Selective intermediate-/small-conductance calcium-activated potassium channel (KCNN4) blockers are potent and effective therapeutics in experimental brain oedema and traumatic brain injury caused by acute subdural haematoma
Abstract
Early deterioration and death after brain injury is often the result of oedema in the injured and peri-lesional tissue. So far, no pharmacotherapy is available that exhibits significant brain oedema-reducing efficacy in patients. We selected two low molecular weight compounds from different chemical classes, a triazole (1-[(2-chlorophenyl)diphenylmethyl]-1,2,3-triazole) and a cyclohexadiene (methyl 4-[4-chloro-3-(trifluoromethyl)phenyl]-6-methyl-3-oxo-1,4,7-tetrahydroisobenzofuran-5-carboxylate) to characterize their pharmacological properties on KCNN4 channels (intermediate/small conductance calcium-activated potassium channel, subfamily N, member 4) in vitro as well as in vivo. In vitro we replaced potassium by rubidium (Rb+) and determined Rb+ fluxes evoked by 10 µm of the calcium ionophore A23187 on C6BU1 rat glioma cells. Compared with known KCNN4 blockers, such as clotrimazole (IC50 = 360 ± 12 nm) and charybdotoxin (IC50 = 3.3 ± 1.9 nm), the triazole and cyclohexadiene were considerably more potent than clotrimazole and displayed similar potencies (IC50 = 12.1 ± 8.8 and 13.3 ± 4.7 nm, respectively). In the rat acute subdural haematoma model, both the triazole and cyclohexadiene displayed reduction of brain water content (−26% at 0.3 mg/kg and −24% at 0.01 mg/kg) and reduction of the intracranial pressure (−46% at 0.1 mg/kg and −60% at 0.003 mg/kg) after 24 h when administered as a 4-h infusion immediately after brain injury. When infarct volumes were determined after 7 days, the triazole as well as the cyclohexadiene displayed strong neuroprotective efficacy (−52% infarct volume reduction at 1.2 mg/kg and −43% at 0.04 mg/kg, respectively). It is concluded that blockade of KCNN4 channels is a new pharmacological approach to attenuate acute brain damage caused by traumatic brain injury.
Abbreviations
-
- BBB
-
- blood–brain barrier
-
- BKCa
-
- large-conductance calcium-sensitive potassium channel
-
- DHP
-
- dihydropyridine
-
- DMEM
-
- Dulbecco's modified Eagle's medium
-
- d.w.
-
- dry weight
-
- FCS
-
- foetal calve serum
-
- HEPES
-
- N-[2-hydroxyethyl]piperazine-N′-[2-ethanesulphonic acid]
-
- IKCa
-
- intermediate-conductance calcium-sensitive potassium channel
-
- KCa
-
- calcium-sensitive potassium channels
-
- KCNN4
-
- potassium intermediate/small-conductance calcium-activated channel, subfamily N, member 4
-
- ICP
-
- intracranial pressure
-
- RT-PCR
-
- reverse transcriptase-polymerase chain reaction
-
- SDH
-
- acute subdural haematoma
-
- TBI
-
- traumatic brain injury
-
- SKCa
-
- small-conductance calcium-sensitive potassium channel
-
- w.w.
-
- wet weight
Introduction
Traumatic brain injury (TBI) and stroke are major causes of mortality and severe disability. As TBI affects mainly young people, it constitutes a major health and socioeconomic problem. Poor neurological outcome is only partly explained by the volume and localization of cerebral tissue that is damaged, but early deterioration and death are often the result of oedema in the infarcted and peri-lesional tissue (Hacke et al., 1996; Xiao, 2002). Although large efforts were made, no pharmacological approach showed significant brain oedema-lowering efficacy in patients (Morley et al., 2004) so far.
Potassium channels play a critical role in a wide variety of physiological processes (Wickenden, 2002). Based on the conductance, calcium-sensitive potassium channels (KCa) can be divided into three major groups: (i) large-conductance (> 200 pS; BKCa); (ii) small-conductance (5–20 pS; SKCa); and (iii) intermediate-conductance (20–40 pS; IKCa) channels.
The first description of a IKCa was provided by Gardos (1958). Compared with SKCa channels, IKCa channels have a distinct pharmacology, being insensitive to apamin, almost insensitive to iberiotoxin, but blocked by charybdotoxin, clotrimazole, some dihydropyridines and 4-phenyl-4H-pyrans (Alvarez et al., 1992; Ellory et al., 1994; Ishii et al., 1997; Rittenhouse et al., 1997; Urbahns et al., 2003). The potassium intermediate/small conductance calcium-activated potassium channel (subfamily N, member 4, KCNN4) was first cloned in 1997 (Logsdon et al.; 1997), and based on the expression pattern, physiological properties and low homology to other SKCa channel proteins, Joiner and co-workers proposed that this channel belongs to a new subfamily of SKCa channels (Joiner et al., 1997). IK1, IKCA1, KCA4, KCa3.1, SK4, hIKCa1, hKCa4, hSK4 and Gardos channel are synonyms. In the present study, we use the term KCNN4 according to the human genome organization.
