Central sprouting of uninjured small fiber afferents in the adult rat spinal cord following spinal nerve ligation
Abstract
Partial nerve injury results in chronic pain that is difficult to treat effectively. To investigate the anatomic basis of this phenomenon we used wheat germ agglutinin–horseradish peroxidase (WGA-HRP) to label the central projections of uninjured small fibers (Aδ and C) in a well-established model of neuropathic pain created by selective spinal nerve ligation in the adult. We found extensive sprouting of uninjured WGA-HRP-labeled afferents into the central termination field in lamina II of dorsal horn normally occupied by L5 afferents whose peripheral axons had been ligated distal to the dorsal root ganglion. The formation of new projections by uninjured fibers into a functionally but not anatomically deafferented field in the adult may play a role in the development of chronic pain.
Abbreviations
-
- DAB
-
- diaminobenzidine
-
- DRG
-
- dorsal root ganglia
-
- PBS
-
- phosphate-buffered saline
-
- WGA-HRP
-
- wheat germ agglutinin–horseradish peroxidase
Introduction
It is generally accepted that intact adult primary sensory neurons in the dorsal root ganglion (DRG) have limited ability to reorganize central projections in the spinal cord (McMahon, 1992). Each nerve has a restricted projection that creates a rostrocaudal somatotopic map of A (myelinated) and C (unmyelinated) fibers in the dorsal horn (Swett & Woolf, 1985; Molander & Grant, 1986; Woolf & Fitzgerald, 1986; LaMotte et al., 1991). In the neonate, after peripheral nerve section the central terminals of both A and C fibers from adjacent intact nerves may sprout into the denervated territory in dorsal horn normally occupied by the central projections of the axotomized fibers (Fitzgerald, 1985; Shortland & Fitzgerald, 1994), but a similar injury in the adult causes only limited superficial sprouting of A fibers in the dorsoventral plane (Woolf et al., 1992, 1995) without rostrocaudal expansion of the projection field (Doubell et al., 1997) unless the peripheral branch of the axon has been induced to regenerate by a prior ‘conditioning’ lesion (LaMotte et al., 1989; McMahon & Kett-White, 1991).
Chronic neuropathic pain is an important clinical problem that is difficult to treat effectively. Selective spinal nerve ligation (Kim & Chung, 1992) in which the spinal nerve is tightly ligated and/or cut at a point before it joins the common nerve provides a rodent model that recapitulates many of the essential features of neuropathic pain and has been widely used to study pathogenesis and treatment of this condition (Yaksh, 1999). Spinal nerve ligation results in ectopic electrical activity in the axotomized DRG (Han et al., 2000; Liu et al., 2000a,b) and in the non-injured DRG adjacent to the ligation whose peripheral axons share the same nerve (Ali et al., 1999; Li et al., 2000; Wu et al., 2001), both of which may contribute to the development of various pain phenomena in this condition (Gold, 2000). Substantial alterations have been defined in transcription (Fukuoka et al., 2002; Wang et al., 2002) and post-translational processing of proteins (Gold et al., 2003) in the non-injured DRG adjacent to the ligated DRG in this model.
We used transganglionic labeling by injection of a wheat germ agglutinin–horseradish peroxidase conjugate (WGA-HRP) into the sciatic nerve to define the central projections of the L4 and L6 DRG before and after selective L5 spinal nerve ligation. WGA-HRP labels small neurons (15–25 µm) in the DRG whose axons are unmyelinated afferents representing 84% of the labeled axons in the dorsal root to the spinal cord (LaMotte et al., 1991). A few myelinated afferents are also labeled by WGA-HRP; these fibers are also small diameter (0.5–2 µm), and their projections are restricted to laminas I–III in the superficial dorsal horn (LaMotte et al., 1991). We observed that L5 spinal nerve ligation results in a substantial rostrocaudal redistribution of unmyelinated projections from L4 and L6 DRG into the core of the L5 projection field not normally overlapped by projections from L4 or L6.
