Nxf and Fbxo33: novel seizure-responsive genes in mice
Abstract
Much is understood about the response of the brain to seizure but little is known in relation to the underlying molecular mechanisms involved. We used microarray technology to investigate the complex genetic response of the brain to generalized seizure. For this investigation a seizure-specific mouse brain cDNA library was generated and spotted onto microarray slides with the aim of increasing the likelihood of identifying novel genes responsive to seizure. Microarray analysis was performed on mouse hippocampus 1 h after generalized seizure pharmacologically induced by pentylenetetrazol (PTZ). Using the custom microarray slides, six genes were identified as being up-regulated in this seizure model and results were validated by real-time PCR. Four of the seizure-responsive genes had previously-reported roles in apoptosis, proliferation or differentiation of neural cells. Two of the genes were novel and in situ hybridization analysis demonstrated heightened mRNA expression in the hippocampus 1 h following generalized convulsive seizure, in a pattern which is typical for other activity-dependant genes expressed in this structure. In addition to being up-regulated postseizure, the genes described in this paper appear to be expressed normally in the adult hippocampus and during development.
Abbreviations
-
- b-HLH
-
- basic helix–loop–helix
-
- DG
-
- dentate gyrus
-
- PTZ
-
- pentylenetetrazol
-
- SIM2
-
- single-minded 2
Introduction
The response of the central nervous system to seizure is complex, with the induction of activity-dependent genes (Kaminska et al., 1994), neuronal cell death (Filipkowski et al., 1994; Pollard et al., 1994; Gorter et al., 2003) and the proliferation, migration and differentiation of neural stem cells (Parent et al., 1997) which ultimately direct plastic changes in the brain (Parent & Lowenstein, 2002). However, to date little is understood regarding the molecular mechanisms that drive these seizure-associated phenomena.
Complex neural responses like those seen following seizure would be attributable to the actions of numerous genetic loci. Accordingly a functional genomics approach would be appropriate to identify genes associated with generalized seizure. In some studies of postseizure responses, microarray analysis has been employed to interrogate gene expression changes following seizure or during epileptogenesis. One study specifically examined the difference in gene expression patterns following seizure between different strains of laboratory mice (Sandberg et al., 2000), while another focused on differential gene expression following electroshock-evoked seizure (French et al., 2001). Finally, gene expression patterns have been investigated at various time points after prolonged seizure, during the period of epileptogenesis (Elliott et al., 2003; Lukasiuk et al., 2003).
The aim of this study was to specifically identify seizure-responsive genes that are novel. To achieve this a murine model was investigated in which pentylenetetrazol (PTZ) was administered to pharmacologically induce generalized seizures (Squires et al., 1984). To isolate novel genes, a seizure-specific mouse brain cDNA library was generated and spotted onto slides that were subsequently interrogated by microarray hybridization. Using this experimental paradigm six genes were identified as being differentially expressed 1 h following generalized seizure. Two of the seizure-responsive genes were novel, having no previous association with seizure. The four remaining genes encode transcription factors that, in addition to being responsive to seizures, have documented roles in the developmental processes of apoptosis, proliferation and differentiation. Bioinformatic data suggests that the two novel genes are also expressed during neural development.
Materials and methods
Mice
This investigation was performed under the guidelines of the University of Adelaide Animal Ethics Committee (ethics approval number S-03-2002). Experimental and control male C57BL/6 mice (7 weeks) were supplied by The University of Adelaide Laboratory Animal Services Unit. Mice were weighed and housed individually in separate boxes to habituate overnight under a 12 : 12 h light : dark cycle with food and water available ad libitum to the time of injection. On the day of seizure induction the appropriate volume of PTZ (Sigma, MO, USA 20 mg/mL in saline) was administered by i.p. injection into experimental mice to a final dose of 70 mg/kg. Convulsive seizures were typically observed within 5 min of PTZ administration and characterized by loss of posture and clonic activity of the forelimbs and hindlimbs followed by mild hindlimb extension, collectively lasting up to 20 s. Typically a single round of clonic convulsive activity was observed. Control mice were injected i.p. with an equivalent volume of saline. Mice were maintained for 1 h and then killed by cervical dislocation. Once dead, mice were decapitated; whole brains were extracted and placed on an ice-cold surface where the hippocampus was removed under a dissecting microscope using blunt instruments.