Although KCNN4 channels are expressed in a wide variety of cell types (Schwab, 1998), the physiological roles of IKCa channels are not exactly understood. Evidence emerged that these channels play a role in a variety of physiological processes, such as migration (Schwab et al., 1999), immune cell activation (Logsdon et al., 1997), mitosis (Kayser et al., 1998), erythrocyte volume regulation (Brugnara et al., 1996) and a beneficial role on the respiratory burst of microglia when stimulated with phorbol 12-myristate 13-acetate (Khanna et al., 2001). The latter finding suggests an involvement of KCNN4 channels in the pathogenesis of brain damage, which was proven very recently when a 4-phenyl-4H-pyran was characterized as a potent KCNN4 blocker with neuroprotective properties in a rat model of TBI (Urbahns et al., 2003).
The aim of the present study was to characterize two prototypes of newly identified KCNN4 blocker and to investigate their therapeutical potential against acute brain oedema and brain tissue damage in a rat model of acute brain injury.
Materials and methods
Chemicals and reagents
All chemicals were of the highest commercially available purity and were, if not otherwise indicated, purchased from Merck KGaA, Darmstadt, Germany. The triazole (1-[(2-chlorophenyl)(diphenylmethyl)]-1H-1,2,3-triazole), the cyclohexadiene (methyl 4-[4-chloro-3-(trifluoromethyl)phenyl]-6-methyl-3-oxo-1,3,4,7-tetrahydro-2-benzofuran-5-carboxylate) and clotrimazole (1-[(2-chlorophenyl)diphenylmethyl]-1H-imidazole) were synthesized by the Department of Chemical Research, Bayer Health Care (Wuppertal, Germany), A23187 (5-(methylamino)-2-[(2R,3R,6S,8S,9R,11R)-3,9,11-trimethyl-8-[1S-1-methyl-2-oxo-2-(1H-pyrrol-2-yl)-ethyl]-1,7-dioxaspiro[5.5]undec-2-yl]methyl-4-benzoxazolecarboxylic acid) was obtained from Sigma (Sigma-Aldrich Chemie GmbH, Steinheim, Germany). Reference compounds used for characterization of the C6BU1 cell line are summarized in Table 1.
| Compound | Block of | Compound | Block of |
|---|---|---|---|
| Agitoxin-2* | Some voltage-gated K+ channels | Margatoxin* | Voltage-gated (Kv1.3 clone) K+ channels |
| Apamin2 | Apamin-sensitive SKCa | Nicardipine2 | Ca2+ channel blocker, L-type |
| Bumetanide2 | Na+–K+–Cl– co-transporter inhibitor | Nifedipine3 | Ca2+ channel blocker, L-type |
| Charybdotoxin3 | Non-specific blocker of KCNN4 | Nimodipine3 | Ca2+ channel blocker, L-type |
| Chlorotoxin* | Blocks Cl– channels expressed in gliomas | Nitrendipine3 | Ca2+ channel blocker, L-type |
| Clotrimazole3 | Blocks Ca2+-activated K+ channels, antifungal | Noxiustoxin* | Some Ca2+-activated and voltage-gated K+ channels |
| Dendrotoxin2 | Some voltage-gated K+ channels | Ouabain2 | Na+–K+ ATPases blocker |
| Iberiotoxin2 | Selective for BKCa | Tetrodotoxin2 | Sodium channels |
| Kaliotoxin* | Some Ca2+-activated and voltage-gated K+ channels |
- Indices refer to the source from where the compounds were obtained. *Alamone Laboratories (Jerusalem, Israel); 2Sigma-Aldrich GmbH (Deisenhofen, Germany); 3Bayer AG (Leverkusen, Germany).
Rubidium efflux on C6BU1 rat glioma cells
For the pharmacological characterization of the compounds in vitro, rat C6BU1 glioma cells [No ACC 108, Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (DSMZ), Braunschweig, Germany] were cultivated in 48-well plates in Dulbecco's modified Eagle's medium (DMEM)/10% foetal calf serum (FCS) for 3 days. Cells were washed with wash-buffer containing (in mm): N-[2-hydroxyethyl]piperazine-N′-[2-ethanesulphonic acid] (HEPES), 20; NaCl, 150; NaH2PO4, 0.84; CaCl2, 1.8; glucose, 5.5; MgCl2, 0.8; pH 7.4; and loaded with rubidium (Rb+) for 2 h at 37 °C using Rb+-loading buffer (in mm: HEPES, 20; NaCl, 150; NaH2PO4, 0.84; CaCl2, 1.8; RbCl, 5.4; glucose, 5.5; MgCl2, 0.8; pH 7.4). Cells were washed three times with wash-buffer (see above) and incubated for 10 min with test compounds and 10 µm A23187, both diluted in wash-buffer. Supernatant of each well was rapidly collected and the probes were diluted 1 : 10 in 0.1% CsCl, 1% HCl. Rb+ content was determined using atomic absorption spectroscopy (ATIUnicam 939, Analytical Technology, Monrovia, CA, USA). Rb+ concentrations of the samples were normalized by using a calibration curve, which was measured previous to each experiment. Basal Rb+ release was subtracted from A23187-evoked release, the obtained control values were set to 100% (maximal release) and compared with values obtained with test compounds.
