Long-term motor cortex reorganization after facial nerve severing in newborn rats
Abstract
Using the model of facial nerve injury, we have compared the effect of injury in newborn and adult rats on the adult rat motor cortex (M1). To this end, the facial nerve was severed in 10 newborn rats 2 days after birth (Newborn group) and in 10 adult rats (Adult group). In both the Control (contralateral to untouched nerve) and the Experimental (contralateral to severed nerve) hemisphere of each rat, the M1 output organization was assessed by intracortical microstimulation. Our findings demonstrated that: (i) there is no statistical difference in the percentage of movement sites and in current thresholds required to evoke movement in Control hemispheres between the Adult and Newborn groups of rats; (ii) in Adult Experimental hemispheres, neck sites expand in the medial part of the vibrissae representation more extensively than shown in Newborn Experimental hemispheres; (iii) in Newborn Experimental hemispheres eye sites expand in the medial part of the vibrissae representation more extensively than in Adult Experimental hemispheres (these sites overlap the cortical region where electrical stimulation evokes neck movement in Adult Experimental hemispheres) and (iv) in both Newborn and Adult Experimental hemispheres, forelimb sites expand similarly thereby overlapping the same cortical region, corresponding to the lateral part of the vibrissae representation. We conclude that, when the facial nerve injury is performed in the newborn rat, the pattern of movement representation differs from that obtained with the same lesion in the mature brain only in the frontal cortex corresponding to the medial part of the normal vibrissae representation.
Abbreviations
-
- ICMS
-
- intracortical microstimulation
-
- ML
-
- medial–lateral
Introduction
During development and also throughout adult life, the mammal nervous system is capable of widespread functional reorganization (Kaas, 1999; Sanes & Donoghue, 2000). In most studies, a common experimental strategy has been to produce a restricted motor or sensory loss by denervating a small portion of the body. As a general feature of mammalian cerebral cortex remodelling after peripheral nerve lesion, the corresponding disconnected cortex adopts the functional properties of its immediate cortical surroundings. To date, most of the studies of plasticity in developing cortical maps have dealt with the mutability of the sensory systems (Hubel et al., 1977; Waite & Cragg, 1982; Bronchti et al., 1992; Innocenti, 1995; Cusick, 1996) while fewer have delved into the mutability of the motor system (Donoghue & Sanes, 1988). These studies demonstrated that changes in visual and S1 maps appear to be most noticeable when peripheral manipulations are carried out during a critical perinatal period. Nevertheless, little is known about the effect which neonatal motor nerve lesions have on cortical output and motor system reorganization.
In the developing cerebral cortex, the formation of a topographic map involves establishing initial coarse maps which are refined during perinatal life (Crair et al., 1998) and it is the pattern of neural activity that refines sensory–motor maps in the developing cortex throughout perinatal life (Carvel & Simons, 1996; Keller et al., 1996; Nicolelis et al., 1996; Huntley, 1997). This process could be profoundly altered in young animals when peripheral axons are severed during a period of sensitivity to axonal injury (Sendtner et al., 2000) as the nervous system is more sensitive to peripheral manipulations than in the adult state. However, we would expect a lack of motor cortex to muscle output early in life to lead to different, more extensive adaptive mechanisms in the developing motor system than the same lesion in the mature brain and this would be seen as a different pattern in the M1 representation of movements.
In the rat, exploring behaviour exhibits a sequence of movements usually referred to as ‘sniffing’ (Welker, 1964). Behavioural observations indicate that sniffing is a relatively fixed, stable motor pattern involving the spatiotemporal coordination of different movements. Indeed, during sniffing behaviour, discrete head and eye movements work together with the ample excursions of mystacial vibrissae (whisking) and are accompanied by rapid respiratory movements and body postural adjustments. Whisking appears around the middle of the second postnatal week (Welker, 1964). The relative immaturity of the neonatal vibrissae system makes it a useful model for studying the mechanisms of plasticity in developing mammals.
The present study investigates whether the pattern of functional abnormalities in the cortical representation of movements, previously described after facial nerve injury in the adult rat (Sanes et al., 1990; Franchi, 2000b), is similar or different when facial nerve injury is performed at a critical stage of whisking maturation in the newborn rat.
A preliminary report of these data has appeared elsewhere (Veronesi & Franchi, 2003).
Materials and methods
Animals and surgery
Experiments were carried out on 20 Albino rats weighing 250–300 g. The facial nerve was severed, respectively, in 10 newborn rats (Newborn group) and 10 adult rats (Adult group). The experimental plan was designed according to the Italian law for the care and use of experimental animals (DL116/92) and approved by the Italian Ministry of Health. Whole litters, including both male and female rats, were used for the neonatal and adult groups. Neonatal animals were produced in-house from breeding stock. All animals were maintained with unlimited access to food and water pre- and postsurgery. Post-surgical care included local anaesthetic (Lidocaine), local and systemic antibiotics (Rifamicina SV; Lepetit), and fluids to prevent dehydration.
For all experimental procedures adult rats were anaesthetized initially with ketamine HCl (100 mg/kg i.p.). For the duration of the experiment, anaesthesia was maintained by supplementary ketamine injections such that a long-latency, sluggish hindlimb withdrawal was sometimes achieved only with severe pinching of the hindfoot. Under anaesthesia, the body temperature was maintained at 36–38°C with a heat lamp.
The general procedures were as follows.
- (i)
Two days after birth, newborn rats were anaesthetized by hypothermia, cooling them on ice for 5–10 min. Under an operating microscope, the facial nerve of one side was exposed at the foramen stilomastoideum, ligated using 10-0 surgical sutures and transected distally from this ligature. A drop of Histoacryl tissue adhesive (Braun Melsungen, Germany) was applied to the proximal nerve stump to counteract axon regeneration. The skin was closed with acrylic tissue adhesive (Histoacryl). At the end of the surgical procedure, all pups were warmed and then returned to the dam. Postoperatively, none of rats displayed complications or the inability to suck milk. In the subsequent postsurgical period, these rats lacked facial movement on the operated side and showed remarkable face and jaw asymmetry. The paralysed vibrissae appeared to be atonically backward positioned and showed normal growth in vibrissae number and good growth in vibrissae length. In the subsequent postsurgical weeks, these rats displayed slower than normal growth so that at 4 months of age only rats weighing 250–300 g were taken into account for the mapping procedures.
