Cannabinoids modulate spontaneous neuronal activity and evoked inhibition of locus coeruleus noradrenergic neurons
Abstract
The noradrenergic pathway arising from the locus coeruleus (LC) is involved in the regulation of attention, arousal, cognitive processes and sleep. These physiological activities are affected by Cannabis exposure − both in humans and laboratory animals. In addition, exogenous cannabinoids, as well as pharmacological and genetic manipulation of the endocannabinoid system, are known to influence emotional states (e.g. anxiety) for which a contributory role of the LC-noradrenergic system has long been postulated. However, whether cannabinoid administration would affect the LC neuronal activity in vivo is still unknown. To this end, single-unit extracellular recordings were performed from LC noradrenergic cells in anaesthetized rats. Intravenous injection of both the synthetic cannabinoid agonist, WIN55212-2, and the main psychoactive principle of Cannabis, Δ9-tetrahydrocannabinol, dose-dependently increased the firing rate of LC noradrenergic neurons, with WIN55212-2 being the most efficacious. Similar results were obtained by the administration of these drugs into a lateral ventricle. Cannabinoid-induced stimulation of LC noradrenergic neuronal activity was counteracted by SR141716A, a cannabinoid receptor antagonist/reverse agonist, which by itself slightly reduced LC discharge rate. Moreover, WIN55212-2 suppressed the inhibition of noradrenergic cells produced by stimulation of the major γ-aminobutyric acid (GABA)ergic afferent to the LC, the nucleus prepositus hypoglossi. Altogether, these findings suggest the involvement of noradrenergic pathways in some consequences of Cannabis intake (e.g. cognitive and attention deficits, anxiety reactions), as well as a role for cannabinoid receptors in basic brain activities sustaining arousal and emotional states.
Introduction
The majority of noradrenaline (NA)-containing neurons of the CNS are concentrated in the pontine nucleus locus coeruleus (LC) (Dahlström & Fuxe, 1964). By means of a widespread efferent network, the LC provides NA throughout the entire brain and, noteworthy, represents the only source of NA to the hippocampus and cerebral cortex (reviewed in Berridge & Waterhouse, 2003). Because NA neurons are active specifically during waking, and activation of the noradrenergic pathway stimulates upper brain structures (Berridge & Foote, 1991), the projection from the LC has long been considered an arousal-related system. Moreover, the gating and tuning actions of NA at postsynaptic targets highly suggest a primary role for the LC in attention, learning and memory (Aston-Jones et al., 1991; Aston-Jones, 2002; Berridge & Waterhouse, 2003; Bouret & Sara, 2004). Conversely, a dysregulation of the LC-noradrenergic system is thought to contribute to cognitive, emotional and attentive dysfunctions associated with a variety of neuropsychiatric illnesses (reviewed in Berridge & Waterhouse, 2003).
Impairment of attention and cognitive functions, and influences in affective (e.g. anxiety) and arousal states, are well-documented consequences of Cannabis intoxication both in humans and animals (Adams & Martin, 1996; reviewed in Hall & Solowij, 1998; reviewed in Chaperon & Thiébot, 1999; Martin et al., 2002; D'Souza et al., 2004; reviewed in Viveros et al., 2005). All these effects might involve alterations in central noradrenergic neurotransmission mediated via activation of specific cannabinoid receptors (Howlett, 2002). Consistent with this ‘noradrenergic hypothesis’, it has been shown that cannabinoid agonists, as well as antagonists, modulate extracellular NA levels in brain structures relevant for cognition and emotions (frontal cortex, hippocampus, hypothalamus), even though biphasic, species- and/or strain-specific experimental conditions (e.g. in vitro vs. in vivo), and stress-dependent actions have been reported (Poddar & Dewey, 1980; Jentsch et al., 1997; Schlicker et al., 1997; Kathmann et al., 1999; Trendelenburg et al., 2000; reviewed in Schlicker & Kathmann, 2001; Tzavara et al., 2001, 2003; Moranta et al., 2004; Oropeza et al., 2005).
Although the influence of cannabinoids on the release properties of noradrenergic terminals has been widely explored, little is known about their functional interactions with NA neurons in the LC area. Recently, evidence has emerged that systemic cannabinoid administration induces robust Fos expression in the rat LC (Patel & Hillard, 2003; Oropeza et al., 2005), where endogenous and exogenous cannabinoids modulate N-methyl-d-aspartate-induced excitation of NA neurons (Mendiguren & Pineda, 2004), whose spontaneous firing rate is increased by blockade of the endocannabinoid anandamide metabolism (Gobbi et al., 2005).
Ergo, we sought to verify whether cannabinoid compounds affect neuronal activity of LC NA cells in vivo. In addition, based on the prominent role of cannabinoids in modulating γ-aminobutyric acid (GABA) neurotransmission (see Freund et al., 2003 for a review), we also investigated cannabinoid influence on the inhibition of LC evoked by stimulation of the nucleus prepositus hypoglossi (PrH), which is both a major source of GABA inputs to the LC, and a significant station in the control of sleep and visual attention processing (see Aston-Jones et al., 1991; Kaur et al., 2001; reviewed in Berridge & Waterhouse, 2003).