Rubidium influx on human erythrocytes
Human erythrocytes were freshly prepared from the blood of female volunteers (blood group A Rh+). The erythrocytes were washed three times in buffer containing (in mm): HEPES, 20; NaCl, 150; glucose, 5.5; NaH2PO4, 0.8; KCl, 5.4; MgCl2, 0.8; CaCl2, 1.8; ouabain, 100; pH 7.4; and then resuspended in Rb+-loading buffer (wash-buffer, in which KCl was replaced by the same amount of RbCl), the final concentration of erythrocytes was 1%. Test compounds were added for 120 min and Rb+ influx was triggered by mobilization of intracellular Ca2+ stores by 10 µm A23187. Intracellular Rb+ was measured as described above after sedimentation and lysis of the erythrocytes.
Binding studies
Receptor and enzyme interaction screenings for selected compounds were performed at Panlabs (Taipei, Republic of China) and CEREP (Celle L'Evescault, France), as described in the respective assay protocols. The initial concentrations of test compounds were 10 µm.
Reverse transcriptase-polymerase chain reaction (RT-PCR) investigations
Total brain tissue from injured and non-injured rats was harvested and snap frozen with liquid nitrogen. Microglia were prepared according to standard protocols. In brief, cortical tissue of rat pups (days 1–2) was prepared and incubated in DMEM (10% inactivated FCS) for 11 days. Cells were than gently shaken for 1 h and the supernatant was centrifuged for 10 min at 200 g. The supernatant was discarded and the cell pellet was frozen in liquid nitrogen. For RNA isolation, tissues and cells were homogenized for 30 s on ice (Homogenisator Polyltron PT 1200, Kinematica, Switzerland). The RNA was isolated from the tissue using a RNA-Matrix method according to the manufacturer's protocol (Bio101TM, Qbiogene, Heidelberg, Germany). The quality of the total RNA was controlled on a 1% agarose gel, and 1 μg of total RNA was reverse transcribed using 400 U of Superscript II reverse transcriptase, Oligo dT Primer (0.5 μg) and 10 U RNAsin in 40 μL at 37 °C for 1 h (Invitrogen, Karlsruhe, Germany). β-Actin served as an intrinsic control for variations in cDNA amounts. The rat KCNN4 primers were designed according to the rat KCNN4 mRNA sequence (NM 023021) and were sense CAC-CCG-GGT-CGC-CTG-CTT-CT and antisense GCG-GCC-GAC-TCC-TTC-ATC-TCT-TTG-TTT, yielding a 341-bp product. Primers for rat β-actin were designed according to the mRNA sequence (J00691) and were sense CTA-TCG-GCA-ATG-AGC-GGT-TC and antisense CTT-AGG-AGT-TGG-GGG-TGG-CT, resulting in a 762-bp product. PCR reactions were performed in 25-μL volumes with a final concentration of 300 nmol for each primer, with an initial denaturing step of 95 °C for 4 min, then 95 °C for 30 s, 60 °C for 30 s and 72 °C for 30 s, for 40 cycles (β-actin 28 cycles), followed by a final step of 72 °C for 4 min.
Brain oedema and neuroprotection studies
Animals
Wistar rats (230–300 g, Harlan-Winkelmann, Borchen, Germany) were used for the acute subdural haematoma (SDH) experiments. The animals were allowed to adapt to housing conditions for at least 1 week before they were subjected to the study. They were housed in groups of three to five individuals in macrolon cages (type III, Ebeco, Castrop-Rauxel, Germany) bedded with sawdust. The animal housing room and the laboratory for surgery were climate controlled and continuously illuminated from 06.00 h until 18.00 h. Room temperature was about 21 °C, relative humidity was about 50%. Food (Altromin 1324, Altromin Spezialfutterwerk GmbH, Lange, Germany or R/M-H; V1534-00 DDb, Ssniff Spezialdiäten GmbH, Soest, Germany) and water were available ad libitum. On the day of surgery, the rats were randomly assigned to one of the treatment groups. For pharmacokinetic investigations, male Wistar rats (200 g, Harlan Winkelmann, Borchen, Germany) were used. All animals received solid feed (Altromin® 1324, Altrogge, Lage/Lippe, Germany) as restricted feeding (15 g per rat and day). Experimental protocols and conditions conformed with the German regulations on animal welfare.