- (ii)
In adult rats, the facial nerve was exposed at the point where it exits the stilomastoid foramen, ligated using 6-0 surgical sutures and transected just beyond the point of ligature. The proximal stump was dried and covered with acrylic tissue adhesive (Histoacryl) to prevent the proximal axons from sprouting. The skin was closed with 6-0 sutures and the wound was cleansed with antibiotic solution (Rifamicina SV; Lepetit). Postoperatively, none of the rats displayed complications or the inability to eat. Following surgery, the animals lacked vibrissae movement and blinking on the operated side but spontaneous vibrissae fibrillation was observed a few days later. For the following 4 weeks the de-efferented vibrissae were atomically backward positioned and mild puffs directed at the animal's denervated hemiface did not elicit any evident blinking responses.
Intracortical stimulation mapping
In each animal, the movements evoked by intracortical microstimulation (ICMS) in the frontal agranular cortex were mapped in both Control and Experimental hemispheres (Control, hemisphere contralateral to the untouched side; Experimental, hemisphere contralateral to the denervated side). In both the Newborn and Adult groups, mapping was performed 4 months and 4 weeks, respectively, after the facial nerve had been severed. Previous studies showed that M1 reorganization in the adult rat stabilized in the fourth week after the facial nerve was severed (Sanes et al., 1990; Franchi, 2000b). The anaesthetized animal was placed in a Kopf stereotaxic apparatus and a craniotomy was performed over the frontal cortex of the Experimental hemisphere. At the end of this initial mapping step, the same animal was subjected to motor cortex mapping of the Control hemisphere. The mapping procedure was similar to that described by Donoghue & Wise (1982) and Sanes et al. (1990) and detailed elsewhere (Franchi, 2000a). Briefly, the dura remained intact and was kept moist with a 0.9% saline solution. The electrode penetrations were regularly spaced out over a 500-µm grid. Alteration in the coordinate grid, up to 50 µm, was sometimes necessary to prevent the electrode from penetrating the surface blood vessels. These adjustments in the coordinate grid were not reported in the reconstructing maps. When the adjustment was over 50 µm, the penetration at this site was not performed. Glass-insulated tungsten electrodes (0.6–1 MΩ impedance at 1 kHz) were used for stimulation. The electrode was lowered perpendicularly into the cortex to a depth of 1.5 mm below the cortical surface and adjusted ± 200 µm so as to evoke movement at the lowest threshold. In preliminary experiments this depth was found to correspond to layer V of the frontal agranular cortex (Franchi, 2000a).
Monophasic cathodal pulses (30 ms train duration at 300 Hz, 200 µs pulse duration) of a maximum of 60 µA were passed through the electrode with a minimum interval of 2.5 s. Starting with a 60 µA current, the intensity was decreased in 5-µA steps until the movement was no longer evoked and the intensity was then increased to a level at which approximately 50% of the stimulations elicited movement. This level defined the current threshold. If no movements or twitches were evoked with 60 µA, the site was recorded as negative. Mapping was initiated at a high current because the initial polysynaptic recruitment of remote neurones optimizes the detection of movements in this 500-µm step grid mapping. When movement was observed in two different body parts, current thresholds were determined for each component. Body parts activated by microstimulation were identified by visual inspection and/or muscle palpation. When eye movement was observed, the current threshold was determined under an optical microscope. The terms ‘forelimb movement’ and ‘hindlimb movement’ refer collectively to distal and proximal joint movements. Forelimbs and hindlimbs were approximately half-way between flexion and extension and were alternately flexed and extended, particularly at the representational borders.
Histology
At the end of the experimental procedure, the animals were perfused transcardially. In all animals, gross postperfusion examination of the injured nerve showed no continuity of the nerve at the acrylic stopper level. The brains were then removed, postfixed and transferred to a 30% sucrose solution until they sank. They were then sectioned coronally into 50-µm-thick slices and stained with thionine or neutral red to verify microelectrode positions and depths. The sections containing the facial nuclei were also determined. Morphological examination of these sections (Axioskop Zeiss and DMC Polaroid camera for image acquisition) showed extensive degenerative processes involving facial nuclei on the side of the lesion (1, 2). In comparison to the Adult group of rats, in the Newborn group facial nerve severing caused the death of all facial motoneurones.

Example of a coronal section, 50 µm thick, of the facial nucleus (7) in the Adult group of rats stained with neutral red. 4V, fourth ventricle; g7, genu facial nerve; MVe, medial vestibular nucleus; py, pyramidal tract; Sp5O, nucleus spinal tract trigeminal nerve, oral. Note the smaller number of motoneurones in the nucleus on the severed side.
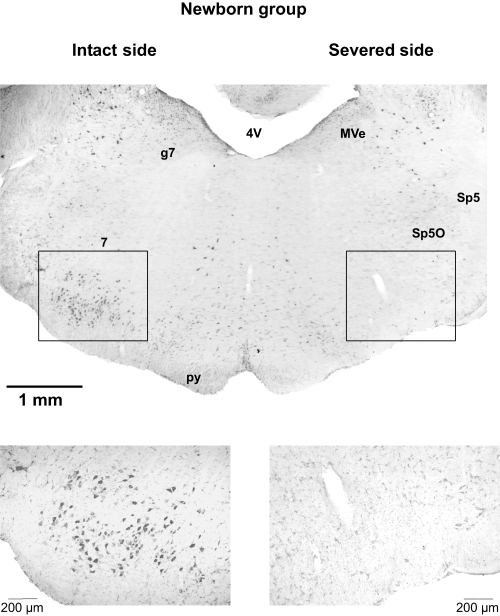
Example of a coronal section, 50 µm thick, of the facial nucleus (7) in the Newborn group of rats stained with neutral red. 4V, fourth ventricle; g7, genu facial nerve; MVe, medial vestibular nucleus; py, pyramidal tract; Sp5, spinal tract trigeminal nerve; Sp5O, nucleus spinal tract trigeminal nerve, oral. Note the absence of facial motoneurones on the severed side.