RNA extraction
RNA extractions for the preparation of the subtracted cDNA library were performed using the TRIzol (Invitrogen; CA, USA) method and mRNA purified using the Oligotex poly A+ extraction system (Qiagen; Hilden, Germany). For microarray experimentation, total RNA extractions were performed using a modified protocol combining the TRIzol method with the RNeasy extraction system RNA cleanup protocol (Qiagen). RNA samples were quantified by spectrophotometer (Eppendorf; Hamburg, Germany) and RNA integrity checked on 1% agarose gels using a deionized formamide-based loading buffer.
Seizure-specific mouse brain cDNA library
A seizure-specific mouse brain cDNA library was constructed by suppression-subtractive hybridization (Diatchenko et al., 1996) using the PCR-select cDNA subtraction system (Clontech; CA, USA). Forward subtractions were performed using a PTZ-treated sample as tester and a saline-treated control sample as driver, while reverse subtractions were performed in the opposite configuration. The library was generated at the 1-h time point after treatment with PTZ or saline and cloned into pGEM-T easy vector (Promega, WI, USA).
One thousand and fifty-six bacterial colonies were isolated from agar dishes into 96-well plates and grown in superbroth. Clones were amplified directly in 100-µL PCR reactions (Sambrook & Russell, 2001) by adding 1 µL of culture to the PCR mix. M13 forward (GTT TTC CCA GTC ACG AC) and M13 reverse (CAG GAA ACA GCT ATG AC) primers were synthesized locally (GeneWorks; South Australia, Australia) and used with 3 mm MgCl2 at an annealing temperature of 52 °C. Products were checked for contamination on 1% agarose gels, and then purified using Multiscreen PCR cleanup plates (Millipore; MA, USA).
Microarray analysis
The seizure-specific mouse brain cDNA library was printed in quadruplicate on ultraGAPS-coated slides (Corning; NY, USA) using a Vertik SEDC-2 microarray spotter with quill-tipped microarray pins (Telechem; CA, USA). A known activity-dependant immediate–early gene which is up-regulated in mouse brain in response to convulsive seizures, c-fos (Morgan et al., 1987; Clough et al., 1997), was also printed on microarray slides as a positive control. Differential expression of c-fos was used as a control to show that the seizure had successfully induced transcriptional changes in the mouse hippocampus. A dilution series of c-fos (1.5 µg−75 ng) was also printed on the slides to control for signal intensity. Numerous other controls were also printed on the slides including nondifferentially expressed controls (β-actin, GAPDH), a universal control comprising all clones in the library, DNA controls including mouse genomic DNA, mouse Cot-1 DNA, salmon sperm DNA and plant genomic DNA. Poly A RNA was also spotted in addition to a 50% DMSO-only control.
Microarray hybridizations (Schena et al., 1995) were conducted implementing pairwise comparisons of gene expression in hippocampus from seizure-challenged mice (n = 2) against vehicle-treated control mice (n = 2), 1 h after treatment. Superscript III reverse transcriptase (Invitrogen) was used to generate cDNA from 50 µg of total RNA in conjunction with amine-modified random hexamers (Xiang et al., 2002) and amine-modified oligo-d(T) (GeneWorks), 4 and 2 µg, respectively. Amino-allyl dUTP (4 µm; Sigma) along with dTTP (6 µm) was incorporated into reverse transcripts (a 2 : 3 ratio of unlabelled dTTP nucleotide to amino-allyl dUTP). dATP, dCTP and dGTP were used at a final concentration of 16.6 µm each. cDNA was post-labelled with Cy5 or Cy3 monofunctional reactive dye (Amersham; Freiburg, Germany). Slides were prehybridized for 1 h in a mixture of 10 mg/mL BSA, 25% deionized formamide, 5 × SSC and 0.1% SDS. Hybridization solution contained labelled cDNA resuspended in 3 µL deionized formamide, 3 µL 6.25 × SSC and 0.2 µL 10% SDS with yeast tRNA (15 µg), polyA RNA (8 µg) and Cot-1 DNA (20 µg). Hybridizations were performed in a humidified chamber at 42 °C overnight.