Materials and methods
Animals and surgery
Male Sprague–Dawley albino rats (Harlan Nossan, Milano, Italy) weighing 250–300 g were used in all experiments. Subjects were kept on a 12 : 12 h light : dark cycle with food and water available ad libitum. Experimental protocols were approved by the Ethical Committee at the University of Cagliari and performed in strict accordance with the care and use of animals approved by the American Physiological Society and EEC Council Directive of 24 November 1986 (86/609). Rats were anaesthetized with chloral hydrate (400 mg/kg i.p.), and the femoral vein was cannulated for i.v. administration of pharmacological agents and supplemental doses of the anaesthetic. Rats were mounted in a conventional stereotaxic apparatus (Kopf, Tujunga, CA, USA), the skull surface was exposed and a burr hole was drilled over the LC (3.5–4.0 mm posterior to lambda, 1.2–1.5 mm lateral to the midline, 5.5–6.5 mm ventral to the cerebellar cortex) for the insertion of a recording electrode at posteroanterior angle of 15 ° (Faiers & Mogenson, 1976). For orthodromic stimulation experiments, a Formvar-coated stainless steel bipolar electrode (250 µm tip diameter) was inserted, from the contralateral hemisphere, in the ipsilateral PrH (3.0–3.5 mm posterior to lambda, 1.5 mm lateral to the midline, 6.0–6.5 mm ventral to the cerebellar cortex), at a lateromedial angle of 16 °. For intracerebroventricular (i.c.v.) drug administration, a guide cannula (23-gauge stainless steel) was placed into the ventricle homolateral to the recording side (1.0 mm posterior, 1.4 mm lateral to bregma and 4.0 mm ventral to the cortical surface). Structures were localized according to the stereotaxic atlas of Paxinos & Watson (1997). I.c.v. injections were made through a prefilled inner cannula (30-gauge stainless steel tubing) connected to a 50-µL Hamilton microsyringe and extending 1.0 mm below the tip of the guide into the ventricle. Infusion rate was set at 2.5 µL/min by an electrically driven mini-pump.
Electrophysiological experiments
Single-unit activity of LC NA cells was recorded extracellularly by glass micropipettes filled with 2% Pontamine sky blue dye dissolved in 0.5 m sodium acetate (impedance 4–6 MΟhm). NA neurons were identified according to well-established electrophysiological characteristics (Cedarbaum & Aghajanian, 1977; Jodo et al., 1998). These included: (i) the presence just lateral to the LC of the mesencephalic nucleus of the V nerve, whose cells were activated by proprioceptive stimulation of the face (jaw stretch); (ii) a broad (3–4 ms in duration), often notched, biphasic waveform; (iii) slow spontaneous discharge (0.5–3.0 Hz); (iv) a typical response to noxious stimuli such as contralateral foot or tail pinch by an increase in activity followed by a quiescent interval; and (v) the inhibition by the α2-adrenoceptor agonist clonidine and subsequent reversal by the α2-adrenoceptor antagonist idazoxan.
The extracellular neuronal signal was filtered (bandpass 500–5000 Hz) and amplified (Neurolog System, Digitimer, UK), displayed on a digital storage oscilloscope (Tektronix, TDS 3012) and recorded on tape. Experiments were sampled on- and off-line by a computer connected to CED Power 1401 laboratory interface (Cambridge Electronic Design, Cambridge, UK). The spontaneous firing rate was recorded for 5 min to establish a baseline measure of firing rate. Drugs were then administered either i.v. (1 mL/kg of body weight), at exponentially increasing doses at 120-s intervals, or i.c.v. (10 µL/rat). Changes in firing rate were calculated by averaging the effects of the drugs for the 2-min period following drug administration and comparing them with the mean of the pre-drug baseline. Only one cell/rat was recorded.
For PrH stimulation experiments, the protocol previously described by Ennis & Aston-Jones (1989a,b) was applied. Briefly, after baseline firing rates were obtained for 5 min, responses to electrical stimulation of PrH were evaluated and a peri-stimulus time histogram (PSTH) was generated on-line (Spike 2 software, CED) for each neuron. PSTHs, typically generated at 1.5 times the threshold for apparent inhibition, were accumulated for 50 consecutive stimulus sessions (100-s sweeps; 8-ms bins) at 0.5 Hz. Stimuli were monophasic square wave pulses, 0.5 ms in duration, 0.5–1.0 mA in amplitude. Stimulus-evoked spiking probability was calculated off-line by dividing the number of spikes in the 35 consecutive bins after the stimulus (based on the mean duration of inhibition) by the total number of spikes in all 250 bins (2 s).
Drugs
Chloral hydrate (Carlo Erba, Milano, Italy), clonidine and idazoxan (Tocris Cookson, Bristol, UK) were freshly diluted in a saline solution. Δ9-tetrahydrocannabinol (Δ9-THC), in ethanol solution, was purchased from Research Biochemical International (Natick, MA, USA). The ethanol was evaporated immediately before use under argon and the residue, as were WIN55212-2 (RBI, Natick, MA, USA) and SR141716A (Sanofi-Aventis, Montpellier, France), dissolved in 1% Tween 80 and then diluted in a saline solution.