SDH
The animals were anaesthetized with the inhalation anaesthetic isofluran (Forene®, Abbott GmbH, Wiesbaden, Germany or Isofluran-Baxter, Baxter Deutschland GmbH, Unterschleißheim, Germany mixed with ≅28% O2 in N2O to 1.5–5% v/v concentration). SDH was induced on the left hemisphere according to a standard surgical procedure (Miller et al., 1990), with the following minor modifications. The top of the head was shaved, the skin was disinfected and opened with a longitudinal midline cut. A small part of the periosteum was removed and a burr hole was drilled into the skull with the stereotaxic coordinates: −1 mm caudal, −2.8 mm lateral to the bregma (Paxinos & Watson, 1996). The dura was carefully opened, and a specially designed plastic cannula was inserted into the subdural space between the dorsal surface of the brain and the dura. Thereafter the cannula was fixed with a tissue glue (Histoacryl®, B. Braun Surgical GmbH, Melsungen, Germany). Non-heparinized autologous blood was collected by puncture of the tail vein and injected directly via the prefixed cannula into the subdural space (total volume of 0.2 mL within 4 min). After that, the probe was shortened and closed with cyanacrylate glue (Histoacryl®). The skin wound was closed with suture clips. During the surgery and the continuous i.v. infusion of the drug or vehicle, the body temperature was monitored and maintained within the physiological range (37.0 ± 0.5 °C) using a warming pad. After recovery from anaesthesia, the animals were returned to their home cage. With the exception of blood injection, animals belonging to the control group were passed through the same procedure.
Determination of intracranial pressure (ICP)
The method was slightly modified according to a published method (Zwienenberg et al., 1999; Mauler et al., 2003) and was performed by an operator blinded to the groups. The ICP measurements were conducted 24 h after surgery. ICP was assessed with a commercial available pressure transducer (Micro-Tip 2F SPR-612, Millar Instruments, TX, USA) with an external diameter of 0.44 mm. For the quantification of ICP, animals were anaesthetized with an i.p. injection of Ketavet (72 mg/kg ketamine HCl; Pharmacia & Upjohn) and Rompun (9.6 mg/kg xylacin; Bayer AG, Germany) solved in physiological salt solution (6 mL/kg). During ICP assessment, the body temperature was maintained at 37 °C with a warming pad. The plastic cannula, which was used for blood injection, was removed from the skull and the drill hole was cleaned from the tissue glue. With fine scissors the dura mater was carefully dissected and the pressure transducer was lowered into the drill hole using an automatic, microprocessor-controlled system (proprietary development). The tip of the pressure transducer was placed 2 mm into the brain parenchyma directly located under the blood clot. Due to the automated process, the procedure and the insertion speed was the same for all animals. After the sensor tip was lowered, the system was calibrated and the sensor was allowed to adapt to the pressure in the parenchyma for 10 min. After this period, the ICP was recorded for 30 min via a microprocessor-controlled amplifier, which was developed in cooperation with the Center for Sensor Systems (ZESS, University of Siegen, Siegen, Germany). After the measurement, the animal was decapitated and the brain was removed for determination of brain water content. If a gross-pathological assessment of the removed brain revealed that the blood clot was too small or if the blood clot drifted into the cistern, the animals (1–2%) were excluded from analysis by the operator. For comparison of individual experiments, ICP of treatment groups were expressed as a percentage of the respective controls, which were set to 100%.
Determination of brain water content
The wet weight/dry weight (w.w./d.w.) method was modified according to a published method (Xi et al., 2001; Mauler et al., 2003). For the experiments, rats were decapitated 24 h after surgery and the whole brain was dissected, the olfactory bulb and the cerebellum were removed, both hemispheres were separated and weighted (w.w.). After drying for 24 h at 115 °C, the hemispheres were weighted again (d.w.). The percentage water content of both hemispheres was calculated according to the formula [100 × (w.w. − d.w.)/w.w.] by an operator blinded to the groups. The difference in percentage water content between the two hemispheres (left − right) served as a parameter to determine the severity of the brain oedema. For comparison of individual experiments, brain water contents of treatment groups were expressed as a percentage of the respective controls, which were set to 100%.
Determination of infarct volume
Seven days after surgery the rats were decapitated, their brains were rapidly removed and frozen in 2-methylbutane cooled down to −30 °C on dry ice. Serial coronal sections (20 µm thick) were cut throughout the entire infarcted area with a standard distance of 500 μm using a cryostat microtome (Leica CM 3050, Leica Vertrieb GmbH, Bensheim, Germany and Microm HM 500 OM, Microm Laborgeräte GmbH, Walldorf, Germany). Slide-mounted brain sections were stained with Cresyl fast violet. The volume of the cortical infarct was determined by an operator blinded to the group's composition with a computer-assisted image analysis system (Optimas, BioScan, Edmonds, WA, USA). Infarct volumes were expressed in mm3 (mean ± SEM). For comparison of individual experiments, infarct volumes of treatment groups were expressed as a percentage of the respective controls, which were set to 100%.