Materials and methods
Selective L5 spinal nerve ligation was performed in adult male Sprague–Dawley rats 200–250 g at the time of operation as previously described (Kim & Chung, 1992; Hao et al., 2003). Briefly, under chloral hydrate anesthesia the L5 spinal nerve was identified and carefully dissected free from the adjacent L4 spinal nerve, tightly ligated using 6-0 silk sutures, and the incision closed with wound clips. Sham controls were dissected in an identical manner but the L5 spinal nerve was not ligated. The experiment was conducted with five animals in each of four groups: (i) sham operated; (ii) spinal nerve ligation followed by 3-day survival; (iii) spinal nerve ligation followed by 7-day survival; (iv) spinal nerve ligation followed by 21-day survival. The entire experiment was repeated a second time (for the serial reconstruction) with an additional five animals per group for each of the four groups. A separate set of 16 animals were used for behavioral studies of mechanical allodynia and thermal hyperalgesia as described below. All of the studies were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Pittsburgh and conform to the guidelines of animal studies as described by the Society for Neuroscience.
Mechanical allodynia and thermal hyperalgesia
An additional eight animals received spinal nerve ligation performed as described above, and eight sham controls operated in a similar manner but without nerve ligation were evaluated separately for mechanical allodynia and thermal hyperalgesia. Tactile threshold was determined using calibrated von Frey filaments (0.4, 0.7, 1.2, 1.5, 2.0, 3.6, 5.5, 8.5, 11.8 and 15.1 g) presented serially to the hind paw in ascending order of strength; and mechanical threshold determined according to the method described (Chaplan et al., 1994), with a tactile stimulus producing a 50% likelihood of withdrawal determined by using the up–down method (Dixon, 1980). A positive response was defined as a rapid withdrawal and/or licking of the paw immediately upon application of the stimulus. Whenever a positive response occurred the next finer von Frey hair was applied, and whenever a negative response occurred the next higher force was applied. In the absence of a response to pressure of 15.1 g animals were assigned to this cutoff value. Thermal nociceptive thresholds were assessed using the Hargreaves method (Hargreaves et al., 1988). The animals were placed in a transparent express box with a thin glass floor and the withdrawal latency to focused beam of radiant heat applied to the plantar surface of the paw measured.
Spinal nociceptive projections were labeled by the uptake of WGA-HRP (Molander & Grant, 1985). Four microliters of WGA-HRP (Vector Laboratories, Burlingame, CA, USA) was injected into both sciatic nerves. Animals that were to be killed at 3 or 7 days after ligation were injected with WGA-HRP immediately after spinal nerve ligation; animals that were to be killed at 21 days after spinal nerve ligation and sham-operated animals were injected with WGA-HRP 14 days after the ligation or sham operation.
The distribution of WGA-HRP-labeled afferents in the dorsal horn was determined by immunocytochemical staining of transverse sections of lumbar spinal cord using a biotinylated primary antibody against WGA followed by a biotinylated secondary antibody amplified with an avidin–biotin–HRP complex and detected using diaminobenzidine (DAB, Vectastain Kit, Vector Laboratories). On 3, 7 or 21 days after surgery, the animals were perfused transcardially with ice-cold phosphate-buffered saline (PBS) followed by 4% paraformaldehyde. The spinal cord and L4 and L5 DRG were removed, kept in fixative for 2 h at room temperature, cryoprotected in 30% sucrose for 24–48 h at 4 °C, and embedded in OCT embedding medium. Forty-micrometer transverse sections cut on a cryostat (Leica, Germany) were washed four times for 5 min in PBS-T (PBS + 0.1% Tween 20), treated with 0.1% H2O2 in PBS for 15 min followed by washing four times for 5 min in PBS-T, and then placed in blocking buffer containing 2.5% normal serum and 0.1% Triton X-100 in PBS-T for 1 h at room temperature. Sections were incubated with biotinylated goat anti-WGA (1 : 500, Vector Laboratories) in antibody solution (PBS-T + 2.5% normal serum) overnight at 4 °C, rinsed four times for 10 min in PBS-T then incubated with biotinylated anti-goat IgG (1 : 500, Vector Laboratories) for 1 h at room temperature, rinsed four times for 10 min in PBS-T, then incubated with VECTASTAIN ABC Reagent for 2 h at room temperature and finally washed three times for 10 min with PBS-T at room temperature. Sections were incubated with DAB for 2–5 min at room temperature and the reaction stopped by the addition 0.1 m Tris buffer.
