss et al., 1998). The higher concentrations of [Leu31, Pro34]NPY and (Ala31, Aib32)-NPY were chosen based on the ED50 and EC50 reported for these agonists [in nm: 2.01 compared with 1.29 (NPY) and 98 compared with 18.5 (NPY), respectively] (Wyss et al., 1998; Cabrele et al., 2000). In addition, this higher concentration of (Ala31, Aib32)-NPY is sufficient to inhibit light-induced phase advances at CT 20 to the same extent as NPY (Gamble et al., 2005). Injections were given in the dark housing room to hamsters gently restrained by hand in dim red illumination (provided by a red Sylvania 15-Watt light bulb mounted into a foil-covered lamp with slits in the foil ∼ 3 feet from the animal's head; < 5 lux measured by Sekonic Digilite model L-318B). The needle was left in place for at least 20 s after injection. The red light was only used during the experimental treatments and tissue preparation. Animals that served as positive controls (light-exposed, but no injection) and negative controls (no light exposure, no injection) were run concurrently with every experiment. The results from these positive and negative controls were very similar to the light + vehicle- and sham-light + vehicle-treated animals, respectively. All procedures and protocols were conducted according to the NIH Guidelines, and approved by the Georgia State University Institutional Animal Care and Use Committee.
Tissue preparation
For analysis of Per1 mRNA and melatonin levels, animals were killed by decapitation 1 h after injection and/or the onset of the light pulse. Pineal glands and brains were immediately removed and frozen on dry ice. These procedures were performed under dim red illumination (< 5 lux measured by Sekonic Digilite model L-318B). Coronal sections (20 µm thickness) of the SCN region were cut using a cryostat and collected on Suprafrost slides. Whole brains, brain slices and pineal glands were stored at −80 °C. Every fourth brain section was stained with Cresyl violet to determine the location of the needle tract. The injection sites were evaluated using a light microscope. Only animals with injection sites that did not penetrate the third ventricle, did not damage the SCN, but were within 300 µm of the SCN were included in the statistical analysis.
In situ hybridization
For in situ hybridization, a fragment of hamster Per1 cDNA, which had been cloned into the ApaI (anti-sense) and BstX (sense) sites of the pBluescriptII KS(–) plasmid vector (generously donated by Dr Joseph Takahashi, Northwestern University, Evanston, IL, USA) was linearized using ApaI and BstX restriction endonucleases (Promega, Madison, WI, USA). In vitro transcription of the linearized templates was used to synthesize [35S]-UTP (1250 Ci/mmol)-labeled riboprobes using T3 (sense) and T7 (anti-sense) RNA polymerases. Sections mounted on slides were fixed in 4% paraformaldehyde, acetylated by 0.25% acetic anhydride in 0.1 m triethanolamine, and dehydrated in ethanol. Each slide was then hybridized in a solution consisting of 10 mm dithiothreitol, [35S]-hPer1 (500 000 cpm), and buffer [50% formamide, 100 mg/mL dextran sulfate, 2 × standard sodium citrate (SSC), 1 × Denhardt's solution, 0.5 mg/mL tRNA, 0.5 mg/mL heparin sodium salt and 0.4 mg/mL ssDNA], and then incubated overnight at 55 °C. For post-hybridization, sections were washed (50% formamide in 2 × SSC at 52 °C) and treated with Rnase A (1 µg/mL) in a bath at 37 °C. The slides were then air-dried and exposed to Kodak BioMax MR films with 14C microscales (Amersham Life Science) for 10 days at room temperature (Paul et al., 2004). Autoradiograph images were scanned into Adobe Photoshop 6.0, and optical density units (ODU) were measured using Scion Image for Windows. The region of interest was defined using a box (0.20 mm2) in the middle of the SCN. Background ODU measured in the lateral hypothalamus were subtracted from SCN values. The three sections closest to the mid-SCN were averaged and analysed in each animal. A value of 100 was assigned to the maximum ODU, and the other values were adjusted relative to the maximum. The dorsomedial and the ventrolateral regional measurements were assessed as previously described (Paul et al., 2003, 2004). In order to measure regional differences, a mean OD value was obtained using a box (0.10 mm2) that was placed in the middle of the dorsomedial or ventrolateral region of the SCN. Background OD values measured in the lateral hypothalamus were subtracted from SCN regional values. These measurements were then normalized relative to the maximum as before.