Histology
At the end of recording sessions, DC current (10 µA for 15 min) was passed through the recording micropipette in order to eject Pontamine sky blue for marking the recording site, and through the stimulating electrode (30 µA for 1 min) for lesioning the stimulation site. Brains were then rapidly removed and fixed in 8% paraformaldehyde solution. The position of the electrodes was microscopically identified on serial sections (60 µm) stained with Cresyl violet.
Statistical analysis
Statistical significance was evaluated by anova for repeated measures, followed by Dunnett's test as post hoc.
Results
Figure 1 shows placement of the recording (A) and stimulating (B) sites. Fifty-five rats had recording electrodes in the LC, primarily in the rostral part of the pons (at the level of the motor nucleus of the trigeminal nerve, see Fig. 1A, bottom panel). Of these, 12 rats also had stimulating electrodes properly placed in the medullary PrH (Fig. 1B, bottom panel). Action potential characteristics and discharge rate of LC NA single units corresponded to those previously found (Faiers & Mogenson, 1976; Cedarbaum & Aghajanian, 1977; Jodo et al., 1998). Action potentials were mostly biphasic and large in duration (3–4 ms), and often notched (see Fig. 2A for a representative example). LC NA cells displayed a mean firing rate of 1.36 ± 0.05 Hz (n = 55) and responded to contralateral paw and tail pinch with a brief burst of activity followed by a post-activation interval of quiescence (see Fig. 2B). Systemic administration of clonidine (0.0025–0.01 mg/kg, i.v., cumulative doses) (Fig. 2C), which binds to inhibitory α2-adrenergic autoreceptors, produced a marked depression of activity (Cedarbaum & Aghajanian, 1977). This inhibition was abruptly reversed by the subsequent injection of the α2-adrenergic antagonist idazoxan (0.3 mg/kg, i.v.) (Fig. 2C).
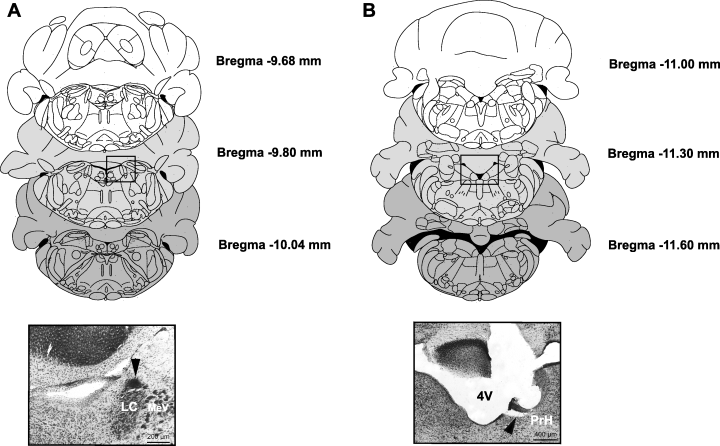
Schematic representation of the anatomical location of recording (see square in A) and stimulation (see square in B) areas. Brain sections correspond to the atlas of Paxinos & Watson (1997) and represent the boundaries used in the cranio-caudal axis. Distance from bregma is indicated. Insets: photomicrographs of coronal sections through the pons (A) and the rostral medulla (B) of an experimental rat brain corresponding to the squares in the above panels. The black spot (arrow on the A inset) marks the position of the recording electrode, while the arrow on the (B) inset shows the stimulation site. LC, locus coeruleus; MeV, mesencephalic nucleus of the V nerve. PrH, nucleus prepositus hypoglossi; 4V, fourth ventricle.
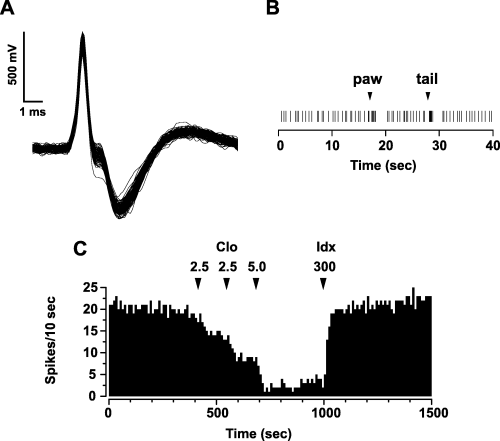
Electrophysiological and pharmacological identification of recorded neurons. (A) Superimposed traces, acquired from a digital storage oscilloscope, showing the spontaneous firing of an LC NA neuron recorded from a chloral hydrate-anaesthetized rat. Note the typical, broad, notched waveform. (B) NA cells responded to painful stimuli (contralateral paw and tail pinch) with a brief excitation followed by a prolonged period of post-activation inhibition. (C) Example of a pharmacological verification of the recording from an LC NA neuron. Note the dramatic decrease of cell activity after systemic administration of the α2-adrenoceptor agonist clonidine (Clo) and the abrupt reversal by the α2-adrenoceptor antagonist idazoxan (Idx). Numbers above arrows indicate dosages expressed in µg/kg i.v.