Map construction and data analysis
Using a dedicated plotting program (written with the Laboratory View Development System, see Acknowledgements), an on-line grid map was constructed by labelling electrode penetrations according to the distance (in mm) from the bregma. At a current intensity of 60 µA or less, threshold values were recorded on a sheet scrolling below the map grid. This procedure considers the cortical surface subdivided into a square grid where each movement threshold point was the centre of a 500-µm-wide square. In each hemisphere, vibrissae and forelimb movements were mapped in order to determine the extent and location of these representations. Penetrations not performed in correspondence to superficial large vessels within the motor map were not taken into account in the computations. This procedure presents several potential sources of variability that could affect the configuration accuracy and size of the movement representations. To reduce the effect of experimental sources of variability, a similar mapping density was maintained across all animals. This procedure cannot prevent electrode track distortions arising from the curvature in the lateral part of the frontal cortex. This mainly affects the forelimb sites situated in the more lateral position in rat M1. As the goal of this study was to document reorganization in that part of M1 where the electrode was lowered perpendicularly into the cortex, no attempt was made to correct for any potential distortion in the more lateral sites. The cortex was not systematically explored less than 1 mm lateral from the midline. In Control hemispheres, the cortex medial to the vibrissae representation was occupied by a small representation of eye, eye–eyelid movements (Hall & Lindholm, 1974; Donoghue & Wise, 1982; Toldi et al., 1996; Guandalini, 1998) and a thin strip of cortex where ICMS evoked miosis (Gioanni & Lamarche, 1985; Guandalini, 2001, 2003). In any case, eye, eye–eyelid, pupilar movements and unresponsive points formed the basis for delineating the medial border of the vibrissae representation.
To quantitatively assess the difference in the movement representation between the Adult and Newborn groups, we compared the percentage of unresponsive and movement sites, expressed as a fraction of the number of sites from 1 to 3 mm from the midline (Fig. 5). This cut-off was applied because 3 mm from the midline corresponded to the most lateral significant point of vibrissae site distribution in the plot of the medial–lateral (ML) movement frequency distribution in Control hemispheres of Adult as well as Newborn groups (see Fig. 6C). To avoid interindividual biases related to differences in the motor map size and ML position, the unresponsive points situated around the map were not taken into account in the plotting frequency histograms (Fig. 5).
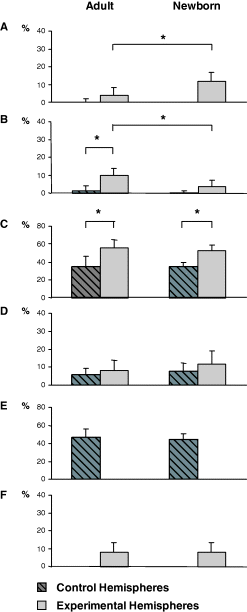
Comparison between the percentages of effective sites devoted to various movements represented in M1 (up to 3 mm from the midline) for Adult and Newborn groups of rats. (A) Eye; (B) neck; (C) forelimb; (D) miosis; (E) contralateral vibrissae; (F) ipsilateral vibrissae. Data are mean + SD. *Statistically significant differences within and between the Adult and Newborn groups of hemispheres using the posthoc Scheffé test (P < 0.05). In comparison to Adult Experimental hemispheres, Newborn Experimental hemispheres showed a significant increase in the percentage of eye sites (A) and a significant decrease in the percentage of neck sites (B). In contrast, this comparison shows no significant difference in the percentage of forelimb (C), miosis (D) and ipsilateral vibrissae sites (F). The percentage of hindlimb movement sites is not shown in the graph because this movement was not extensively explored.
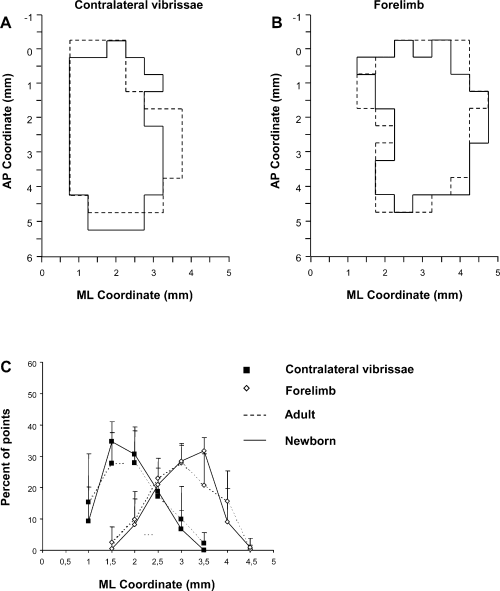
Overview of the size, location and topographic overlapping of the vibrissae (A) and forelimb (B) representation in Control hemispheres of Adult (dashed line) and Newborn (unbroken line) groups of rats. These are schematic composite maps overlaying the vibrissa and forelimb representation of each Control hemisphere of the Adult and Newborn rats. Note that the location of the vibrissae and forelimb representations overlaps in similar cortical territory in both Adult and Newborn Control hemispheres. (C) Medial–lateral frequency distribution of penetrations eliciting vibrissa (▪) and forelimb (◊) movements in Adult (dashed line) and Newborn (unbroken line) Control hemispheres. For each hemisphere, penetrations were distributed into 0.5-mm bins extending from the midline (0 mm) to 4.5 mm lateral of the midline, irrespective of the antero-posterior coordinate. The numbers of penetrations falling into each bin were tailed and expressed as a percentage of the total number of penetrations for that movement (+ SD). Note that there is no significant difference in distribution between groups (P > 0.05, χ2 test) and between each bin of distributions (P > 0.05, Scheffé test). ML, medial–lateral; AP, antero-posterior.
To visualize differences in the shaping and localization of representational movements between the Adult and Newborn group hemispheres, cumulative maps were constructed for each group by overlapping and plotting the size and location of the individual movement representations for all Control and Experimental hemispheres in each group (6, 7).
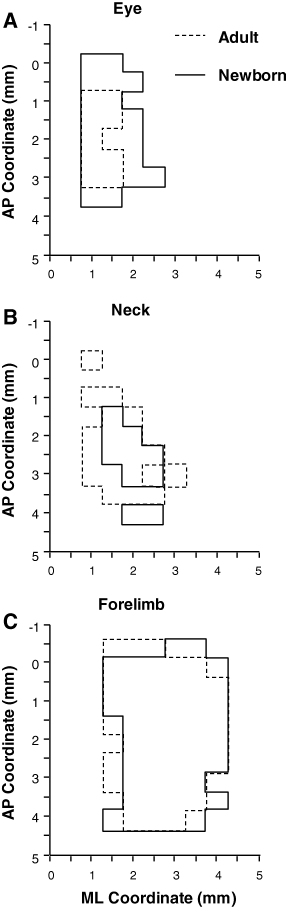
Overview of the size, location and topographic overlapping of the eye (A), neck (B) and forelimb (C) representation in Experimental hemispheres of Adult (dashed line) and Newborn (unbroken line) groups of rats. These are schematic composite maps overlaying the eye, neck and forelimb representations of each Experimental hemisphere of the Adult and Newborn rats. Note that the Newborn cumulative eye map encompasses the Adult eye cumulative map in the antero-posterior and lateral cortical coordinates and the Newborn neck cumulative map overlaps the lateral part of the Adult neck cumulative map. In contrast, the location of the cumulative forelimb map overlaps similar cortical territory in both Adult and Newborn Experimental hemispheres. ML, medial–lateral; AP, antero-posterior.