Radioimmunoassay
To measure melatonin levels in the pineal gland, each pineal gland was homogenized by sonication in 250 µL phosphate-buffered saline. Levels of pineal melatonin were measured using a radioimmunoassay method described in Paul et al. (2004).
Phase-shifting analysis
A phase-shift control group of animals received two treatments: NPY or saline microinjection followed by a light pulse. The group assignment was random and counterbalanced. A minimum of 10 days separated the two treatments. Phase shifts were quantified using the linear regression method (Daan & Pittendrigh, 1976). Two regression lines were determined: one for the daily activity onsets for 7 days before the injection, and another for the daily activity onsets for 9–10 days after the injection. Activity onsets were defined as the first of at least three consecutive 5-min periods of more than 10 wheel revolutions. The first three activity onsets after treatment were not included in the second regression line in order to avoid the influence of transient or unstable onsets. If the standard error of either regression line was greater than 20 min, the injection was not included in the analysis. In this study, the phase shift from one injection was excluded for this reason, having a regression line with a standard error of 49 min. Phase shifts were calculated as the difference between onsets of activity predicted for the day following injection by each of the two regression lines. Animals that were used for determining phase shifts in wheel-running rhythms were killed at the completion of the experiment, at which time they were given an overdose of sodium pentobarbital and microinjected with 200 nL of India ink. The brains were removed, stored in 10% formalin, and sectioned on a vibratome (100 µm thick) and mounted on slides.
Data analysis
Statistical significance (ascribed at P < 0.05) was determined using one-way analyses of variance, and Tukey HSD tests were used for post hoc analyses. To determine whether the injection site distance from the SCN was correlated with melatonin levels, Spearman's correlation coefficient was used because the data points were not normally distributed. For the analysis, SPSS 11.0 (SPSS, Chicago, IL, USA) and Microsoft Excel were used.
Results
NPY microinjected into the SCN at CT 20 mimics the effects of light on pineal melatonin and inhibits the effects of light on Per1 mRNA levels
There was a significant effect of treatment on the level of melatonin in the pineal gland (F3,24 = 9.58, P < 0.01, Fig. 1). As predicted, animals presented with a light pulse (and microinjected with vehicle) had significantly lower levels of pineal melatonin than those that received a sham light pulse and vehicle (P < 0.01). Surprisingly, microinjection of NPY into the SCN region did not attenuate photic suppression of melatonin. Furthermore, microinjection of NPY into the SCN region alone (with no light presented) resulted in significantly lower pineal melatonin than microinjection of vehicle (P < 0.01). Pineal melatonin levels for animals in the Veh/Light, NPY/Light and NPY/Sham conditions did not significantly differ (P > 0.05). In Fig. 1, all but two of the pineals collected were from the same animals whose brains were processed for in situ hybridization and depicted in Fig. 2.
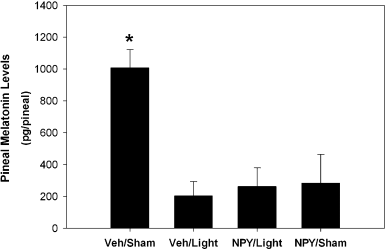
Effects of neuropeptide Y (NPY) into the SCN on light-induced suppression of melatonin levels in the pineal gland. Animals received either: vehicle and sham light (n = 6); vehicle and light (n = 10); NPY and light (n = 7); or NPY and sham light (n = 5). Bars represent mean ± SEM pineal melatonin levels measured in pg/pineal. *Significantly different from all other groups, P < 0.05.