Systemic administration of cannabinoids stimulates LC noradrenergic activity
We first studied whether i.v. administration of two different cannabinoid type 1 (CB1) receptor agonists, WIN55212-2 and Δ9-THC, affects the LC NA neuronal activity in anaesthetized rats. Both systemic and intraventricular (see below) doses of cannabinoid compounds were chosen based on their relevance to behavioural, neurochemical and neurophysiological actions of cannabinoids (Jentsch et al., 1997; Diana et al., 1998; Manzanares et al., 1999; Pistis et al., 2001, 2002; Arguello & Jentsch, 2004; Moranta et al., 2004; Oropeza et al., 2005). Acute injection of WIN55212-2 (0.0625–1.0 mg/kg, i.v. cumulative doses; n = 12) dose-dependently increased the spontaneous firing rate of LC neurons (anova for repeated measures F6,132 = 55.45; P < 0.0001) (Fig. 3A and B). Maximal stimulation (142 ± 8.4%, n = 12) occurred at the cumulative dose of 1.0 mg/kg (Fig. 3A). Similarly, Δ9-THC (0.0625–1.0 mg/kg, i.v. cumulative doses; n = 9) elicited activation (anova for repeated measures F5,65 = 13.19; P < 0.0001), which reached statistical significance at doses higher than 0.0625 mg/kg (121 ± 3.3%, n = 9, at 1.0 mg/kg cumulative dose) (Fig. 3A and C). The cannabinoids' effects (both WIN55212-2 and Δ9-THC) of a significant increase in neuronal activity persisted throughout the recording time (anova for repeated measures: WIN55212-2 F4,44 = 9.85; P < 0.0001, n = 6; Δ9-THC F5,65 = 82.25; P <0.0001, n = 6) (Fig. 3D). The stimulant response induced by both cannabinoids was attenuated (WIN55212-2: three out of five cells tested; Δ9-THC: three out of three cells tested) by SR141716A (1.0 mg/kg i.v.; see Fig. 3C), a CB1 receptor antagonist (Rinaldi-Carmona et al., 1994), thus demonstrating the involvement of the activation of this receptor in the observed effects. Furthermore, to evaluate if LC NA neurons were under a tonic control by endocannabinoids, we administered SR141716A (0.625–1.0 mg/kg, i.v. cumulative doses; n = 6) alone in drug-naïve animals. As depicted in Fig. 3A, SR141716A by itself caused a slight, but significant, reduction of LC neuronal activity (anova for repeated measures F5,41 = 4.68; P < 0.05) (see also Fig. 3E). This last observation might be ascribed to the presence of an endogenous cannabinoid tone (see Gobbi et al., 2005) or to the reported inverse agonist properties of this compound (Rinaldi-Carmona et al., 1994; MacLennan et al., 1998; Pan et al., 1998; Pertwee, 2005). Importantly, after the injection of SR141716A, the subsequent administration of either WIN55212-2 or Δ9-THC failed to stimulate LC cells firing rate (see Fig. 3E and F).
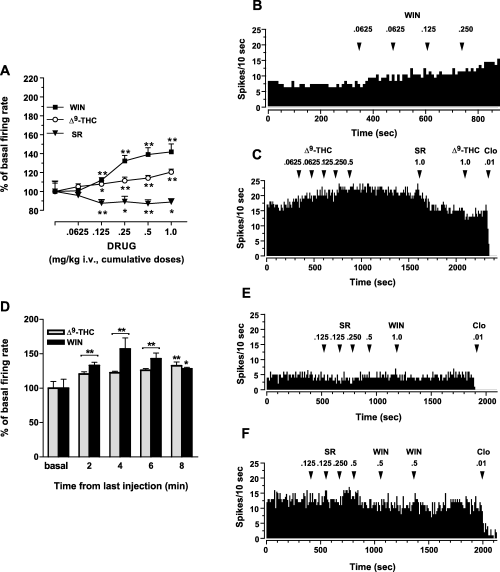
Systemic cannabinoids administration dose-dependently stimulates LC noradrenergic neuronal activity. (A) Intravenous injection of both the synthetic cannabinoid agonist, WIN55212-2 (WIN, closed square; n = 12), and the main psychoactive principle of Cannabis, Δ9-tetrahydrocannabinol (Δ9-THC; open circles; n = 9), dose-dependently increased the firing rate of LC noradrenergic neurons, with WIN being the most efficacious. Conversely, the CB1 cannabinoid receptor antagonist/inverse agonist SR141716A administered by itself (SR, closed triangles; n = 6) caused little reduction of the LC neuronal activity (see also E). All data are expressed as percentage of basal firing rate (mean ± SEM). *P < 0.05, **P < 0.01 with respect to pre-drug level (anova for repeated measures and Dunnett's test). (B and C) Representative rate histograms illustrating the increase in frequency of LC NA neurons after i.v. administration of cumulative doses of WIN and Δ9-THC. The CB1 antagonist SR attenuated cannabinoid-induced excitation (as shown in C). Note that Δ9-THC injection after SR was without effect, while the α2-adrenoceptor agonist clonidine (Clo) had a powerful inhibitory action on cannabinoid-enhanced LC neuronal activity. Arrows indicate the time of injection and numbers above arrows indicate dosages expressed in mg/kg i.v. (D) The bar graph shows the over time stimulation of LC cells discharge rate induced by both WIN and Δ9-THC. The cannabinoid effect was still significant 8 min after last dose administration. All data are expressed as percentage of basal firing rate (mean ± SEM). *P < 0.05, **P < 0.01 with respect to pre-drug level (anova for repeated measures and Dunnett's test). (E and F) Illustrative rate histograms showing the mild inhibitory response of LC NA cells to the cannabinoid antagonist SR administered in drug-naïve animals. As depicted in (E), the injection of SR alone was followed by a slight, but significant, depression of LC neuronal activity (see also A). Notably, when injected after SR, the cannabinoid agonist WIN did not modify the spontaneous LC discharge rate. On completion of the experiment, in order to verify the pharmacological profile of recorded neurons, the α2-adrenoceptor agonist Clo was administered. Arrows indicate the time of injection and numbers above the arrows indicate dosages expressed in mg/kg i.v.