Pharmacokinetic studies
Total concentrations of parent drugs in blood and brain were determined after acetonitrile precipitation of proteins. Briefly, 100 μL blood was added to 200 µL acetonitrile containing the internal standard for analytics and extracted. After centrifugation the supernatant was diluted (1 : 1) with sample solvent for the analytics. When the brain concentration was determined, 1 g brain tissue was homogenized in 2 mL acetonitrile and centrifuged, and aliquots of the supernatant were diluted with sample solvent. The analysis was performed by liquid chromatography coupled to an atmospheric pressure ionization/tandem mass spectrometer via the TurboIonspray interface (SCIEX), using similar procedures as previously described (Schuhmacher et al., 2003).
Drug application
For neuroprotection and brain oedema experiments, compounds were dissolved in 10% Cremophor EL® (Sigma-Aldrich Chemie GmbH), 90% physiological saline. Ready-made solution was administered i.v. as continuous infusion, the application volume was 2 mL/kg/h for brain oedema and 4 mL/kg/h for neuroprotection experiments. In all in vivo experiments control animals were injured and received the same volume of vehicle as the verum groups. For pharmacokinetic examinations, test compounds were diluted in 5% ethanol/5% solutol/90% saline and administered i.v. (lateral tail vein) at a dose of 0.2 mg as a bolus injection (< 30 s).
Data analysis
If not otherwise indicated, all in vitro biochemical experiments were performed in triplicate and were repeated at least three times. IC50 values were calculated from concentration–response curves with at least four concentrations. Data analyses were performed using GraphPad Prism version 3.00 for Windows (GraphPad Software, San Diego, CA, USA). For studies on ICP, brain water content and neuroprotection significance of the differences of means were assessed by anova followed, where appropriate, by post-hoc least-significance difference comparison (systat Version 10 SPSS). P ≤ 0.05 was defined as a level of significance.
Results
Rubidium efflux on C6BU1 cells
On C6BU1 cells, Rb+ efflux experiments revealed the classical pharmacological trias of KCNN4 channels, i.e. the channel was blocked by clotrimazole and charybdotoxin and not or only marginally by iberiotoxin (Table 1). As shown in Fig. 1 and Table 2, both the triazole and the cyclohexadiene could be identified as potent KCNN4 blockers and displayed similar IC50 values (12.1 ± 8.8 and 13.3 ± 4.7 nm, respectively). The involvement of other than KCNN4 channels was excluded by several control experiments using known ion channel blockers. The channel blockers iberiotoxin, apamin, dendrotoxin, kaliotoxin, agitoxin-2, margatoxin and noxiustoxin altogether had no effect on A23187-induced Rb+ efflux. In addition, investigations concerning endogenous sodium (tetrodotoxin), calcium (nimodipine, nicardipine, nifedipine, nitrendipine), and chloride channels (chlorotoxin) or pumps (ouabain, bumetanide) revealed, with the exception of nitrendipine (IC50 = 736 ± 148 nm), no significant effects on A23187-triggered Rb+ efflux.
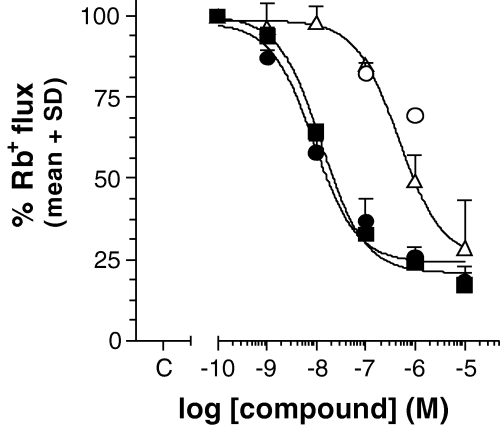
Inhibition of A23187-evoked Rb+ flux on C6BU1 cells by cyclohexadiene (▪), triazole (•), IbTx (○) and clotrimazole (). Values are the mean and standard deviation of at least three independent experiments, each performed in triplicate. The respective IC50 values are given in Table 2.
| Compound | C6BU1 (nm) | Erythrocytes (nm) |
|---|---|---|
| Charybdotoxin | 3.3 ± 1.9 | 5.6 ± 3.6 |
| Iberiotoxin | > 1000 | > 300 |
| Clotrimazole | 360 ± 12 | 255* |
| Triazole | 12.1 ± 8.8 | 20* |
| Cyclohexadiene | 13.3 ± 4.7 | n.t. |
- Given is the mean of the IC50 of at least three experiments, each performed in triplicate. *Mean of two independent experiments, n.t. not tested.