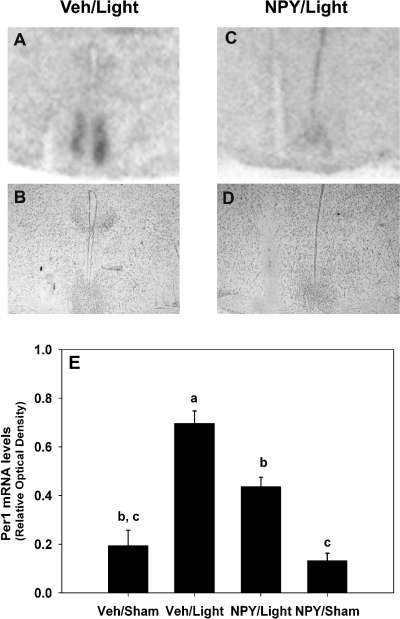
Effects of neuropeptide Y (NPY) on light-induced increase of Per1 mRNA levels in the SCN at CT 20 (8 h after activity onset). Representative autoradiograph images of the effects of NPY on light-induced Per1 mRNA in SCN. Sections are representative of the groups in the bar graph (E): vehicle and light (A and B), and NPY and light (C and D). Cresyl violet-stained sections appear below each autoradiograph (B and D) and show the site of injection. Autoradiograph images were scanned into Adobe Photoshop 6.0 and cropped to display only the SCN and surrounding areas. Cresyl violet-stained sections were digitally photographed. (E) Bar graph representing the mean ± SEM Per1 mRNA levels measured in relative optical density units (ODU). Animals received either: vehicle and sham light (n = 5); vehicle and light (n = 15); NPY and light (n = 14); or NPY and sham light (n = 4). The letters a, b and c denote groups that are significantly different from one another (P < 0.05).
As can be seen in Fig. 2, there was a significant effect of treatment on Per1 mRNA levels in the SCN (F3,34 = 13.84, P < 0.01). Light presentation at CT 20 resulted in significantly greater levels of Per1 mRNA than in all other groups (P < 0.01). NPY significantly attenuated light-induced Per1 mRNA levels (P < 0.01), resulting in levels that were not significantly different from levels in Veh/Sham-treated animals (P > 0.05). Despite this significant reduction, levels of Per1 mRNA were still higher for NPY/Light animals than for NPY/Sham animals (P < 0.05). Microinjection of NPY alone resulted in similar levels of Per1 mRNA as those resulting from vehicle (P > 0.05). To test for regional differences of Per1 mRNA levels in the SCN, a two-way anova (Region × Treatment) was performed. Consistent with the one-way anova, there was a significant main effect for Treatment, F3,34 = 21.04, P < 0.01. While there was a significant main effect for Region (F1,34 = 6.77, P < 0.05), the Region–Treatment interaction was not significant (P > 0.05). Overall, the dorsal region of the SCN had slightly, but significantly, lower levels of Per1 mRNA than the ventral region. Therefore, NPY appears to reduce Per1 mRNA levels equally in both the dorsal and ventral SCN.
To ensure that the microinjection of NPY that we gave significantly inhibited light-induced phase shifts as well as increases in Per1 mRNA, two groups of animals were used. One group of animals (the phase-shift control group) remained in their cages after treatment, and the second group was killed 1 h after treatment, and their brains were processed for Per1 hybridization. As illustrated in Fig. 3, light presented at CT 20 resulted in a phase advance (58.9 ± 18.4 min; mean ± SEM), which was significantly decreased by microinjection of NPY before the light pulse (4.8 ± 11.8 min; t12 = 2.60, P < 0.05, two-tailed).
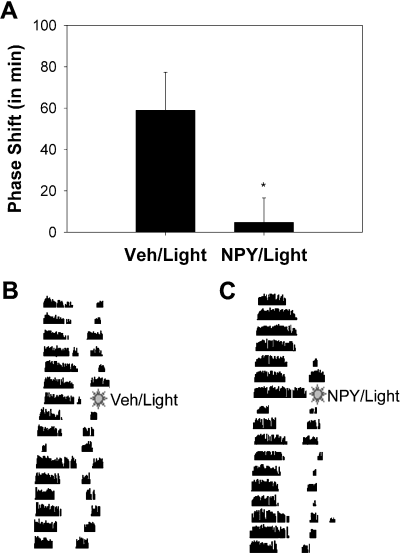
The effects of neuropeptide Y (NPY) on light-induced phase shifts in behavior. (A) Phase advances in min (mean ± SEM) calculated by linear regression in animals that received vehicle and light (Veh/Light), or NPY and light (NPY/Light). *P < 0.05. Below, representative actograms of wheel-running rhythms demonstrating the effects of vehicle (B) or NPY (C) on light-induced phase advances. Each line represents a 24-h period, and successive days are shown from top to bottom. All treatments were administered at CT 20.