Slides were scanned using a GenePix dual-laser scanner driven by GenePix Pro 4.0 software (Axon Instruments; CA, USA). All analyses were performed in the R statistical computing environment (http://www.r-project.org). The Spot package was used for image analysis (http://spot.cmis.csiro.au/spot/index.php) implementing a seed-growing algorithm for spot image segmentation and a morphological opening algorithm for background subtraction. All further analysis was performed using the SMA package (http://www.stat.berkeley.edu/users/terry/zarray/Software/smacode.html). Locally weighted scatter plot smoother function (LOWESS) was used for within-slide normalization. The data from the three individual arrays were scaled to make them directly comparable. Bayesian analysis was used to identify clones showing consistent differential expression both within and between arrays (Lönnstedt & Speed, 2002).
Real-time PCR analysis
The clones corresponding to the seizure-responsive genes were retrieved from the subtracted library and identified by sequencing (BigDye III; Applied Biosystems; CA, USA). Real-time PCR was then performed to validate the microarray results, adapting the method from Chiang et al. (1996). Hippocampi were dissected from the brains of PTZ-treated mice (n = 6), saline-treated control mice (n = 6) and sham-treated mice (n = 6). The sham-treated mice were pierced i.p. with a needle without injection of any solution, to control for any effects that saline alone may have on gene expression.
RNA was extracted in the same manner as for microarray experimentation (above) and reverse-transcription reactions were performed using Superscript III reverse transcriptase (Invitrogen). cDNA samples were diluted to a uniform concentration of 50 ng/µL. Real-time PCR reactions were performed using TaqMan master mix on an ABI SDS 7000 light cycler driven by ABI prism SDS v1.1 (Applied Biosystems). TaqMan primers and probes (Heid et al., 1996) were designed using Primer Express v2.0 (Applied Biosystems) and synthesized locally (Table 1; GeneWorks). Probes were generated with a 5′ FAM fluorescent reporter and a 3′ BHQ1 quencher. Primers and probes were used at a final concentration of 300 and 250 nm, respectively, and reactions for each sample were performed in quadruplicate. PTZ-17 was used as an internal reference to control for loading and facilitate relative quantification using the ddCt approach. PTZ-17 was selected as an internal reference because, although expression is down-regulated in mouse neocortex in response to PTZ-induced seizures (Kajiwara et al., 1995), transcript levels remain unaffected in hippocampus (data not shown). Primers for the assayed genes were similar in reaction efficiency to the PTZ-17 internal reference primers.
| Gene | Forward primer | Probe | Reverse primer |
|---|---|---|---|
| c-fos | TCCCGCTCTGTGCCAGAT | ACCTGTCCGGTTCCT | GCTCCCAGTCTGCTGCATAGA |
| Ptz17 | CTGGTAGGTCAATTACAGTTTTGTGATT | CCCGCTACCGTGACT | CTGGCCCTGGTGGATGTG |
| Fbxo33 | TGGCGAGAGTCTTGACCGATA | CAACCATGTGCCTTTGCAGCGACT | GAAGCATTGTGGACCAGAAGAGA |
| Nxf | AGCATTCCAGGCTCATCTGAA | AGAGCAACTGAGCCC | GGCGAAGTAAGTCTTGGTAGGATT |
| Egr1 | CAAAGCCTCCCCCAAAACA | AGCGTGTCCCTCACAT | TGCCGATGGCTTGACATG |
| Nr4a1 | GTTATCCGAAAGTGGGCAGAAA | CCCTGGCTTCATTG | TCTTGGTCTCCTGGGCAAAG |
| Btg2 | AGTTTGAGAGACTTGAGGCCTTCTA | AAGCCCTCATCAGTGTC | AATGGCTTTCCTTCAGGTTCAG |
| Sgk | CGCCAAGTCCCTCTCAACA | ATCAACCTGGGTCCGTC | TTGGCGTGAGGGTTGGA |
Kruskal–Wallis analysis of variance was used to determine whether there were significant differences in expression among the studied groups for a given gene. When significant differences were found, the Mann–Whitney U-test was employed post hoc to identify the group(s) significantly different from the saline-treated control samples for a given gene.