For 3D reconstruction of labeled structures in the spinal cord, the experiment was repeated with five animals per group, the L4–6 spinal cord segment identified by tracing the L4 and L6 dorsal roots back to the spinal cord, removed, kept in fixative for 2 h at room temperature, cryoprotected in 30% sucrose for 24–48 h at 4 °C and embedded in OCT. Fifty-micrometer cross-sections were cut on a cryostat. The distribution of stained nerve terminals in the dorsal horn appeared identical to the first experiment. For three animals at each time point every fourth section was collected in serial order (approximately 30 sections per animal), stained with DAB as described above, and a digital image of each section obtained with a Zeiss Axiophot using a 1.25 ×/0.035 lens and a PC-based imaging system (MCID, Imaging Resources, Brock, Ontario, Canada). The serial spinal cord images were normalized for differences in background densities using Adobe Photoshop, the images loaded into a stack and the image stack aligned using the M3D image reconstruction software (MCID, Imaging Resources). Stained structures in dorsal horn and motor neurons in the ventral horn were defined and a pseudocolor 3D reconstruction of the defined structures representing the motor neuron root and the labeled projection into dorsal horn created. The three reconstructed images were very similar, and a single representative reconstruction is presented.
In order to determine the amount of WGA-HRP transported to the L4 DRG following L5 spinal nerve ligation, the experiment was repeated and the bilateral L4 DRG collected from three animals at each time point killed 3, 7 and 21 days after L5 spinal nerve ligation; as a control the L4 DRGs were collected from three animals without injection of WGA-HRP. The individual L4 DRG were first homogenized by using a Teflon-glass homogenizer in 50 µL of lysis buffer containing 10 mm Tris buffer, 50 mm NaCl, 2.5 mm MgCl2 (TBS) and proteinase inhibitors (Cocktail Tablets, Roche, Mannheim, Germany). The DRG lysates were incubated on ice for 30 min and then centrifuged at 14000 g at 4 °C for 30 min to remove tissue debris. Two microlitres of supernatant from each sample containing equal amounts of protein was applied to a nitrocellulose membrane prepared according to the manufacturer's instructions. A serial dilution of peroxidase WGA from 6 pg/µL to 4 ng was used to create a standard curve (R2 = 0.9935). After blocking the membrane with 5% non-fat milk and washing with TBS the membrane was incubated with biotinylated goat anti-WGA solution (1 : 1000 in 2% non-fat milk, Vector Laboratories) at 4 °C overnight. After washing the membrane three times with TTBS buffer (1 × TBS with 0.1% Tween-20) the membrane was incubated with donkey anti-goat IgG-HRP (1 : 8500 in 2% non-fat milk, Santa Cruz Biotechnology) for 1.5 h at room temperature, washed three times for 10 min at room temperature, and developed in substrate solution (Super Signal Dura Extended, Pierce Biotechnology, Rockford, IL, USA) for 2 min at room temperature. The blot was exposed to BioMax film (Kodak, Rochester, NY, USA) for 90 s, and the amount of product quantified using a PC-based image analysis system (Imaging Resources), using the standard curve for quantification. To ensure that equivalent amounts of protein had been loaded for each sample, the membrane was subsequently stripped (Restore Western Blot Stripping Buffer, Pierce Biotechnology) for 3 min at 30 °C and, after washing with TTBS, reprobed with a mouse monoclonal anti-β actin antibody (Sigma).
Results
By 1 week after the operation the animals developed mechanical allodynia and thermal hyperalgesia that persisted unchanged for several weeks (Fig. 1). Sham-operated animals killed 7 days after nerve injection of WGA-HRP showed extensive labeling of the superficial laminae of dorsal horn on both sides of the spinal cord, consistent with uptake by unmyelinated and thin myelinated afferents that project to that region (Fig. 2) as previously described (Robertson & Grant, 1985; LaMotte et al., 1991). Motor neurons projecting into the nerve at that level were also strongly labeled with the retrograde tracer (Fig. 2). Spinal nerve ligation interrupts motor axons and the peripheral branch of the bipolar sensory axons from the DRG at that level. There was an absence of WGA-HRP-labeled afferents in the dorsal horn of spinal cord at the L5 level in animals that had been injected with WGA-HRP immediately after spinal nerve ligation and killed 3 days later (Fig. 2). The somatotopic level (L5) of spinal cord was identified by the absence of labeled motor neurons at that level resulting from the ligation of the motor component in the L5 spinal nerve. 3D reconstruction of labeled profiles from serial sections demonstrated the small area of small fiber overlap into the L5 distribution predominantly from the caudal projection of L4 afferents (Fig. 3).