Activation of Y5-like receptors mimics NPY in the ability to suppress pineal melatonin
To follow up on the surprising finding that NPY suppresses the levels of pineal melatonin, a separate experiment examined whether NPY acts on the same receptor subtype for the suppression of melatonin as for the inhibition of phase shifts (Yannielli & Harrington, 2001). Animals received treatment of either a light pulse, microinjection of NPY, a high or low dose of a Y5 agonist [(Ala31, Aib32)-NPY], a high or low dose of a Y1/5 agonist ([Leu31, Pro34]NPY), a Y2 agonist (NPY 13–36) or vehicle. The anova revealed a significant main effect of treatment on the levels of melatonin in the pineal gland (F7,67 = 11.23, P < 0.05, Fig. 4). As found in the previous experiment (see Fig. 1), pineal melatonin levels for animals that received either vehicle, the Y2 agonist or the low concentration of the Y1/5 agonist were significantly higher than all other groups (P < 0.05). In addition, the levels for animals that received either light, NPY, the low concentration of the Y5 agonist, or the high concentrations of the Y1/5 and Y5 agonists, did not significantly differ from one another. Evaluation of the histology indicated that the reduction of melatonin induced by NPY injection was specific to the SCN. In addition, there was a significant positive correlation between the injection site distance from SCN and melatonin levels (Spearman's rho; r2 = 0.59, P < 0.05), indicating that sites that were closer to the SCN were more likely to suppress melatonin.
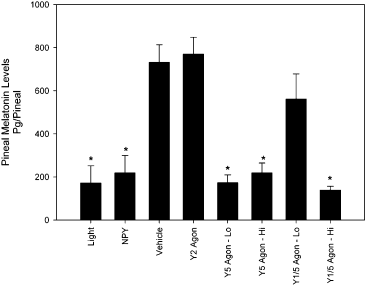
Effects of neuropeptide Y (NPY) agonists into the SCN on melatonin levels in the pineal gland. Animals received either: light (n = 7); NPY (n = 7); vehicle (n = 6); Y2 agonist (n = 5); Y5 agonist (0.23 mm; n = 4); Y5 agonist (1.15 mm; n = 4); Y1/5 agonist (0.23 mm; n = 4); Y1/5 agonist (1.15 mm; n = 4). Bars represent mean ± SEM pineal melatonin levels measured in pg/pineal. *Significantly different from the vehicle, Y2 agonist or Y1/5 agonist (0.23 mm) (P < 0.05).
Discussion
These data are the first to demonstrate the paradoxical effects of NPY within the SCN. NPY simultaneously mimicked the effects of light on pineal melatonin and inhibited the effects of light on the induction of Per1 mRNA and phase shifts in circadian rhythmicity. In addition, these results provide the first evidence that a peptide released in the SCN may be involved in the regulation of pineal melatonin production. The only other data on how NPY released in the SCN might influence pineal melatonin come from studies where the IGL was lesioned. Lesions of the IGL significantly reduced the ability of brief photic stimulation (< 1 min duration) to suppress levels of the melatonin precursor, N-acetylserotonin, in the pineal gland. However, IGL lesions did not inhibit the ability of 15-min light pulses to suppress pineal N-acetylserotonin (Cipolla-Neto et al., 1995).
In the present study, NPY injected into the SCN region had potent effects on pineal melatonin, clock gene expression and phase shifts in circadian rhythmicity. The same microinjection of NPY mimicked the ability of light to completely suppress melatonin levels and, at the same time, essentially eliminated the ability of light to induce phase shifts in circadian rhythmicity. It is interesting that although NPY also significantly reduced Per1 mRNA levels, Per1 mRNA levels were not reduced to the level of the controls. Thus, these data support the hypothesis that light-induced phase shifts in circadian rhythmicity require increased levels of Per1 mRNA in the SCN, and suggest that only a partial reduction in Per1 mRNA levels is required to eliminate light-induced phase shifts. This possibility is consistent with evidence that tetrodotoxin (TTX; which blocks Na+-dependent action potentials) blocks light-induced phase advances in circadian rhythmicity in the late night, but does not fully reduce the light-induced increase in Per1 mRNA levels in the SCN (Gamble et al., 2003; Paul et al., 2004). Taken together, these data indicate that complete suppression of Per1 mRNA levels in the SCN may not be necessary to inhibit the effects of phase-shifting stimuli during the night.