In situ hybridizations
PTZ seizure-challenged brains (n = 6) and brains from saline-treated mice (n = 6) were used for in situ analysis of the novel genes. PTZ seizure-challenged (1 h postseizure) and saline-treated control mice were killed with Nembutal® (Rhone Merieux; Queensland, Australia) overdose 1 h postseizure and then transcardially perfused with saline, followed by 4% paraformaldehyde (Sigma) in 0.1 m phosphate buffer (pH 7.4). Brains were removed, postfixed overnight in the same fixative at room temperature and embedded in paraffin. Sagittal sections were cut (10 µm) using a rotary microtome (Leica Microsystems AG; Heerbrugg, Switzerland).
Clones corresponding to the seizure-responsive genes were retrieved from the subtracted library and used as templates to transcribe sense and antisense probes utilizing the DIG RNA labelling system (Roche; Mannheim, Germany). In situ hybridizations were performed adapting the method from Gee & Roberts (1983). In brief, hybridizations were incubated overnight at 60 °C with riboprobe at 1 µg/µL in hybridization buffer. Slides were washed in 0.2 × SSC for 1 h at 72 °C and incubated overnight at 4 °C with AP-conjugated anti-DIG antibody (Roche) at a dilution of 1 : 4000 with 10% horse serum in 0.15 m NaCl and 0.1 m TrisCl (pH 7.5). Colour reactions were allowed to develop to the point when colour started to emerge in the sense controls. Results were analysed blind by an independent researcher using a visual rating system. Images were acquired on a Zeiss Axiophot microscope (Zeiss; Jena, Germany) using a Fujix HC-1000 3CCD image-capturing camera driven by Fujix Photograb software (Fujifilm; Tokyo, Japan).
Bioinformatics
Bioinformatic analyses were performed using utilities and databases at the National Centre for Biotechnology Information (http://www.ncbi.nlm.nih.gov).
Results
Three microarray hybridizations were performed, consisting of two individual comparisons and a dye-swap configuration for one of the experimental groups, using custom slides spotted with a seizure-specific mouse brain cDNA library. The intensity of most cDNA spots was within the analysable range and extended to levels of high intensity, confirming that probe labelling was efficient (Fig. 1). All dilutions of the seizure-induced c-fos control printed on the array were identified. Bayesian analysis indicated that expression data from individual experiments were highly reproducible and clearly identified differentially expressed genes (Fig. 2). The selection criterion used in this study was based on Bayesian posterior log odds such that no up-regulated clones appeared to be down-regulated at similar odds. From this analysis, six genes were shown to up-regulate in the mouse hippocampus 1 h following generalized seizure (Table 2).
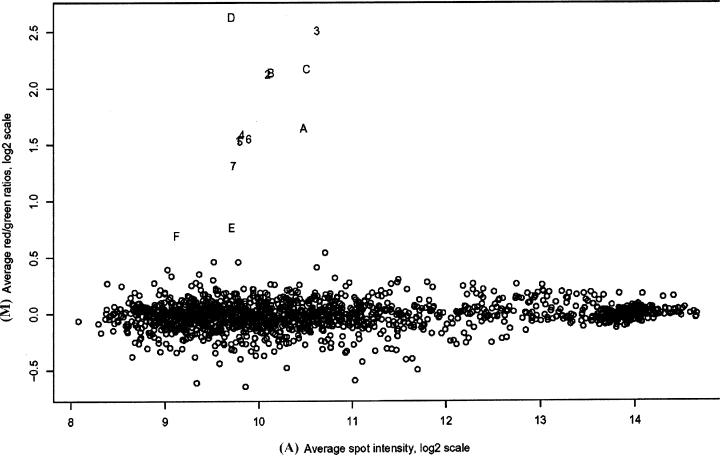
M vs. A plot of normalized microarray data comparing hippocampus from seizure-challenged and vehicle-treated mice. Y axis (M), log ratio of red/green intensities. X axis (A), log average of the sum of the red (Cy5) and green (Cy3) intensities. All differentially expressed genes were up-regulated in seizure-challenged hippocampus and are represented by the alphanumeric outliers. Negative controls, though not plotted, were included in the analysis. A, Egr1; B, Btg2; C, Nr4a1; D, Nxf; E, Sgk; F, Fbxo33; 1–7, dilution series of c-fos positive control.