The effects of systemic administration of WIN55212-2 and Δ9-THC are mimicked by central application of the drugs
Though the first group of experiments clearly showed modulation of LC NA neuronal activity as a result of systemically administered cannabinoid agonists, the data provided no indication whether WIN55212-2 or Δ9-THC were acting through a central site of action, or not. Therefore, we also applied these compounds intraventricularly.
The injection of WIN55212-2 (10 and 25 µg/10 µL/rat) into the ventricle ipsilateral to the recording side caused a clear-cut increase in LC NA neuronal activity (anova for repeated measures, F6,27 = 16.65 and F6,48 = 14.45, respectively, P < 0.0001) (Fig. 4A and B). The effect was dose-dependent, the maximal stimulation being 141 ± 8% (at 10 min, n = 4) and 184 ± 18% (at 8 min, n = 7) after 10 and 25 µg/rat of WIN55212-2, respectively (Fig. 4A).

Central injection of cannabinoids increases LC NA neurons firing frequency. (A) Time-course of the dose-dependent stimulating effect produced by i.c.v. administration (at arrow) of 10 (open squares) and 25 (closed squares) µg/10 µL WIN on LC NA neurons discharge rate. Δ9-tetrahydrocannabinol (Δ9-THC; 25 µg/10 µL i.c.v.; closed circles) induced a milder excitation of LC NA activity, while i.c.v. infusion of vehicle (10 µL 1% Tween in saline; open triangles) did not lead to a statistically significant effect. All data are expressed as percentage of basal firing rate (mean ± SEM). *P < 0.05, **P < 0.01 with respect to pre-drug level, anova for repeated measures and Dunnett's test. (B) Example of the activation induced by WIN (10 µg/10 µL/rat, i.c.v.) on an LC neuron. The stimulatory response was reversed by the i.v. administration of the cannabinoid antagonist SR. The subsequent injection of the α2-adrenoceptor agonist clonidine (Clo) strongly inhibited NA cell firing. (C) A representative firing rate histogram displaying the lack of effect of vehicle (10 µL 1% Tween in saline, i.c.v.) on the discharge activity of an LC NA cell. Numbers above arrows indicate dosages expressed in mg/kg i.v., except where specified.
Systemic injection of SR141716A (1.0 mg/kg, i.v.) blunted cannabinoid-induced stimulation in all neurons tested (see Fig. 4B). Not surprisingly, Δ9-THC (25 µg/10 µL/rat) applied intraventricularly produced a weaker, but statistically significant, enhancement of LC neuronal activity (anova for repeated measures F6,34 = 10.68; P < 0.0001) (Fig. 4A), which reached a maximum 10 min after injection (129 ± 1.3%, n = 5). The infusion of vehicle-control (1% Tween 80 in saline solution, 10 µL/rat, n = 4) into the lateral ventricle did not significantly affect LC NA neurons firing rate (anova for repeated measures F7,16 = 1.42; P = 0.26) (Fig. 4A and C).
WIN55212-2 blocks LC inhibition from the PrH
Anatomical and electrophysiological evidence demonstrates that the core of the LC receives its main afferent projections from the medullary nuclei PrH and paragigantocellularis (see for a review Ennis et al., 1998). While the innervation from nucleus paragigantocellularis is predominantly excitatory (Ennis & Aston-Jones, 1988), the input arising from PrH is mostly inhibitory (Ennis & Aston-Jones, 1989a,b). Inhibition of LC neurons following PrH stimulation is mediated by GABA acting through GABAA receptors (Ennis & Aston-Jones, 1989b), and implicated in the modulation of rapid eye movement sleep (Kaur et al., 2001), attention to external stimuli and orienting behaviours (Aston-Jones et al., 1991; see Berridge & Waterhouse, 2003).