Although all the injection sites in this study were histologically confirmed to be within 300 µm of the SCN, it remains possible that NPY may have spread and exerted its effects in areas outside of the SCN. For example, it might be possible that NPY acted on the paraventricular nucleus of the hypothalamus (PVN), which is a component of the multisynaptic pathway that regulates melatonin production (Larsen et al., 1998). However, several lines of evidence suggest that the PVN is not likely to be the site of action of NPY in the present study. First, a previous study has shown that microinjection of NPY into the PVN in rats inhibits circulating levels of corticosterone, while NPY injection into the SCN region does not (Albers et al., 1990). Because NPY injected in a volume of 300 nL in these previous studies did not spread to the PVN, it seems unlikely that NPY injected in the smaller volume of 200 nL in the present study would reach the PVN. Second, the present study found that the distance of the injection site from the SCN was positively correlated with melatonin levels (i.e. injections closer to the SCN were more likely to suppress melatonin). Third, a previous study has shown that diffusion from a 300-nL injection into the SCN region is limited to a region not much larger than the SCN, suggesting that distances within 0.5 mm of the SCN (compared with 0.3 mm in the present study) are relatively specific to the SCN (Rea et al., 1993).Taken together, these data suggest that the SCN is the most probable site of action of NPY in the present study.
The results of the present study are consistent with recent evidence that the mechanisms for communication of photic information to the pacemaker and the pineal are separate and independent from one another within the SCN. For example, TTX is able to inhibit NMDA-induced suppression of melatonin, but not the NMDA-induced increase in Per1 mRNA (Paul et al., 2004). In addition, injection of NMDA antagonists into the SCN does not block the ability of light to suppress melatonin, although NMDA antagonists attenuate light-induced increases in Per1 mRNA (Paul et al., 2003). The present data suggest that NPY receptor activation is able to inhibit the light-induced increase in Per1 mRNA but not the light-induced suppression of melatonin. Therefore, it is possible to modulate pineal melatonin levels via the SCN without modulating Per1 mRNA levels in the SCN and vice versa. Taken together, these results suggest that the photic transduction pathway within the SCN that results in suppression of pineal melatonin during the night is independent of the photic transduction pathway that upregulates Per1 mRNA.
Although there is considerable evidence that NPY mediates the circadian effects of non-photic stimuli during the day (Rusak et al., 1989; Biello et al., 1994; Huhman & Albers, 1994), the role of NPY in mediating the effects of non-photic stimuli during the night is less clear. NPY can mimic the ability of non-photic stimuli, such as novel wheel running, to inhibit light-induced shifts of circadian phase at night (Ralph & Mrosovsky, 1992; Weber & Rea, 1997; Lall & Biello, 2002; Gamble et al., 2005). However, to date, there is no evidence that inhibition of NPY activity can inhibit the ability of non-photic stimuli to block light-induced phase shifts at night. In fact, serotonin may mediate the circadian effects of non-photic stimuli during the night because injection of a serotonin antagonist prevents the inhibition of light-induced phase advances by exposure to novel activity wheels (Mistlberger & Antle, 1998).
Very little is known about the effects of non-photic stimuli on the regulation of melatonin levels. However, forced swimming at night significantly reduces pineal melatonin levels in rats (Yaga et al., 1993), and in humans, night-time bicycling attenuates the evening increase in plasma melatonin (Monteleone et al., 1990). It will be interesting to determine if other forms of non-photic stimuli are capable of influencing melatonin levels.
Another possible role for NPY released at night is that it contributes to the communication of some form of photic information to the SCN. Support for this idea comes from the finding that transitions from light to dark and dark to light induce significantly higher levels of NPY in the SCN (Shinohara et al., 1993; Shinohara & Inouye, 1995). In addition, NPY binding in the SCN as measured with 125I-PYY significantly increases following a transition from light to dark (Stopa et al., 1995). Finally, NPY may be released when light is presented during the late night because administration of an NPY Y5 antagonist or antisera to NPY enhances advances produced by light at this time (Biello, 1995; Yannielli et al., 2004). Thus, it is possible that NPY is released during some change in photic stimulation during the night.