Rubidium influx on human erythrocytes
On human erythrocytes the pharmacological characterization revealed similar results as obtained on the rat glioma cell line. As shown in Table 2, charybdotoxin was the most potent compound (IC50 = 5.6 ± 3.6 nm), whereas iberiotoxin was at least 150-fold less potent (IC50 > 300 nm), the triazole was 10 times more potent (IC50 = 20 nm) than clotrimazole (IC50 = 255 nm).
Binding studies
Selected compounds, such as the cyclohexadiene, were tested on their ability to bind to other ion channels (Ca2+ channels: dihydropyridine- (DHP-), diltiazem-, verapamil- and N-type-binding sites. K+ channels: ATP-, voltage- and apamin-binding sites. Na+ channels: site 2. Cl– ionophore). No significant interaction at 100 nm, but some minor cross-selectivity (50% competition at Ca2+ channel-binding sites) could be observed at 10 μm. The cyclohexadiene (10 μm) was also tested in a PanLabs receptor and enzyme screen, which revealed a significant interaction with CYP450 2C19 (IC50 = 7.86 ± 0.79 μm) only.
RT-PCR investigations
The reaction product was of the expected size (341 bp), and expression of KCNN4 channels could be detected in C6BU1 cells (Fig. 2A), cultivated microglia (Fig. 2B), in brain preparations of sham-operated animals (Fig. 2B) and injured animals (Fig. 2A and B) as well, when determined 1 day or 7 days after SDH. When the antisense primer was used, no signal could be detected.
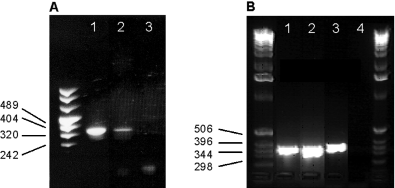
Detection of KCNN4 mRNA by PCR in (A) C6BU1 cells (lane 1) and brain preparations of injured animals 1 day post-surgery (lane 2). (B) Detection of KCNN4 mRNA in sham-operated animals (lane 1), injured animals 7 days post-surgery (lane 2) and rat brain microglia (lane 3). Lane 3 in (A) and lane 4 in (B) represent the negative controls.
Efficacy on brain oedema in the SDH model
The water content for sham-operated animals was 78.53 ± 0.49% (left hemisphere) and 78.51 ± 0.42% (right hemisphere). For vehicle-treated and injured animals, control values were 78.63 ± 0.76% for the right and 81.23 ± 0.68% for the left hemisphere. ICP values for sham-operated animals were 7.46 ± 0.46 mmHg, and for vehicle-treated and injured control animals 24.91 ± 1.17 mmHg. ICP as well as the brain water content were determined 24 h after injury and are concordant with previously reported values (Mauler et al., 2003). The elevated ICP levels were significantly reduced by the triazole (−46%, −43% and −37% at 0.1, 0.3 and 1 mg/kg; Fig. 3A) as well as by the cyclohexadiene (−45%, −60%, −46%, −27% at 0.001, 0.003, 0.01 and 0.03 mg/kg; Fig. 4A). The attenuation of ICP was accompanied by a significant reduction of brain water content when the triazole (−18%, −26% and −20% at 0.1, 0.3 and 1.0 mg/kg; Fig. 3B) as well as when the cyclohexadiene (−20% and −24% at 0.003 and 0.01 mg/kg) was administered (Fig. 4B).
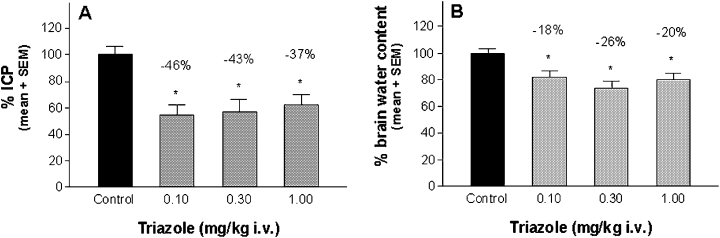
Efficacy of the triazole against brain oedema in the rat SDH model as shown by (A) intracranial pressure (ICP) and (B) brain water content determination 24 h after injury. The triazole was administered as continuous i.v. infusion for 4 h, starting immediately after injury. Control animals received the same volume of vehicle as the verum groups. ICP and brain water content of drug-treated animals was calculated as a percentage of the injured, vehicle-treated control group, which was set to 100%. Values above bars indicate the percentage change compared with controls. *P < 0.05, n = 8–12 per dose group.