In agreement with previous reports (Ennis & Aston-Jones, 1989a,b), single-pulse electrical stimulation (0.5 Hz) of PrH determined a current-dependent (mean current intensity of 0.89 ± 0.07 mA, n = 10), short-latency and temporary inhibition of LC neuronal activity (mean onset latency of 8.00 ± 2.53 ms, mean duration of 284.80 ± 44.02 ms, n = 11) (Fig. 5A and B). Therefore, the aim of our third series of experiments was to determine if cannabinoid receptor activation influenced GABA neurotransmission between the PrH and the LC. Only LC neurons inhibited by PrH stimulation were selected. As shown in Fig. 5A, WIN55212-2 (0.5 mg/kg, i.v.) fully suppressed the inhibition of LC evoked by stimulation of PrH in five out of seven cells tested. The cannabinoid effect was readily eliminated by SR141716A (1.0 mg/kg, i.v.), thus restoring PrH-induced suppression of LC neurons activity (Fig. 5A). Moreover, in order to test for an endocannabinoid tonic control of this neural pathway, we administered the CB1 antagonist by itself. SR141716A did not affect LC inhibition from the PrH (n = 4) (Fig. 5B), but completely prevented the action of WIN55212-2 (1.0 mg/kg, i.v. Fig. 5B). The effect of cannabinoids was also evaluated in terms of changes of PrH-evoked spike probability of LC cells. Indeed, modifications in spike probability are an index of changes induced by the tested compounds over the inhibition of LC neurons evoked by PrH stimulation (see Materials and methods). WIN55212-2 (0.5 mg/kg, i.v.) produced a significant increase in PrH-evoked spiking probability of LC cells (from a baseline value of 0.09 ± 0.02 to a post-drug value of 0.18 ± 0.03 at 200 s, n = 5, anova for repeated measures, F2,14 = 7.40; P < 0.02) (Fig. 5C), whereas SR141716A (1.0 mg/kg, i.v.) administered alone was ineffective (from a baseline value of 0.09 ± 0.02 to a post-drug value of 0.09 ± 0.01 at 200 s, n = 4, anova for repeated measures, P > 0.05) (Fig. 5D). Nevertheless, this compound fully blocked the effect of a subsequent administration of WIN55212-2 (1.0 mg/kg, i.v.) on PrH-evoked spike probability of LC cells (Fig. 5D).
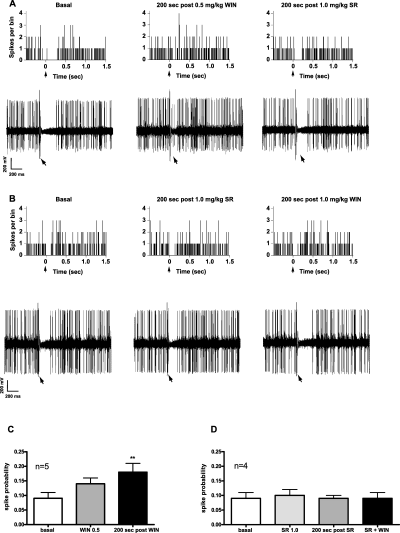
WIN suppresses PrH-evoked inhibition of LC NA neurons. (A) PSTHs (top panels) and representative digital storage oscilloscope traces (bottom panels) showing inhibitory responses elicited in LC neurons by PrH stimulation (delivered at time 0, arrow). The administration of 0.5 mg/kg i.v. WIN blocked the PrH-evoked suppression of the LC NA cells' activity. The effect was fully reversed by the cannabinoid antagonist SR. (B) SR by itself did not influence the PrH-evoked inhibition of LC NA neurons, but it was effective in preventing the action of 1.0 mg/kg WIN. Illustrative PSTHs (top panels) and digital storage oscilloscope traces (bottom panels) are shown. (C and D) WIN increased spike probability of LC NA neurons after PrH stimulation (C), whereas the same parameter was not altered by the injection of SR in drug-naïve animals (D). This compound, however, blocked the effect of a subsequent administration of WIN (D). **P < 0.01 with respect to baseline level, anova for repeated measures and Dunnett's test. PSTH bin 8 ms, 50 sweeps.
Discussion
The present study shows that both systemic and central administration of cannabinoid compounds enhances the spontaneous activity of LC NA cells in vivo. Moreover, it demonstrates that the cannabinoid agonist WIN55212-2 also suppresses the inhibition of NA neurons evoked by stimulation of the PrH. These results provide the first evidence that cannabinoids, exogenously administered, act as positive neuromodulators of LC NA neurons in vivo, adding strength to the hypothesis that central noradrenergic circuits may be implicated in mediating some psychotropic and emotional effects of Cannabis derivatives (see Jentsch et al., 1997; Tzavara et al., 2003; Oropeza et al., 2005).