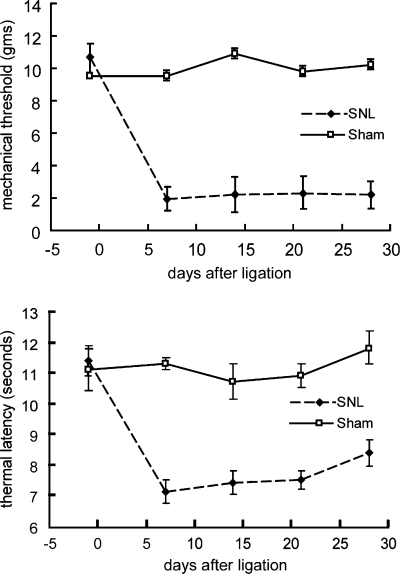
Mechanical allodynia (upper graph) and thermal hyperalgesia (lower graph) in animals after spinal nerve ligation.
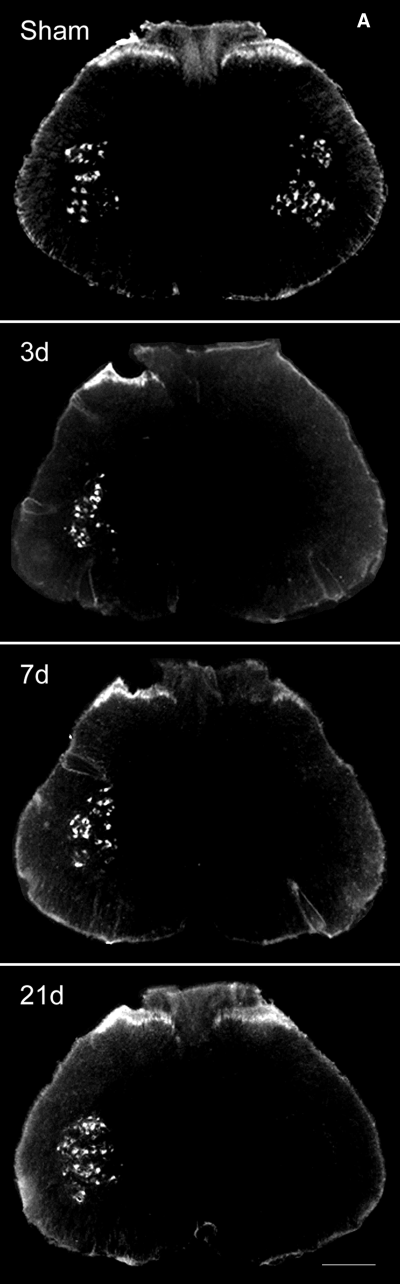
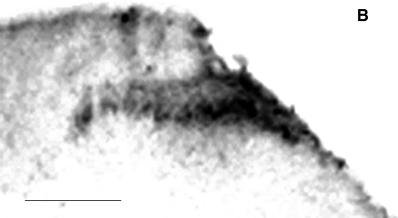
(A) Labeling of L5 spinal cord by sciatic nerve injection of WGA-HRP. The superficial laminas of dorsal horn and motor neuron on both sides show extensive labeling by WGA-HRP (sham). In animals killed 3 days after ligation (3d), no labeled motor neurons or labeled dorsal horn afferents are seen ipsilateral to the spinal nerve ligation. Animals killed 7 days after spinal nerve ligation (7d) show no labeled motor neuron at L5, but labeled sensory afferents in dorsal horn begin to appear. Animals killed 21 days after spinal nerve ligation (21d) and 7 days after WGA-HRP injection into nerve show extensive labeling of dorsal horn afferents in the previously unlabeled L5 distribution; motor neurons at the same level remain unlabeled by retrograde transport of the tracer. A total of 10 animals at each time point were examined, and the results were identical in all cases. Scale bar: 250 µm. (B) Higher power view of 21-day spinal cord. Scale bar, 250 µm.