In order to demonstrate an overall ML movement reorganization across the cortex, in each map, the penetrations were divided into 0.5-mm-wide bins into which all sites eliciting movement were grouped, irrespective of their antero-posterior coordinates. For each bin, starting 1 mm from the midline and extending laterally 4.5 mm, the number of vibrissae, forelimb, neck and eye sites were tallied and converted to frequency by expressing the data as a percentage of the total number of movement sites (Fig. 8). To determine whether any changes in representational movements were related to changes in thresholds for evoking movement, the thresholds for each movement were determined for each group of hemispheres. Across-group comparisons were performed using one-way anova, Scheffé's post-hoc test and χ2 test. A probability value of less than 0.05 was considered statistically significant.
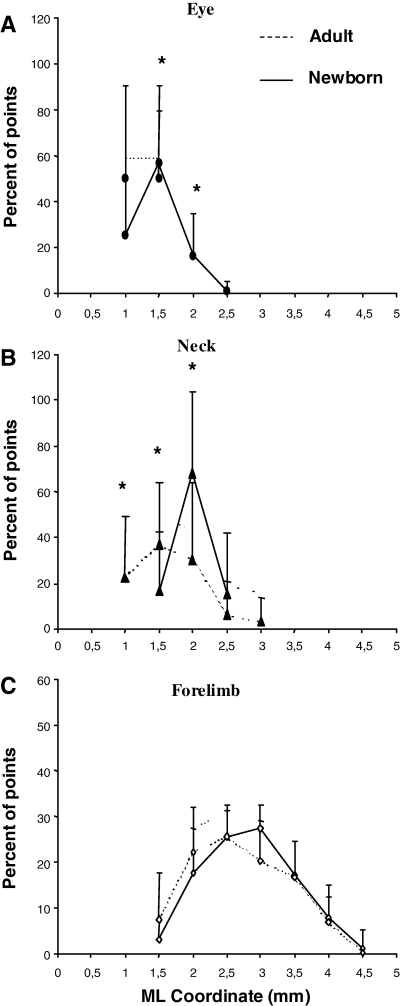
Comparison of medial–lateral frequency distribution of penetration eliciting eye (A), neck (B) and forelimb (C) movement between Adult and Newborn Experimental hemispheres. The medial–lateral movement distribution plot is explained in Fig. 6C. *Statistically significant differences between each bin of Adult compared with Newborn distribution using a posthoc Scheffé test (P < 0.05). Note that: (i) the distribution of eye sites shifted laterally in Newborn hemispheres compared with the distribution in the Adult hemispheres; (ii) the neck site distribution proved greater in the more lateral bins in Newborn hemispheres compared with the distribution in the Adult hemispheres and (iii) there is no difference in the forelimb site frequency distribution between Adult and Newborn Experimental hemispheres or between individual distribution bins. ML, medial–lateral.
Results
Neither the Adult nor the Newborn group of animals displayed natural whisking movement on the severed side as they explored freely in their cages. The paretic mystacial muscle pad proved atrophic compared with those on the control side. Histological examination of the severed facial nucleus showed the loss of a large number of motoneurones in the Adult group and loss of all motoneurones in the Newborn group (1, 2).
Motor representations in Control hemispheres
The mean number of penetrations performed in each hemisphere on this 500-µm sampling grid was 56.2 ± 4. In each rat, both hemispheres were studied and a threshold-evoked movement map was derived from each hemisphere (3, 4).
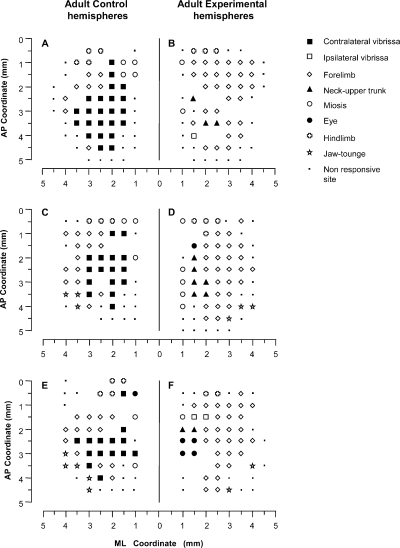
Examples of bilateral M1 mapping of movements evoked at threshold current levels in the Adult group of rats. The Control hemisphere (A, C and E) corresponds to the motor map of movements evoked in the contralateral, untouched side The Experimental hemisphere (B, D and F) corresponds to the motor map of movements evoked in the contralateral, severed side. The microelectrode was sequentially introduced at a depth of 1500 µm. Interpenetration distances were 500 µm. In these M1 mapping schemes, frontal poles are at the bottom. 0 corresponds to the bregma and numbers indicate the rostral or caudal distance from the bregma or lateral distance from the mid-line. The location of movements evoked at each point is indicated by a symbol (see the key), with the small spot indicating sites unresponsive at 60 µ A; where there is no symbol (within or at the border of the maps), penetration was prevented by a large blood vessel. Control hemispheres showed a large contralateral vibrissae movement representation; eye movement was shown in only one of three hemispheres (E). In all Experimental hemispheres there is evidence of persistent neck and ipsilateral vibrissae movement in the medial part of the former vibrissae representation. Note that the forelimb sites expand medially within the lateral part of the former vibrissae representation. Eye sites were shown in two of three hemispheres (D and F) at the medial border of the former vibrissae representation. ML, medial–lateral; AP, antero-posterior.