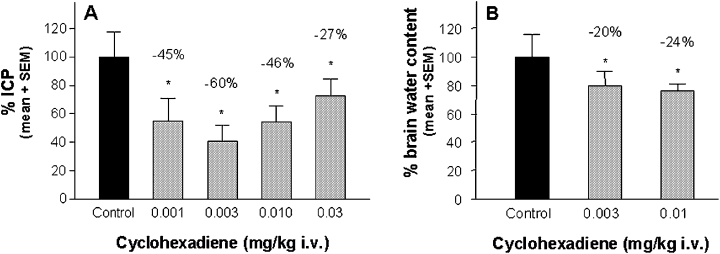
Efficacy of the cyclohexadiene against brain oedema in the rat SDH model as shown by (A) intracranial pressure (ICP) and (B) brain water content determination 24 h after injury. The cyclohexadiene was administered as continuous i.v. infusion for 4 h, starting immediately after induction of SDH. Control animals received the same volume of vehicle as the verum groups. ICP and brain water content of drug-treated animals was calculated as a percentage of the injured, vehicle-treated control group, which was set to 100%. Values above bars indicate the percentage change compared with controls. *P < 0.05, n = 8–12 per dose group.
Neuroprotective efficacy in the rat SDH model
Injection of autologous blood into the subdural space resulted in a clot formation over a part of the ipsilateral frontal cortex (area 2 and area 1), forelimb and hindlimb area and parietal cortex (area 1), with similar consequences as reported previously (Mauler et al., 2002). The infarct volume size of vehicle-treated controls was 118.38 ± 5.05 mm3, and was concordant with previously reported values (Mauler et al., 2003). When the triazole was given as a 4-h infusion immediately after injury, a significant mean infarct volume reduction of −44%, −52%, −51% and −26% was found at 0.4, 1.2, 4.0 and 12.0 mg/kg, respectively (Fig. 5A). When the cyclohexadiene was administered under the same experimental conditions, a significant neuroprotection of −43% could be observed at 0.04 mg/kg i.v. (Fig. 5B).
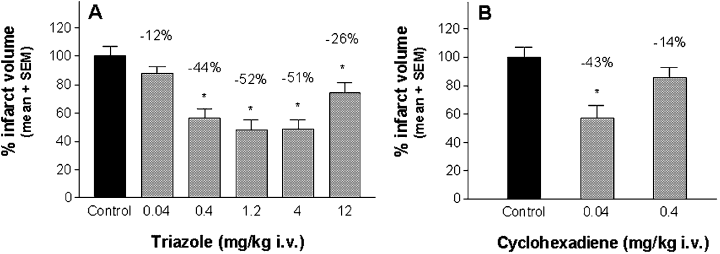
Neuroprotective efficacy of (A) the triazole and (B) the cyclohexadiene in the rat SDH model. The compounds were administered as continuous i.v. infusion for 4 h, starting immediately after induction of SDH and infarct volumes were determined after 7 days. Control animals received the same volume of vehicle as the verum groups. Infarct volumes were calculated as percentage of infarct volumes of the control group, which was set to 100%. Values above bars indicate the percentage infarct volume reduction compared with controls. *P < 0.05, n = 8–12 per dose group.
Pharmacokinetic
Both drugs displayed a strong accumulation in the brain. However, whilst plasma concentrations of the test drugs were similar, the degree of accumulation in the brain was different. The total brain concentration of the triazole exceeded the plasma concentration 3.7-fold (Cbrain/Cplasma = 3.7), the accumulation of the cyclohexadiene was even more pronounced and reached a Cbrain/Cplasma of approximately 10.
Discussion
The characterization of the pathophysiological role of KCNN4 channels in brain damage has so far been hampered by the lack of potent and specific low-molecular-weight inhibitors. The aim of the present study was to characterize two newly identified, specific and structurally independent low-molecular-weight KCNN4 blockers to prove the hypothesis that blockade of this channel can lead to a new pharmacological approach for the therapy of acute brain oedema and brain tissue damage following TBI.
We used a rat glioma cell line, which expressed the rat variant of the KCNN4 and measured Rb+ flux using atomic absorption spectroscopy. As previously reported, this technique is sensitive to measure the functional state of Ca2+-activated K+ channels in rat glioma C6 cells (Tas et al., 1988). In C6BU1 cells we have proven KCNN4 expression by using RT-PCR, shown the functionality of the expressed channel using known channel blocker and have excluded that other than KCNN4 channels mediated Rb+ flux by using various reference compounds and toxins. However, some KCNN4 channel-blocking properties of nitrendipine and related DHP-derivatives were reported previously (Ellory et al., 1994). Thus, a weak signal of nitrendipine (IC50 = 736 ± 148 nm) was expected. Although we have not investigated the electrophysiological properties of KCNN4 channels expressed in C6BU rat glioma cells, the RT-PCR results and the observed pharmacology characterize this channel as the rat variant of the human KCNN4 channel.
Selectivity of the newly identified compounds was confirmed in PanLabs and CEREP screens, which revealed no significant interactions with other tested receptors or enzymes. In particular, binding to other ion channels was weak. Apart from the negligible interaction with the enzyme CYP450 2C19 it can be considered that the new identified compounds are selective KCNN4 blockers.