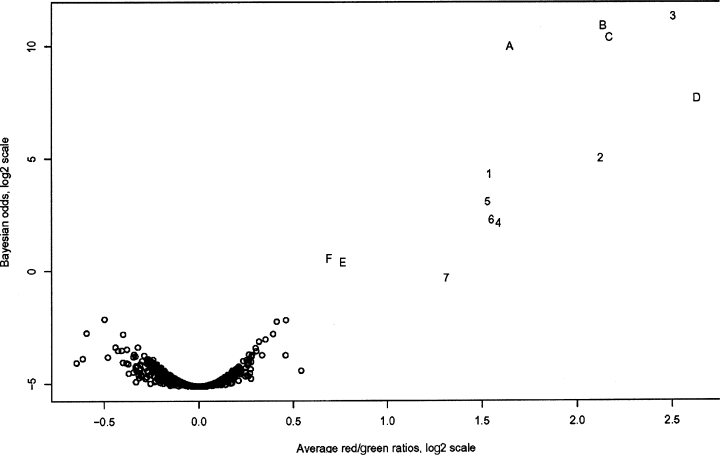
Bayesian analysis of collective microarray seizure data 1 h postseizure. X axis, log ratio of red/green intensities. Y axis, log odds score. Genes that are consistently and significantly up-regulated are indicated in the upper right-hand quadrant. Negative controls, though not plotted, were included in the analysis. A, Egr1; B, Btg2; C, Nr4a1; D, Nxf; E, Sgk; F, Fbxo33; 1–7, dilution series of c-fos positive control.
| Gene | GenBank | UniGene | Function(s) | Reference |
|---|---|---|---|---|
| Fbxo33 | BC020022 | Mm.311026 | Unknown | |
| Nxf | NM_153553 | Mm.287867 | Neural plasticity (putative) | Ooe et al., 2004 |
| Egr1 | NM_007913 | Mm.181959 | Apoptosis Differentiation | Gubits et al., 1993 Sukhatme et al., 1988 |
| Nr4a1 | BC004770 | Mm.119 | Cell cycle re-entry (GO/G1) | Hazel et al., 1988 |
| Btg2 | NM_007570 | Mm.239605 | Apoptosis Differentiation | Wang et al., 1997 el-Ghissassi et al., 2002 |
| Sgk | NM_011361 | Mm.28405 | Cell cycle re-entry (GO/G1) | Webster et al., 1993 |
Of the six genes identified by microarray, two were novel, having no previous association with generalized seizure. F-box only protein 33 (Fbxo33; GenBank accession BC020022), and Nxf (GenBank accession NM_153553) were both up-regulated in the mouse hippocampus 1 h after seizure. Bioinformatic analysis of Fbxo33 demonstrated a longest open reading frame coding for a protein of 501 amino acids and a conserved domain search showed a highly conserved (92.7% identity with consensus) N-terminal F-box ubiquitination domain between residues 13 to 50. Analysis of Nxf revealed that the longest open reading frame encodes a theoretical protein of 802 residues with a conserved N-terminal basic helix–loop–helix (b-HLH) domain at residues 10–52 (79.2% identity with consensus) followed by two PAS domains at residues 72–135 and 216–273 (92.5% and 86.6% identity with consensus, respectively).
Real-time PCR was used to validate the microarray data and accurately determine the level of increased expression, in terms of fold change, for the observed genes in response to seizure. In addition to the PTZ and saline treatments, the inclusion of sham-injected mice in the real-time PCR analysis allowed us to control for any effects that vehicle (saline) may have on gene expression. PTZ-17 was used as an internal reference and average dCt values relative to this reference were highly reproducible between all samples and replicates.