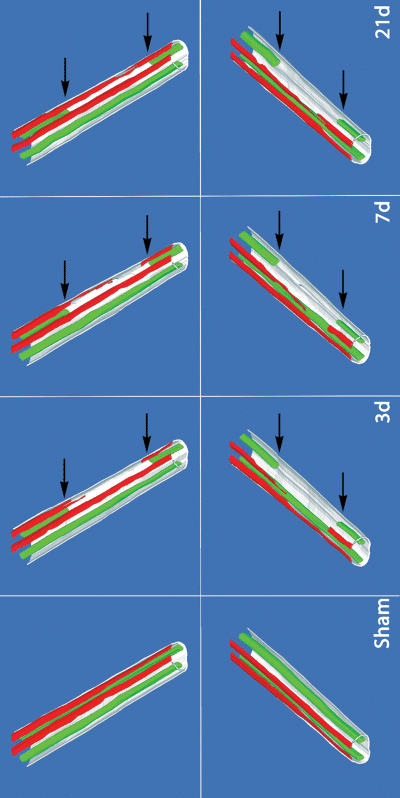
3D reconstruction of the WGA-HRP-labeled sensory afferents and labeled motor neurons in the spinal cord constructed from every fourth 50-µm section. The labeled projections into lamina 2 are shown in red, and the labeled motor neurons in green. The area of unlabeled motor neurons (indicated by the pair of arrows) defines the L5 segment of the lumbar spinal cord. The limited overlap of unmyelinated afferent projections is seen in animals killed 3 days after spinal nerve ligation (3d), as is the gradual extension of labeled terminals over the subsequent 3 weeks. 3D reconstructions (average of 30 sections per reconstruction) were performed on three animals at each time point. Representative reconstructions are shown.
Animals killed 7 days after spinal nerve ligation followed by WGA-HRP injection demonstrated extension of WGA-HRP-labeled afferents into the dorsal horn at the L5 spinal level (Fig. 2). The location of the sections at the L5 level was confirmed by the fact that the motor neurons in the same section were unlabeled (Fig. 2). The extent of ingrowth of labeled afferents into the L5 segment of spinal cord is more clearly delineated in the 3D reconstruction of serial sections (Fig. 3). By 21 days after spinal nerve ligation (WGA-HRP injected 7 days prior to death) there was extensive extension of labeled dorsal horn projections into the previously unlabeled L5 spinal nerve distribution (2, 3).
The appearance of labeled projections in the dorsal horn of the L5 spinal segment ipsilateral to the ligation did not result from transganglionic labeling through the ligated L5. While animals killed 21 days after sham operation and 7 days after WGA-HRP injection into sciatic nerve showed substantial labeling of predominantly small neurons in both L4 and L5 DRGs (Fig. 4), animals killed 21 days after spinal nerve ligation and 7 days after WGA-HRP injection into sciatic nerve showed no labeled cell bodies in L5 (Fig. 4). This confirms the completeness of the ligation and excludes the possibility that sprouting of peripheral projections of the ligated L5 spinal nerve across the ligation could be a source of the labeled afferents in the L5 segment of the dorsal horn.