Several findings corroborate the idea that cannabinoids affect noradrenergic neurotransmission via activation of cannabinoid CB1 receptors. First, two structurally unrelated cannabimimetics shared a stimulating, dose-dependent effect on LC noradrenergic neuronal activity. The observed differences in efficacy are consistent with their affinity for cannabinoid-binding sites (Wiley et al., 1998) and with Δ9-THC being a partial agonist of CB1 receptors (see Howlett, 2002). Second, the stimulation of LC NA cells by the cannabinoid agonists was both attenuated and prevented by SR141716A. The CB1 receptor antagonist, when administered alone in drug-naïve animals, elicited a slight but significant decrease in LC neuronal activity, suggesting the presence of a tonically active endocannabinoid input upon LC NA neurons. Importantly, this possibility is further supported by the latest evidence showing that URB597, a selective inhibitor of the endocannabinoid anandamide degradation (Kathuria et al., 2003), is able to enhance the spontaneous firing rate of LC NA cells in a SR141617A-sensitive manner (Gobbi et al., 2005).
Our findings are also in line with recently reported observations that systemic administration of different cannabinoid agonists induces the expression of FOS protein within the rat LC through activation of CB1 receptors (Patel & Hillard, 2003; Oropeza et al., 2005). In fact, accumulation of FOS protein is augmented as a result of somatodendritic depolarization, thus representing a reliable index of neuronal activation (Morgan & Curran, 1986). Additionally, Δ9-THC and WIN55212-2 have been shown to acutely increase NA turnover in the rat prefrontal cortex and nucleus accumbens ex vivo (Jentsch et al., 1997), and NA release in the rat frontal cortex in vivo (Oropeza et al., 2005; P. Devoto & A.L. Muntoni, unpublished observations), respectively, the latter effect being prevented by the cannabinoid receptor antagonist SR141716A. LC neurons fire spontaneously at low, tonic discharge rates that are linearly correlated with NA release in target areas (Florin-Lechner et al., 1996; Berridge & Abercrombie, 1999). Therefore, our results may provide an impulse-dependent mechanism for the above-mentioned augmentation of NA efflux, as well as for the CB1-mediated enhancement of NA synthesis in the rat LC, hippocampus, cerebral cortex, hypothalamus and cerebellum, as recently reported by Moranta et al. (2004).
To exclude the contribution of potential systemic effects induced by the i.v. cannabinoid administration, we applied WIN55212-2 and Δ9-THC into the ventricle ipsilateral to the recording site. The synthetic cannabinoid agonist produced a robust, dose-dependent excitation of LC NA cells, promptly reversed by the cannabinoid antagonist SR141716A. Also, Δ9-THC induced a significant stimulation of the LC cells firing rate, consistent with the idea that cerebral CB1 receptors were involved in the observed effects. However, because of systemic and central administration, we cannot exclude the possibility that the drugs might act within the forebrain to indirectly activate the LC. Therefore, determining the effect of local administration of cannabinoids on LC cells firing rate in the intact animal, in concert with investigations of their actions on inhibitory and excitatory synaptic transmission in the LC, remain our important future goals. In fact, cannabinoid-induced stimulation of LC NA neurons spontaneous activity might be the consequence of multiple mechanisms, including direct excitation of the neurons by activation of postsynaptic CB1 receptors, neuronal disinhibition due to a reduction of inhibitory control of the cell and/or modulation of excitatory inputs. The fact that CB1 receptor protein (Herkenham et al., 1991) and mRNA (Matsuda, 1993) levels within the rat pons have been found difficult to detect or very low, led us to investigate a possible presynaptic neuromodulatory role for cannabinoids in the LC area. Indeed, the inhibitory function of presynaptic cannabinoid receptors on neurotransmitters' release at many types of synapses is well known (see for a review Schlicker & Kathmann, 2001). In particular, physiological effects of cannabimimetics are often mediated by reduction of GABAergic transmission in the CNS and, congruently, CB1 receptors are found in large amounts on central GABAergic terminals (see Freund et al., 2003 for a review). Cannabinoids have been found to presynaptically inhibit GABA release in brain structures such as striatum (Szabo et al., 1998), rostral ventromedial medulla (Vaughan et al., 1999), periaqueductal grey (Vaughan et al., 2000), hippocampus (Katona et al., 1999; Hájos et al., 2000; Hoffman & Lupica, 2000; Hájos & Freund, 2002), cerebellum (Takahashi & Linden, 2000), nucleus accumbens (Hoffman & Lupica, 2001; Manzoni & Bockaert, 2001), substantia nigra pars reticulata (Chan et al., 1998) and ventral tegmental area (Szabo et al., 2002).