In accordance with the microarray data, the six seizure-responsive genes were confirmed by real-time PCR to up-regulate, relative to controls, 1 h following seizure (Fig. 3). The c-fos gene was used as a positive control in the real-time PCR experiments as for the microarray experiments. As anticipated, c-fos was greatly up-regulated after seizure at a level of ≈ 46-fold. Concerning the experimental genes, up-regulation was observed at levels ranging from 3.8- to 19.6-fold for Sgk and Nxf, respectively. Kruskal–Wallis analysis of variance for average fold change values showed that there were significant differences in gene expression levels among the different experimental groups for each gene (P < 0.05). Post hoc analysis using the Mann–Whitney U-test revealed that, in each case, there were significant differences between saline-treated controls and seizure samples (P < 0.01) while no significant differences were observed between control and sham-treated samples.
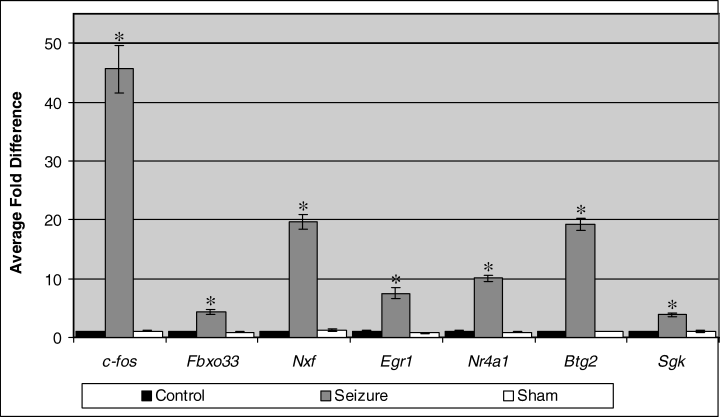
Quantitative real-time PCR analysis of gene induction in response to generalized seizure. PTZ was used to induce seizure and fold differences were relative to saline-injected control samples, 1 h following treatment. Mann–Whitney U-tests were performed against saline-treated control samples; *P < 0.01. Error bars are SEM. Control n = 6, seizure n = 4 and sham n = 6.
In situ hybridizations were undertaken specifically focusing on the spatial mRNA expression patterns of the two novel sequences, Fbxo33 and Nxf, in hippocampus (Fig. 4). Sense probes were used to control for specificity and produced no background staining. When investigating the saline-treated controls, Fbxo33 and Nxf transcripts were observed in the granular cell layer of the dentate gyrus (DG) and in the CA1 region of the pyramidal cell layer, with less intense staining apparent in the CA3 and hilar subfields.
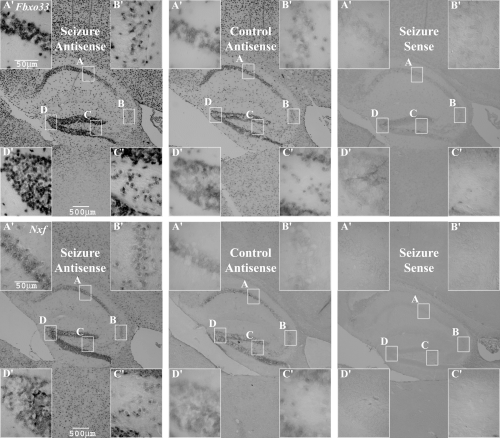
In situ hybridizations representative of hippocampus 1 h after PTZ-induced seizure or saline treatment; 10-µm paraffin sections. Main image, 2.5× objective lens; inserts (A′, B′, C′ and D′), 40× objective lens; A, CA1; B, CA3; C, hilus; D, dentate gyrus. Seizure antisense n = 6, control antisense n = 6, seizure sense n = 6.
When mice had experienced PTZ-induced seizure, a similar pattern of expression was observed in the hippocampus; however, staining was notably more intense. Furthermore, the in situ hybridization results collectively demonstrated that expression of Fbxo33 and Nxf also increased within the thalamus and cerebral cortex (Fig. 4). Though staining intensity increased in the hippocampus for both genes postseizure, it was apparent that staining was less intense for Nxf than for Fbxo33, before and after seizure, even though Nxf demonstrated a greater level of induction postseizure as indicated by real-time PCR.