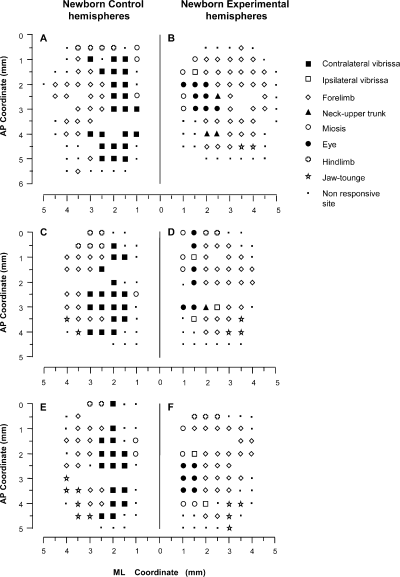
Examples of bilateral M1 mapping of movements evoked at threshold current levels in the Newborn group of rats. The location of movements evoked at each point is indicated by a symbol (see the key), with the small spot indicating sites unresponsive at 60 µ A; where there is no symbol (within or at the border of the maps), penetration was prevented by a large blood vessel. (A, C and E) Control hemisphere. (B, D and F) Experimental hemisphere. Note that, in Experimental hemispheres, the eye site representation clearly expands laterally within the medial part of the former vibrissae representation where neck sites were observed in Experimental hemispheres in the Adult group of rats. Also note that the forelimb expanded medially inside the former vibrissa representation. This expansion qualitatively overlaps the forelimb site expansion in Adult Experimental hemispheres (cf. Fig. 3A, C and E). ML, medial–lateral; AP, antero-posterior.
In the Control hemispheres from both the Adult and Newborn groups, the general features of M1 size, shape and location conformed to previous descriptions of the rat M1 (see 3, 4; for review, see Wise & Donoghue, 1986). As shown in 3, 4, most of the M1 map was occupied by areas from which vibrissae and forelimb movements can be evoked at a threshold current. Both the vibrissae and forelimb representations were antero-posteriorly elongated strips that shared an undulating border with the vibrissae representation medially situated and the forelimb representation laterally situated. In Control group hemispheres, the electrical stimulation of the vibrissae and forelimb representations at a threshold current evoked contralateral movement in all sites. The vibrissae representation was bordered caudally by the hindlimb representation. The jaw and tongue movement representations were rostro-lateral to the vibrissae and forelimb representations. In most Control hemispheres there was evidence of the rostral forelimb representation (Neafsey & Sievert, 1982; see 3, 4). Neck movements were only occasionally observed in sites where contralateral vibrissae movement was evoked at the same threshold current (Adult group, four sites out of two hemispheres, 1.4% of total sites; Newborn group, one site in one hemisphere, 0.45% of total sites; see histograms in Fig. 5B). In the strip of frontal cortex situated medially to the vibrissae representation, miosis, and occasionally eye movement, was induced under the present stimulation condition (Hall & Lindholm, 1974; Donoghue & Wise, 1982; Gioanni & Lamarche, 1985; Guandalini, 2001). Mapping on this 500-µm sampling grain, eye movement was threshold-evoked movement only at one point in two of the Adult animals (one illustrated in Fig. 3E; 0.5% of total sites in histograms in Fig. 5A). Non-responsive points formed the basis for delineating the rostral M1 border.
In spite of interindividual variability, qualitative comparison of Adult and Newborn Control hemispheres showed similar shape and size patterns in all maps of the vibrissae and forelimb representations (3, 4, 6).
The statistical comparison of Adult and Newborn Control hemispheres showed no significant difference in the percentage of movement sites (Fig. 5A–E; P > 0.05, Scheffé test) or in the ML frequency distributions of the contralateral vibrissae and forelimb sites (Fig. 6C; vibrissae, 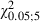 = 9.0; forelimb,
= 9.0; forelimb, 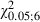 = 8.6; P > 0.05, χ2 test). Similarly, no significant differences in evoked movement thresholds were found between Adult and Newborn Control hemispheres (P > 0.05, Scheffé test).
= 8.6; P > 0.05, χ2 test). Similarly, no significant differences in evoked movement thresholds were found between Adult and Newborn Control hemispheres (P > 0.05, Scheffé test).
Motor representations in Adult Experimental hemispheres
In Adult Experimental hemispheres, movement reorganization clearly emerged in the cortical area corresponding to the vibrissae representation in Adult Control hemispheres (Fig. 3B, D and F). Qualitative and quantitative analysis showed that the pattern of movement reorganization conformed to previous descriptions of long-term M1 reorganization after severing the facial nerve in adult rat (Sanes et al., 1990; Franchi, 2000b). In Adult Experimental hemispheres, the cortical region corresponding to the vibrissae representation showed four noteworthy types of alterations.
- (i)
Increase in eye movement sites (mean sites per hemisphere in Control vs. Experimental, 0.3 ± 0.8 vs. 1.5 ± 1.5) and in sites where ICMS evoked miosis (mean sites per hemisphere in Control vs. Experimental, 2.5 ± 0.9 vs. 3.1 ± 2.3). In comparison to Control hemispheres, the percentage increase of these sites was not significant (
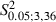 < 8.76, P > 0.05, Scheffé test; Fig. 5A and D). Eye sites were observed in two of 10 Control hemispheres (one illustrated in Fig. 3E) and in seven of 10 Experimental hemispheres whereas miosis sites were seen in all Control and Experimental hemispheres. As shown in Figs 3B, D and F, and 7A, all of these sites were localized at 1 or 1.5 mm in the ML coordinate, corresponding to the medial border of the normal vibrissae representation.
< 8.76, P > 0.05, Scheffé test; Fig. 5A and D). Eye sites were observed in two of 10 Control hemispheres (one illustrated in Fig. 3E) and in seven of 10 Experimental hemispheres whereas miosis sites were seen in all Control and Experimental hemispheres. As shown in Figs 3B, D and F, and 7A, all of these sites were localized at 1 or 1.5 mm in the ML coordinate, corresponding to the medial border of the normal vibrissae representation. - (ii)
Increase in the percentage of sites showing neck movement at the threshold stimulation current (mean sites per hemisphere in Control vs. Experimental, 0.7 ± 1.1 vs. 3.6 ± 1.3). In comparison to Control hemispheres, the percentage increase of the neck sites was significant (Fig. 5B, Control vs. Experimental, 1.4 ± 2.7 vs. 9.8 ± 3.9% of total sites;
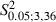 = 40.3, P < 0.05, Scheffé test). Neck sites consisted of contiguous or separate foci in the region nearly corresponding to the medial part of the normal vibrissae representation (3, 7).