Another interesting feature of the present study was the finding that activation of Y5-like receptors in the SCN may mediate the ability of NPY to mimic the effects of light on pineal melatonin. Immunoreactivity for NPY Y5 receptors is found in several locations within the anterior region of the hypothalamus, including: the entire SCN, the lateral magnocellular and parvocellular PVN (but not the ventral PVN), the lateral hypothalamic area (but not the ventrolateral hypothalamus) and the supraoptic nucleus (Wolak et al., 2003). Of these areas, only the PVN is known to project indirectly to the pineal gland; however, for the reasons discussed above, it seems unlikely that NPY acted in the PVN to suppress melatonin in the present study. Within the SCN, Y1 and Y5 NPY receptors have been localized, whereas Y2 receptors have not been found (Wolak et al., 2003; Fetissov et al., 2004, but see Golombek et al., 1996; Parker & Herzog, 1999). The majority of these receptors are thought to be G-protein-coupled, positively coupled to [Ca2+]i and negatively coupled to cAMP (Balasubramaniam, 2003). In addition, the Y1 and Y2 receptors may also act as autoreceptors (King et al., 1999; Landry et al., 2000; St Pierre et al., 2000). It is interesting that NPY application and activation of Y5-like receptors in the SCN cause a significant depression of the spontaneous firing rate (Liou & Albers, 1991; van den Pol et al., 1996; Cutler et al., 1998; Gribkoff et al., 1998), which is necessary for the nightly increase in pineal melatonin synthesis (Perreau-Lenz et al., 2004). Van den Pol et al. (1996) showed that NPY, a Y1/5 agonist ([Leu31, Pro34]NPY), and a Y2/5 agonist [PYY(3–36)] induce long-term depression of cytosolic Ca2+ and glutamate-mediated excitatory activity in SCN neurons. Perreau-Lenz et al. (2004) found that NMDA-type glutamate receptor activation in the PVN is required for the pineal melatonin production during the night, and proposed that a small subset of SCN cells are active at night and responsible for the excitatory input to the PVN that drives night-time melatonin production (Perreau-Lenz et al., 2004). It may be that activation of NPY Y5 receptors inhibits SCN glutamatergic signaling to the PVN that is responsible for night-time melatonin production.
This explanation becomes more complex when taking into consideration light exposure, which in general, increases SCN Per1 gene activity and neuronal activity (Kuhlman et al., 2003). If NPY Y5 receptor activation inhibits SCN excitatory activity, then it makes sense that the resulting increase in Per1 gene activity would also be inhibited. The same would hold true for a shift in circadian phase and, indeed, activation of NPY Y5-like receptors in the SCN also appear to mediate the ability of NPY to inhibit the light-induced phase shifts of circadian rhythmicity (Yannielli & Harrington, 2001; Lall & Biello, 2003; Gamble et al., 2005). How light exposure during the night might affect a smaller subset of SCN cells that may provide glutamatergic signaling to the PVN is not clear. It is possible that NPY could be released from a subpopulation of terminals containing NPY in the SCN in response to light exposure (Shinohara et al., 1993; Biello, 1995; Shinohara & Inouye, 1995; Stopa et al., 1995; Yannielli et al., 2004), and act on NPY Y5 receptors to suppress excitatory activity in a subset of SCN cells that project to the PVN (Perreau-Lenz et al., 2004). Thus, one possible explanation of these paradoxical results is that Y5 receptors are found on two different populations of SCN neurons: one that mediates the suppression of melatonin by light and another that mediates the effects of light on phase shifts.
Acknowledgements
We would like to thank Ryan Sweeney, Muriel Akpa, Deb Smith, Stephanie Peglow, Nicole M. Bullock and Jacopo Aguzzi for technical assistance. The current affiliations for K.L.G. and K.N.P. are Department of Biological Sciences, Vanderbilt University, Nashville, TN (K.L.G.) and Center for Sleep and Circadian Biology, Northwestern University, Evanston, IL (K.N.P.). This work was supported by NIH grants MH67420 (K.L.G.), MH58789 (H.E.A.), NS043459 (G.T.), NS41857 (K.P.) and NSF IBN9876754 (H.E.A.).
Abbreviations
-
- CT
-
- circadian time
-
- IGL
-
- intergeniculate leaflet
-
- GHT
-
- geniculate hypothalamic tract
-
- NMDA
-
- N-methyl-d-aspartate
-
- NPY
-
- neuropeptide Y
-
- ODU
-
- optical density units
-
- PVN
-
- paraventricular nucleus of the hypothalamus
-
- Per1
-
- Period1
-
- RHT
-
- retinohypothalamic tract
-
- SCN
-
- suprachiasmatic nucleus
-
- SSC
-
- standard sodium citrate
-
- TTX
-
- tetrodotoxin