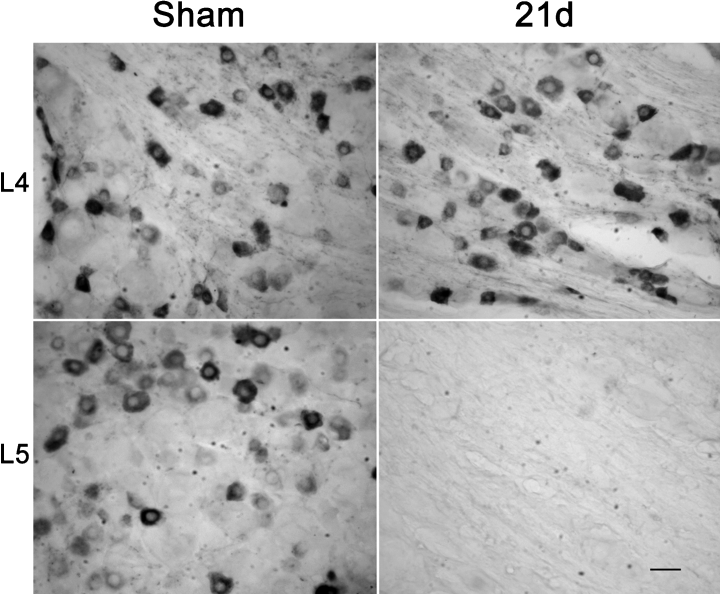
Twenty-one days after L5 spinal nerve ligation or sham operation (7 days after WGA-HRP retrograde labeling), small DRG neurons are extensively labeled in the sham-operated L4 and L5 DRG, and in the L4 DRG from L5 spinal nerve ligated animals. No WGA-HRP labeling is found in L5 DRG from animals that had undergone L5 spinal nerve ligation 14 days before WGA-HRP injection (lower right). Scale bar, 30 µm.
The uptake of WGA-HRP by peripheral axons of L4 and L6 DRG from the sciatic nerve injection was not altered by the ligation of the adjacent L5 spinal nerve. The amount of WGA-HRP in the L4 and L6 DRG at 3 days, 7 days and 21 days after ligation was the same ipsilateral to the L5 spinal nerve that had been ligated as it was in the contralateral DRG (Fig. 5).
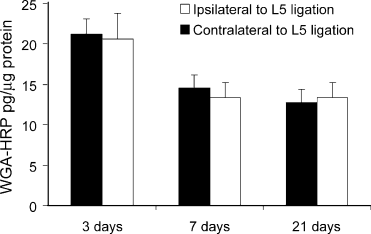
The amount of wheat germ agglutinin–horseradish peroxidase (WGA-HRP) in L4 DRG ipsilateral to L5 ligation (open bars) was no different than the amount in the contralateral L4 DRG (filled bars) in animals injected into bilateral sciatic nerves after L5 spinal nerve ligation. Animals killed at 7 and 21 days after spinal nerve ligation were injected with WGA-HRP 7 days prior to death; animals killed at 3 days were injected with WGA-HRP 3 days prior to death. The difference in amount of WGA-HRP (on both sides) between the 3-day and the 7- and 21-day time points reflects degradation of WGA-HRP. N = 3 animals per group.
Discussion
The central projections to the spinal cord of cutaneous primary afferents are topographically organized, generating somatotopic maps arranged in the rostrocaudal and mediolateral axis to represent the surface of the body (Swett & Woolf, 1985). Cutaneous afferents from the skin of the hindlimb to the substantia gelatinosa of the spinal cord project in a rostrocaudal somatotopic organization, with the most medial digit in caudal L3 and the most lateral digit in rostral L5 and the plantar skin projecting rostral and caudal to the projection of the digits (Molander & Grant, 1985; Swett & Woolf, 1985).
Previous studies utilizing selective peripheral nerve injury or chemical deafferentation and WGA-HRP labeling have failed to demonstrate any evidence of central sprouting of unmyelinated or small myelinated afferents (Molander et al., 1988; Pubols & Foglesong, 1988; LaMotte et al., 1989), unless the denervation was accompanied by a ‘conditioning’ peripheral nerve lesion (McMahon & Kett-White, 1991), but the results of the current study provide unambiguous evidence for sprouting of the central projections of undamaged small fibers into the region occupied by the central projection of fibers whose peripheral projections had been interrupted by spinal nerve ligation. Spinal nerve ligation is different than distal peripheral nerve section, and there are no previous published studies that looked for central sprouting after spinal nerve ligation.
Myelinated afferents to the spinal cord labeled with cholera toxin B-conjugated HRP (B-HRP) project to deeper laminae in the dorsal horn and appear to exhibit substantially greater overlap in the rostrocaudal distribution of adjacent levels than do the small myelinated and unmyelinated fibers labeled with WGA-HRP (Arvidsson & Pfaller, 1990; Rivero-Melian & Grant, 1990), and immunocytochemical studies after rhizotomy of one dorsal root indicate a substantial area of non-overlap in the distribution of those unmyelinated and small myelinated afferents (McNeill et al., 1991; Piehl et al., 1992).