= 40.3, P < 0.05, Scheffé test). Neck sites consisted of contiguous or separate foci in the region nearly corresponding to the medial part of the normal vibrissae representation (3, 7). - (iii)
Sites where ICMS evoked ipsilateral vibrissae movement (mean sites in Experimental hemispheres, 2.9 ± 1.8; 8.1 ± 5.2% of total sites). Ipsilateral vibrissae sites were localized near or intermingled with neck sites and were never observed in corresponding Control hemispheres (3, 5).
- (iv)
Increased forelimb sites occupying regions in the lateral part of the normal vibrissae representation (Fig. 3B, D and F; mean sites per hemisphere in Control vs. Experimental, 13.0 ± 4.5 vs. 20.8 ± 4.4). The percentage of forelimb sites was significantly greater than obtained from the Control hemispheres (Fig. 5C; Control vs. Experimental, 33.5 ± 8.8% vs. 55.8 ± 8.5% of total sites;
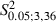 = 21.4, P < 0.05, Scheffé test). The ML distribution of the forelimb sites shifted medially compared with the distribution in the Control hemispheres (Control vs. Experimental hemispheres,
= 21.4, P < 0.05, Scheffé test). The ML distribution of the forelimb sites shifted medially compared with the distribution in the Control hemispheres (Control vs. Experimental hemispheres, 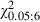 = 15.6, P < 0.05, χ2 test) and the percentage of penetrations yielding forelimb movement significantly increased at 2 and 2.5 mm in the ML coordinate (Control vs. Experimental hemispheres,
= 15.6, P < 0.05, χ2 test) and the percentage of penetrations yielding forelimb movement significantly increased at 2 and 2.5 mm in the ML coordinate (Control vs. Experimental hemispheres, 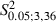 = 19.1 and 13.5 at 2 and 2.5 mm in the ML coordinate, P < 0.05 and
= 19.1 and 13.5 at 2 and 2.5 mm in the ML coordinate, P < 0.05 and 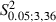 < 8.76, P > 0.05 for other bins in the ML coordinate, Scheffé test).
< 8.76, P > 0.05 for other bins in the ML coordinate, Scheffé test).
Comparison between Adult and Newborn Experimental hemispheres
To show whether severing the facial nerve in the developing brain would give a different pattern of M1 reorganization than the same lesion in the mature brain, a qualitative and quantitative comparison was made of the Adult and Newborn Experimental hemispheres.
The first notable feature was the increase in the number of eye movement sites in all Newborn Experimental hemispheres where the mean value was 4.5 ± 2 eye sites per hemisphere. One of the most obvious examples of this can be seen in Fig. 4B where the stimulation at eight cortical sites evoked eye movements. In contrast, in the corresponding cortical area of the Control hemisphere (Fig. 4A), eye movement was not evoked by stimulation under similar conditions and, in the Adult Experimental hemispheres, a maximum of four cortical eye sites were observed in one hemisphere (Fig. 3F). In all Newborn Experimental hemispheres, the eye sites were localized in the medial part of the corresponding vibrissae representation. In some cases, the eye sites occupied a cortical strip that expanded more into the lateral portion of the former vibrissae representation than into the antero-posterior portion (Fig. 4B) while, in other cases, the eye sites were located in a thin, elongated antero-posterior strip of cortex corresponding to the most medial part of the former vibrissae representation (Fig. 4D). All of these aspects of the eye maps were clearly seen in the cumulative eye sites map showing the cortical area from which eye movement could be evoked from Newborn Experimental hemispheres (Fig. 7A). It is evident that, in newborns, the cumulative eye map encompassed the antero-posterior and lateral cortical positions of the Adult cumulative eye map. In comparison to the Adult, in Newborn Experimental hemispheres the percentage of eye sites was significantly increased (Adult vs. Newborn, 4.1 ± 4.1% vs. 11.8 ± 5.2% of total sites; 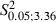 = 27.6, P < 0.05, Scheffé test; Fig. 5A). The ML distribution of the eye sites shifted laterally compared with the distribution in the Adult Experimental hemispheres (Fig. 8A) but the difference between the two distributions was not statistically significant (Adult vs. Newborn Experimental hemispheres,
= 27.6, P < 0.05, Scheffé test; Fig. 5A). The ML distribution of the eye sites shifted laterally compared with the distribution in the Adult Experimental hemispheres (Fig. 8A) but the difference between the two distributions was not statistically significant (Adult vs. Newborn Experimental hemispheres, 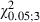 = 4.8, P > 0.05, χ2 test). Nevertheless, penetrations yielding eye movement significantly increased at 1.5 and 2 mm in the ML coordinate (Adult vs. Newborn Experimental hemispheres,
= 4.8, P > 0.05, χ2 test). Nevertheless, penetrations yielding eye movement significantly increased at 1.5 and 2 mm in the ML coordinate (Adult vs. Newborn Experimental hemispheres, 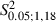 = 10.5 and 6.7 at 1.5 and 2 mm in the ML coordinate, P < 0.05 and
= 10.5 and 6.7 at 1.5 and 2 mm in the ML coordinate, P < 0.05 and 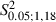 < 4.41, P > 0.05 for other bins in the ML coordinate, Scheffé test; Fig. 8A).
< 4.41, P > 0.05 for other bins in the ML coordinate, Scheffé test; Fig. 8A).
In comparison to Adult Experimental hemispheres, the neck sites significantly decreased in Newborn Experimental hemispheres (Fig. 5B; Adult vs. Newborn, 3.6 ± 1.3 vs. 1.4 ± 1.3; 9.8 ± 4%vs. 3.6 ± 3.3% of total sites; 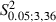 = 20.3, P < 0.05, Scheffé test) and showed an insignificant increase compared with the Newborn Control hemispheres (Fig. 5B; Control vs. Experimental Newborn,
= 20.3, P < 0.05, Scheffé test) and showed an insignificant increase compared with the Newborn Control hemispheres (Fig. 5B; Control vs. Experimental Newborn, 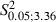 = 6.0, P > 0.05, Scheffé test). Neck sites consisted of contiguous or separate foci localized near the eye, ipsilateral vibrissae or forelimb sites. This aspect was also clearly evident in the plot of the ML distribution of neck sites where the value of the Newborn distribution was higher in the more lateral bins compared with the distribution in the Adult Experimental hemispheres (Fig. 8B). The difference between the two distributions in Fig. 8B was statistically significant (Adult vs. Newborn Experimental hemispheres,
= 6.0, P > 0.05, Scheffé test). Neck sites consisted of contiguous or separate foci localized near the eye, ipsilateral vibrissae or forelimb sites. This aspect was also clearly evident in the plot of the ML distribution of neck sites where the value of the Newborn distribution was higher in the more lateral bins compared with the distribution in the Adult Experimental hemispheres (Fig. 8B). The difference between the two distributions in Fig. 8B was statistically significant (Adult vs. Newborn Experimental hemispheres, 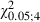 = 51.4, P < 0.05, χ2 test) and sites yielding neck movement were significantly different at 1, 1.5 and 2 mm in the ML coordinate (Fig. 8B; Adult vs. Newborn Experimental hemispheres,
= 51.4, P < 0.05, χ2 test) and sites yielding neck movement were significantly different at 1, 1.5 and 2 mm in the ML coordinate (Fig. 8B; Adult vs. Newborn Experimental hemispheres, 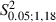 = 6.0, 5.9 and 12.8 at 1, 1.5 and 2 mm in the ML coordinate, P < 0.05 and
= 6.0, 5.9 and 12.8 at 1, 1.5 and 2 mm in the ML coordinate, P < 0.05 and 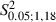 < 4.41, P > 0.05 for 2.5 and 3 mm in the ML coordinate, Scheffé test). In other words, in Newborn Experimental hemispheres, neck sites emerged in the cortical region encompassed by eye sites medially and forelimb sites laterally.