Discussion
We used microarray technology to interrogate a seizure-specific mouse brain cDNA library and isolated six genes from the mouse hippocampus that were up-regulated 1 h following PTZ-induced generalized seizure. Real-time PCR analysis indicated that the six genes isolated by microarray were significantly up-regulated compared to saline-treated controls. As real-time PCR demonstrated no significant differences between saline-treated and sham control groups, the administration of saline was shown to be insufficient to induce the expression of the studied genes.
Of the seizure-responsive genes identified in the hippocampus, two were novel, having no previous association with a neural response to seizure. To identify the regions in which these genes were expressed in the hippocampus, we performed in situ hybridization analysis. Expression of Fbxo33 and Nxf was seen in the dentate gyrus and CA1 regions while expression appeared lower in the CA3 and hilar subfields for both control and seizure groups. Lower expression in the hilus, however, may be directly attributable to lower cell density. The in situ results demonstrate the anticipated increase in staining intensity postseizure, further substantiating the differential expression patterns demonstrated by microarray and real-time PCR.
The primary focus of this study was to identify novel genes in the hippocampus that were responsive to seizure. It is interesting, however, that in addition to up-regulation postseizure, Nxf and Fbxo33 were expressed in the hippocampus of normal adult mice that had not experienced seizure. Recent work indicates that Nxf is expressed in structures of the adult mouse brain, other than the hippocampus, including striatum and the Purkinje cell layer of the cerebellum (Ooe et al., 2004). Furthermore, it was evident from the in situ hybridization results collectively that both novel sequences were up-regulated in the cortex and thalamus postseizure. Nxf and Fbxo33 up-regulation in the cerebral cortex and thalamus after seizure, as for hippocampus, suggests that the function of Nxf is not specific to the hippocampus and that these two structures respond to seizure in a similar way.
From the in situ hybridization results it was apparent that the intensity of staining was lower for Nxf than for Fbxo33. This is in contrast to the microarray and real-time PCR analyses which indicated that seizure-induced up-regulation of Nxf was much greater. The microarray and real-time PCR results specifically address differential expression patterns in response to seizure and do not describe the overall levels of transcript within the cells before or after seizure. Accordingly the lower intensity of staining for Nxf indicates that this transcript was less abundant than Fbxo33, both before and after seizure, even though the observed level of differential expression for Nxf was over four times greater postseizure. This assertion can be made as the riboprobes used in the study were approximately equal in size and hence probe sensitivity was similar for the two genes.
The expression patterns of the novel seizure-responsive genes in the hippocampus were consistent with observations for activity-dependent genes from other studies. Greater levels of expression for Nr4a1 in the dentate gyrus 1 h postseizure has been observed previously (French et al., 2001) and overall this was consistent with the expression pattern of c-fos 1 h after PTZ-induced seizure (Morgan et al., 1987).
During the course of the real-time PCR study, six mice were treated with PTZ to induce generalized seizure; however, only four demonstrated convulsive seizure in response to this treatment and were used in the analysis. It is interesting to note, however, that the two samples which were treated with PTZ but did not demonstrate convulsive seizure also did not show any notable difference in gene expression when compared with saline-treated or sham controls (data not shown).
The Fbxo33 UniGene Cluster (Mm.311026) revealed numerous expressed sequence tags (ESTs) demonstrating embryonic and adult expression in a wide range of normal neural and non-neural tissues, including mammary carcinoma. Mouse Fbxo33 shares 92% identity with the human Fbxo33, which also has no known function.
In the Nxf UniGene cluster (Mm.287867), ESTs were reported from a variety of embryonic and adult neural tissues, suggesting that Nxf is a neural-specific gene. NXF is a putative b-HLH PAS transcription factor with 26% amino acid identity with the mouse single-minded 2 (SIM2) protein and 25% identity with human SIM2. The Sim genes are significant regulators of central nervous system midline cell lineage (Thomas et al., 1988). Mouse Sim2 is located on a region of chromosome 16 which is syntenic with human chromosome 21q22.2 (Dahmane et al., 1995), the so-called Down's syndrome critical region. This region contains the human Sim2 homologue (Yamaki et al., 1996; Chrast et al., 1997) and has been implicated in the pathogenesis of Down's syndrome (Chrast et al., 2000), a significant developmental disorder. Interestingly, it is now clear that the prevalence of seizures in individuals with Down's syndrome is notably higher than for the general population with ≈ 30% of the affected individuals studied, both adult and juvenile, experiencing epileptic seizures (Goldberg-Stern et al., 2001; Puri et al., 2001).