In keeping with these observations, we found that WIN55212-2 abolished the suppression of LC NA neurons' activity induced by electrical stimulation of the PrH in the rostral dorsomedial medulla, the site of origin of the major GABAergic afferent to the LC. The WIN55212-2 ‘suppression of inhibition’ here described was probably mediated through the cannabinoid CB1 receptor as it was both reversed and prevented by the CB1 cannabinoid antagonist SR141716A, which was ineffective when administered alone. PrH neurons, which are spontaneously active in the anaesthetized animals (1–30 Hz) (Ennis & Aston-Jones, 1989a; reviewed in Aston-Jones et al., 1991), are a predominant source of GABA in the LC (see Kaur et al., 2001). Consistently, local application of the GABAA antagonist bicuculline increases the mean spontaneous discharge rate of LC neurons and NA release, suggesting a tonic GABAergic input in vivo (Ennis & Aston-Jones, 1989b; Gervasoni et al., 1998; Kawahara et al., 1999). Thus, it could be suggested that activation of inhibitory cannabinoid receptors located on GABAergic terminals would plausibly lead to a disinhibition of LC NA neuronal activity by a reduction in GABA outflow. If so, our results on GABA-mediated inhibition from PrH would be fully consistent with this hypothesis.
What are the possible behavioural and pathophysiological implications of the present findings? Regardless of the mechanisms involved, the observed stimulation of LC NA neurons may have important consequences because their axons are highly branched and have extensive projections throughout the CNS (see Aston-Jones, 2002 for a review). In particular, as already mentioned, the LC represents the only NA source for brain regions, such as cortex and hippocampus, pivotally involved in higher cerebral functions (reviewed in Berridge & Waterhouse, 2003). Thus, the relevance of the present results is notable because, when taken together with reports showing an increase in rat prefrontal cortical NA outflow following acute cannabinoid exposure (Jentsch et al., 1997; Oropeza et al., 2005; P. Devoto & A.L. Muntoni, unpublished observations), it suggests that stimulation of the LC-noradrenergic system may play a role in the cognitive, affective and attention impairments associated with Cannabis intake in humans (see Hall & Solowij, 1998). Indeed, even small changes in NA levels in the prefrontal cortex profoundly affect cognitive functions (Bouret & Sara, 2004), and inappropriate or long-lasting LC activation can result in hyper-arousal, attentional dysfunction, and disruption of behaviours requiring focused attention (Aston-Jones, 2002) or related to decision processes (see Clayton et al., 2004; Aston-Jones & Cohen, 2005). In addition, modulation of the PrH-LC circuit by cannabinoids might help to understand how these substances affect sleep patterns (Pivik et al., 1972; Feinberg et al., 1975, 1976), arousal states and visuospatial attention processes (Santucci et al., 1996; Ehrenreich et al., 1999; Ploner et al., 2002; Arguello & Jentsch, 2004; D'Souza et al., 2004; Verrico et al., 2004). Indeed, beside regulating rapid eye movement sleep (see Aston-Jones et al., 1991; Kaur et al., 2001), PrH neuronal activity is thought to trigger ‘shift to new targets in the visual fields or relay an arousal or alarm signal to forebrain structures via the LC’ (Berridge & Waterhouse, 2003).
In light of the present and previous results (Jentsch et al., 1997; Gobbi et al., 2005; Oropeza et al., 2005), it is also reasonable to postulate that the LC-noradrenergic system might contribute to modulation of emotional behaviour by cannabinoids (see Hall & Solowij, 1998; Martin et al., 2002; Kathuria et al., 2003; Viveros et al., 2005). Indeed, the LC represents a crucial site for integrating corticotropin-releasing factor and noradrenergic mediation of stress and anxiety responses (Melia & Duman, 1991; Valentino et al., 1993; Jedema & Grace, 2004), it shows reciprocal functional relationships with anxiety and stress-related circuits (e.g. prefrontal cortex, amygdaloid complex; see Jodo et al., 1998; Bouret et al., 2003) targeted by cannabinoids, and it has been implicated in the pathophysiology of mood and anxiety disorders (see Berridge & Waterhouse, 2003).
In summary, we have provided a mechanism for how acutely administered cannabinoids, by increasing the spontaneous activity of LC NA neurons and suppressing their evoked inhibition from the medulla, can regulate NA outflow in forebrain regions. In particular, these effects may provide a rationale for the already reported augment in NA levels in the prefrontal cortex (Jentsch et al., 1997; Oropeza et al., 2005), and be considered as contributing factors to some psychotropic and affective consequences of acute Cannabis intake. Additional investigations of anatomical and chemical substrates underlying cannabinoid stimulation of LC NA neurons may prove beneficial in clarifying the role of cannabinoid receptors in basic brain activities sustaining attentional functions, arousal and emotional processes.
Acknowledgements
This work was partially supported by C.N.R. grant CNRG00722A (Young Investigator Programme 2000, Marco Pistis). We gratefully acknowledge Sanofi-Aventis for the generous gift of SR141716A, Marco Diana for discussions and William T. Dunn III for proof-reading this manuscript.
Abbreviations
-
- Δ9-THC
-
- Δ9-tetrahydrocannabinol
-
- CB1
-
- cannabinoid type 1 receptor
-
- GABA
-
- γ-aminobutyric acid
-
- LC
-
- locus coeruleus
-
- NA
-
- noradrenaline
-
- PrH
-
- nucleus prepositus hypoglossi
-
- PSTH
-
- peri-stimulus time histogram