< 4.41, P > 0.05 for 2.5 and 3 mm in the ML coordinate, Scheffé test). In other words, in Newborn Experimental hemispheres, neck sites emerged in the cortical region encompassed by eye sites medially and forelimb sites laterally.
Comparison between Adult and Newborn Experimental hemispheres showed no significant difference in either the sites that evoked forelimb movement (Fig. 5C; mean sites in Adult vs. Newborn Experimental hemispheres, 20.8 ± 4.4 vs. 20.3 ± 3.6; 55.8 ± 8.5% vs. 52.6 ± 6.3% of total sites; 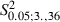 = 0.09, P > 0.05, Scheffé test) or the frequency distribution for ML forelimb sites (Fig. 8C; Adult vs. Newborn Experimental hemispheres,
= 0.09, P > 0.05, Scheffé test) or the frequency distribution for ML forelimb sites (Fig. 8C; Adult vs. Newborn Experimental hemispheres, 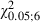 = 0.18; P > 0.05, χ2 test). Differences in sites yielding forelimb movement were not significant in all bins in the ML coordinate (Fig. 8C; Adult vs. Newborn Experimental hemispheres,
= 0.18; P > 0.05, χ2 test). Differences in sites yielding forelimb movement were not significant in all bins in the ML coordinate (Fig. 8C; Adult vs. Newborn Experimental hemispheres, 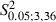 < 8.76 in all ML bins, P > 0.05, Scheffé test). This aspect was also evident in the cumulative map where forelimb sites overlapped the same cortical region in Adult and Newborn Experimental hemispheres (Fig. 7C). Thus, there is evidence that, in Adult and Newborn Experimental hemispheres, forelimb sites similarly occupied regions corresponding to the lateral part of the normal vibrissae representation.
< 8.76 in all ML bins, P > 0.05, Scheffé test). This aspect was also evident in the cumulative map where forelimb sites overlapped the same cortical region in Adult and Newborn Experimental hemispheres (Fig. 7C). Thus, there is evidence that, in Adult and Newborn Experimental hemispheres, forelimb sites similarly occupied regions corresponding to the lateral part of the normal vibrissae representation.
There was no significant difference between Adult and Newborn Experimental hemispheres in regard to the sites where ICMS evoked miosis (Fig. 5D; mean sites in Adult vs. Newborn, 3.1 ± 2.3 vs. 5.1 ± 2.4; 8.2 ± 5.5% vs. 12.1 ± 7.3% of total sites; 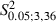 = 2.6, P > 0.05, Scheffé test). In all Newborn Experimental hemispheres, miosis sites were localized antero-posterior or medially to the eye sites (Fig. 4B, D and F).
= 2.6, P > 0.05, Scheffé test). In all Newborn Experimental hemispheres, miosis sites were localized antero-posterior or medially to the eye sites (Fig. 4B, D and F).
Furthermore, there was no significant difference between Adult and Newborn Experimental hemispheres in regard to the sites where ICMS evoked ipsilateral vibrissae movement (Fig. 5F; Adult vs. Newborn Experimental hemispheres, 2.9 ± 1.7 vs. 3.1 ± 2.7; 8.1 ± 5.2% vs. 7.8 ± 5.8% of total sites; 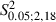 = 0.08, P > 0.05, Scheffé test).
= 0.08, P > 0.05, Scheffé test).
No significant differences in evoked movement thresholds were found in across-group comparisons for all types of movement (P > 0.05, Scheffé test). Thus, in the present experiments there was no evidence of differences in excitability between Adult and Newborn Experimental hemispheres.
Discussion
The primary finding of the present study was that, under identical manipulation in newborn and adult rats, eye movement expands into the medial part of the vibrissae representation more in the newborn-induced map reorganization than it does in the adult-induced map reorganization. In the newborn-induced map reorganization, eye movement expands within the cortical region where neck movement expands in the adult-induced map reorganization. In contrast, in both newborn and adult-induced map reorganization, forelimb movement similarly expands and overlaps the same cortical region corresponding to the lateral part of the vibrissae representation.
Enlargement of the eye representation in Newborn Experimental hemispheres
It is first worth pointing out the possible source of variability that may have contributed to the differences in motor map organization in the Adult and Newborn groups of rats. The fact that there were no statistical differences between Control hemispheres ensures that the experimental source of variability is minor and inconsistent between Adult and Newborn rats. Indeed, in all Control hemispheres, movement representations and the threshold currents required to evoke these movements were substantially consistent with those of previously published M1 maps (Donoghue & Wise, 1982; Gioanni & Lamarche, 1985; Neafsey et al., 1986; Sanes et al., 1990; Franchi, 2000a,b). In cortical exploration, the 500-µm step used in the present experiment is suitable for exploring large representations. The small eye representation, localized at the most medial coordinate of the vibrissae representation (Guandalini, 1998), may go undetected in all hemispheres. This aspect explains the absence of eye representations in the maps of a large number of Control hemispheres.