A series of studies over the past 10 years have suggested that following nerve injury, sprouting of myelinated afferents from deeper laminae in dorsal horn to more superficial laminae might contribute to pain phenomenon resulting from nerve injury (Woolf et al., 1992, 1995), although more recent studies have opened the possibility that the apparent sprouting of myelinated afferents may result in part from changes in uptake of the tracer that result from nerve injury rather than alteration of projections (Tong et al., 1999; Bao et al., 2002; Hughes et al., 2003). Our study, which addresses only the distribution of small myelinated and unmyelinated projections labeled with WGA-HRP, does not provide any insight into the controversy regarding the possibility of sprouting of myelinated fibers, but we did carefully control for the confounding artifact of altered uptake by measuring the amount of WGA-HRP in the DRG, comparing those DRG adjacent to the L5 spinal nerve ligation with the contralateral DRG. We found no influence of adjacent spinal nerve injury on uptake of the WGA-HRP into the uninjured DRG on the same side.
What mechanisms underlie the sprouting of uninjured unmyelinated afferents into the central terminal field of the peripherally injured afferents? Although there is a delayed loss of DRG neurons following permanent peripheral axotomy, this cell loss progresses slowly over several months after the lesion (Coggeshall et al., 1997; Vestergaard et al., 1997; Tandrup et al., 2000). Electron microscopic studies of dorsal horn after peripheral nerve transection have demonstrated ultrastructural changes in the primary afferent endings in the substantia gelatinosa (Knyihar-Csillik et al., 1987; Castro-Lopes et al., 1990), but no evidence of degenerating fibers. Quantitative stereological assessment of DRG after spinal nerve ligation has not been reported. While it is logical to presume that Wallerian degeneration of afferents might be a stimulus for sprouting of uninjured fibers, previous studies using anterograde or transganglionic transport of HRP following limited dorsal rhizotomy have failed to uncover any evidence of sprouting by the uninjured ‘spared’ afferent (Rodin et al., 1983; Rodin & Kruger, 1984; McMahon & Kett-White, 1991); the redistribution of peptidergic afferents in spinal cord after root lesioning suggests that such sprouting must occur (McNeill et al., 1991; Piehl et al., 1992; Belyantseva & Lewin, 1999).
There is evidence that peripheral nerve injury increases expression of neurotrophins in the injured DRG (Sebert & Shooter, 1993; Michael et al., 1999); following spinal nerve ligation the level of nerve growth factor mRNA in DRG increases fourfold and remains elevated for at least 3 weeks (Shen et al., 1999). Neurotrophins released from the central terminal of peripherally axotomized afferents could induce sprouting of neighboring trk-responsive afferents. Alternatively, in the developing visual system synaptic activity is required for the initial formation and subsequent refinement of axonal projections (Sengpiel & Kind, 2002), and in the developing neuromuscular junction the more powerful input to a synaptic target cell destabilizes the less powerful input, so that competitive processes result in the elimination of supernumerary synapses (Buffelli et al., 2003). Spinal nerve ligation results in substantial ectopic electrical activity in lesioned fibers, but all the spontaneous activity is found in A-fibers (Liu et al., 2000a) while the small fibers in the axotomized DRG are electrically silent (Liu et al., 2000a). It is possible that the progressive enlargement of the central projection of the uninjured small fibers may be a consequence of this imbalance in electrical activity.
Selective spinal nerve ligation results in a chronic pain state that has many of the features of neuropathic pain in the human, and has been used successfully to model the effect of therapeutic interventions with good predictive validity (Yaksh, 1999). A recent report demonstrated that in the neonate, pain caused by peripheral inflammation results in a persistent expansion of the segmental projection of nociceptive primary afferents to the spinal cord (Ruda et al., 2000), and suggested that the expanded field may play a role in the perception of pain in the adult. The mechanism and the consequences of the expanded unmyelinated projection caused by spinal nerve ligation require further investigation.
Acknowledgements
This work was supported by grants from the NIH (M.M. and D.J.F.), the Department of Veterans Affairs (M.M. and D.J.F.) and the JDRF (D.J.F.). We thank Drs H. Richard Koerber and Brian Davis for helpful discussions.