Nxf was recently identified from a predicted exon database, isolated from a human foetal brain cDNA library and the mouse homologue subsequently isolated from a mouse genomic library (Ooe et al., 2004). Nxf appears to be expressed late in mouse development, beginning on E17, suggesting that this transcription factor is important in neuronal functions after birth. However, the fact that the gene was first isolated from a foetal brain library argues that this transcript should also be expressed during early development. Nxf functions as a heterodimer, most probably with Arnt2, to positively self-regulate as well as promote the expression of drebrin, a gene that is expressed in a pattern which overlaps with Nxf and is implicated in neural plasticity (Imamura et al., 1992; Harigaya et al., 1996; Hayashi et al., 1996; Sasaki et al., 1996). Furthermore, it is apparent that Sim2 is a negative regulator of NXF/Arnt-mediated transcription, implicating Nxf as a causative factor in Down's syndrome. Nxf regulation of drebrin could also suggest that this transcription factor may promote plastic changes in the hippocampus that are observed after ongoing temporal lobe seizures, for instance mossy fibre sprouting.
The other genes identified as being responsive to seizure in the mouse brain in this study also have known or implicated roles in neural development, as they have been shown to have some function in apoptosis, proliferation or differentiation, though it is not clear that they are exclusively involved in these processes. Egr1 (Sukhatme et al., 1988) and Btg2 (Fletcher et al., 1991) both appear to play dual roles in programmed cell death and differentiation in neuronal cell lines (Mesner et al., 1995; Pignatelli et al., 1999; Malatesta et al., 2000; Corrente et al., 2002; el-Ghissassi et al., 2002). Egr1 is rapidly up-regulated after growth factor-induced differentiation of neural cells (Sukhatme et al., 1988) and Btg2 acts to reduce proliferation rate and promote differentiation (el-Ghissassi et al., 2002). Sgk was the first gene observed to code for a putative protein kinase that is transcriptionally regulated by glucocorticoids, which have important roles in homeostasis and the control of differentiation and development (Helman et al., 1987). Nr4a1 has also been implicated in cell cycle regulation and is specifically expressed during the GO/G1 cell cycle re-entry phase (Hazel et al., 1988).
Btg2, Egr1 and Nr4a1 have been seen previously to up-regulate in the hippocampus, cerebellum and cerebral cortex in response to electroconvulsive shock (Jung et al., 1996), and microarray analysis in another study has shown that c-fos, Egr1 and Sgk are differentially expressed, at different levels in the hippocampus postseizure, between different strains of mice (Sandberg et al., 2000). Independent in situ analysis has also shown that Egr1 and Nr4a1 are up-regulated in the rodent hippocampus following kainic acid-induced seizure (Honkaniemi & Sharp, 1999) and microarray analysis has shown that Nr4a1 is up-regulated following electroshock-evoked seizure (French et al., 2001). The present study, however, is the first to collectively show up-regulation of these genes in response to seizure.
The roles of Fbxo33, Nxf and the other genes responsive to generalized seizure described herein remain to be elucidated. It is apparent that genes which are expressed in the adult brain and may also be involved in neural development are significantly up-regulated in the adult brain following generalized seizure and, hence, are shown to be involved in the first step of a directed response to this form of neurological challenge. The downstream pathways which are in turn regulated by these genes must now be determined to further our understanding of the mechanisms employed by the central nervous system to respond to seizure.
Acknowledgements
We thank Dr Ashley Connolly and Mr Mark vanderHoek for technical advice concerning microarray, and Associate Professor Patricia Solomon for her assistance in establishing the microarray experimental design. We also thank Dr John Mulley and Professor Sam Berkovic for their invaluable guidance in the preparation of this manuscript. This work was supported by NHMRC program grant 207703.