In previous experiments, the M1 reorganization in the adult rat was examined after facial motor nerve lesion, with rats surviving for hours to months after facial nerve surgical transection (Sanes et al., 1988, 1990; Toldi et al., 1996; Franchi, 2000b). These experiments showed that 4 weeks of recovery provides ample time for a long-term modulatory influence on M1 reorganization. In this light, 4 weeks latency after severing the facial nerve is an adequate period of time to stabilize motor cortex reorganization in the present form of plasticity. Threshold data also support this conclusion as the thresholds required to evoke movements in the Adult Experimental hemispheres were similar to those needed to evoke movements in the Newborn Experimental hemispheres. In the present experiment, long-term M1 reorganization after facial nerve severing in the adult rat was consistent with previous observations (Sanes et al., 1990; Franchi, 2000b). In adult animals, the long-term alterations in M1 representation were the larger medial extension of the forelimb representation and a modest lateral expansion of the eye representation. In the intermingled cortical region, localized between these representations, neck and ipsilateral vibrissae were commonly the threshold movement evoked by electrical stimulation.
The long-term M1 reorganization after facial nerve severing early in life led to a significantly larger eye movement representation in the newborn animals studied than the same lesion in the adult rat. On average, in the Newborn Experimental hemispheres the eye movement develops a representation that is more than twice the size of that in the Adult Experimental hemispheres. The abnormally large eye representation is located in the medial part of M1, the part normally occupied by the vibrissae representation in Control hemispheres. The larger expansion of the eye movement representation in the Newborn Experimental hemispheres is further supported by the finding that eye movements were obtained at the M1 coordinate where eye movements were never found in the Adult Experimental hemispheres. It seems clear that the physical spread of stimulus current cannot adequately account for this difference between Adult and Newborn Experimental hemispheres. Thus, we conclude that the relationship between the cortical eye movement topography and age at facial nerve severing is causal. The fact that supra-threshold stimulation current never elicited eye movement in the corresponding sites of the Control hemispheres indicates that the newly expanded eye representation in the Newborn Experimental hemispheres is not produced by uncovering a pre-existing high-threshold representation (Schroeder et al., 1995; Coq & Xerri, 1999; Qi et al., 2000). The observation that the Newborn Experimental hemispheres show a significantly lower percentage of neck sites than the Adult Experimental hemispheres supports the conclusion that, in the Newborn Experimental hemispheres, the eye sites occupy a part of the cortical region where neck movement sites are represented in the Adult Experimental hemispheres. In both Adult and Newborn Experimental hemispheres the percentage difference in forelimb sites was not statistically significant and the expansion of the forelimb representation overlaps the same motor cortex topography in the lateral part of the normal vibrissae representation. This supports the conclusion that the differences in the adult-induced compared with newborn-induced map reorganization are limited in the region of the motor cortex where eye and neck movements emerge after facial nerve severing.
Possible mechanisms and significance
Previous studies have shown that adult rats fail to lose a significant number of motoneurones after peripheral nerve severing (Johnson & Duberley, 1998). In contrast, in newborn rats peripheral nerve injury leads to marked, rapid neuronal death (Li et al., 1998; Sendtner et al., 2000). More than 80% of the axotomized facial motoneurones are rapidly lost when axotomy is performed at birth (Sendtner et al., 1990). In the present experiment, all facial motoneurones were lost in the adult rat when the facial nerve was severed at birth and axonal regeneration was prevented. This effect can result in reorganization of the premotor neurones (Hattox et al., 2003) as well as all cortical and subcortical structures related to motor control of whisking behaviour (Hattox et al., 2002). Facial nerve severing abolished whisker movements without affecting the vibrissae sensory innervation and the barrel field in the primary somatosensory cortex (Rice, 1984). However, abnormal vibrissae muscular control early in life persistently alters active touch during adulthood (Carvel & Simons, 1996; Nicolelis et al., 1996). In this light, newborn rats have normal sensory innervation and normal cytoarchitecture of the barrel cortex but abnormal space recognition on the severed side. The present study provides evidence that facial nerve severing early in life changes the relative size and position of representations within the preserved overall position of the motor cortical area.
It is a commonly expressed view that, in adult animals, changes in the size of motor representations reflect effects that mainly occur at the cortical level, whereas changes in excitability may reflect effects at any level of the motor pathway (Sanes et al., 1990; Hamdy et al., 1998). The fact that different, age-related effects were found in adult compared with newborn motor representations indicates that different mechanisms are probably involved. The adult lesions probably cause changes in the efficacy of synaptic interaction (Jacobs & Donoghue, 1991; Hess & Donoghue, 1994, 1996), unmasking of existing intracortical connections (Calford & Tweedale, 1988; Kimberle & Donoghue, 1991) and forming of new synapses (Keller et al., 1992). The neonatal lesions probably also maintain transient juvenile connections although other mechanisms cannot be excluded (Murakami et al., 1992; Innocenti, 1995; Antonini et al., 1999; Restrepo et al., 2003). The enlarged cortical eye movement representation could reflect not only modifications in local cortical circuits but also changes in the subcortical structures that all cooperated to produce the eye and whisker movements (Guandalini, 1998, 2001; Hattox et al., 2002).
Many fundamental questions about the reorganization of motor maps following peripheral damage in both the adult and developing brain remain unanswered. The precise factors that guide the reorganization in adult and newborn rats are not immediately clear and, in particular, what factor induces the larger expansion in the eye representation in the developing cortex. Moreover, the significance of such reorganization, and specifically the significance of the enlarged eye movement representation in Newborn Experimental hemispheres, is not clear. Many studies have revealed a relationship between motor cortical topography and motor behaviour (Nudo et al., 1996; Kleim et al., 1998, 2002; Remple et al., 2001). It is possible that differences in cortical topography could be related to differences in behavioural experience induced by time-difference facial nerve severing. According to this use-dependent view, the difference in map reorganization between adult and newborn rats could be strictly linked to differences in behavioural asymmetries in exploring peripersonal space (Milani et al., 1989; Symonds & Tees, 1990). Alternatively, the effect of early sensory experience on the developing motor cortex and on its neural substrates could explain the differences in the M1 reorganization of adult and newborn rats (Keller et al., 1996; Huntley, 1997).
In this light, further experiments are needed to provide an understanding of the anatomical substrate and the functional significance of these plastic changes in the adaptive mechanisms in the developing motor system.
Acknowledgements
The authors thank Dr C. Lucchetti for revision of the manuscript and Ing. E. Lodi who wrote the software for the on-line mapping procedures. We thank Dr V. Muzzioli for his assistance with preparation of the figures. We are also grateful to S. Zanellati for her assistance during histological procedures. This study was funded by a grant from Università degli Studi di Ferrara.