To clarify whether or not the compounds were also effective on the human target we used human erythrocytes. Previous reports described azoles, other imidazole derivatives and some DHPs as effective KCNN4 blockers on erythrocytes (Gardos channel; Brugnara et al., 1993a; Ellory et al., 1994) in vitro and discussed these compounds as a putative pharmacological approach for the therapy of sickle cell disease, which was confirmed in animal models as well as in humans (Brugnara et al., 1996). As expected, clotrimazole (Brugnara et al., 1993a) and the triazole blocked A23187-induced Rb+ flux on human erythrocytes. Brugnara and co-workers (Brugnara et al., 1993b) reported that iberiotoxin was not effective on erythrocytes up to a concentration of 10 nm. We could confirm these results; however, Rb+ flux blocking properties of iberiotoxin could be observed when the concentration was increased above 100 nm, which suggests either an iberiotoxin-sensitive channel on erythrocytes, KCNN4 blocking properties at high concentrations, or other non-specific actions of this toxin.
Based on the observation that KCNN4 channels are involved in the modulation of the state of the cytoskeleton (Schwab et al., 1999) and that KCNN4 expression on leucocytes is considerably increased by immunological challenges (Logsdon et al., 1997), we assumed that KCNN4 blocker could be effective against brain oedema formation and tissue damage following TBI by attenuation of the blood–brain barrier (BBB) integrity and the inflammatory response. We have used the SDH model, because one main mechanism involved in the development of cerebral oedema in this model is the reduction of regional cerebral blood flow due to direct compression. This will lead finally to ischaemia and disruption of the BBB. A second mechanism involved in the pathogenesis is the inflammatory response, at least in the first stage (Holmin et al., 1995). As readouts we have used the more clinical relevant method of direct ICP monitoring 24 h after injury (Zwienenberg et al., 1999; Mauler et al., 2003), as well as the classical determination of brain water content (Xi et al., 2001; Mauler et al., 2003) and infarct volume determination 7 days after injury (Miller et al., 1990).
Both compounds significantly reduced ICP, which was also accompanied by a reduction of brain water content when administered as a 4-h infusion immediately after brain injury. In order to rule out that the peak values were not attenuated but potentially only shifted to later without any significant effect on reduction of tissue damage, the infarct volume was determined 7 days post-SDH in separate experiments. The observed neuroprotective efficacy of the clotrimazole derivative (triazole) in vivo is concordant with the previously reported in vitro neuroprotective properties of clotrimazole itself (Isaev et al., 2002). Because the cyclohexadiene as a selective KCNN4 blocker displayed neuroprotective properties as well, it is likely that the observed in vivo efficacy is mediated by blockade of KCNN4 channels.
Although both compounds were similar in potency in vitro, in vivo experiments suggested that the cyclohexadiene is approximately 10-fold more potent than the triazole. We assumed that this difference was attributed to different pharmacokinetic properties. Preliminary results of pharmacokinetic investigations in healthy animals support this assumption and revealed that the cyclohexadiene is enriched approximately 10-fold in brain tissue, whereas the triazole is enriched 3.7-fold.
In some experiments the dose–response curve suggests a u-shape curve type. The reason remains unclear, but similar curve types have been shown for a variety of other compounds (e.g. Horváth et al., 1997; Mauler et al., 2002, 2003). However, one option is that unspecific mechanisms are triggered, which counterattack the primary neuroprotective efficacy when a certain brain tissue concentration is exceeded.
It is demanding to put our results in the context of published data, because the techniques, models and readouts used throughout the literature are highly diverse. As reported by Kawai and co-workers (Kawai et al., 2000), who used a similar model and readout but a different time frame (0–240 min), the N-methyl-d-aspartate channel blocker MK-801 or moderate hypothermia were effective on brain water content reduction by approximately 25%, but not on ICP. A comparison with our data is possible in a limited way only, as we have chosen a time point when the vasogenic brain oedema is fully developed (Schneider et al., 2002). Other investigators have used different experimental designs, such as fluid-percussion brain injury (Okiyama et al., 1997), cold injury (Murakami et al., 1999), closed head trauma (Eilig et al., 2001) and controlled cortical impact (Başkaya et al., 2000;Dempsey et al., 2000). Compounds such as citicoline, ifenprodil or CP 89,113 investigated in the above-mentioned studies were effective in a similar range when brain water content was used as readout. However, we observed a strong efficacy on ICP but moderate efficacy on brain water content. This can be explained by the fact that after an initial compression and regional displacement of tissue, very small increases in water content will result in superproportional ICP increase. Contrariwise, a strong ICP reduction leads to similar moderate reduction of brain water content, but to a considerable improvement of cranial perfusion and cranial perfusion pressure.
However, although the mechanism of action needs clarification, some speculation based on general mechanisms in which KCNN4 channels are thought to be involved can be made. (i) Block of KCNN4 channels attenuates pathophysiological processes of activated microglia, which have been shown to lead, at least in part, to neuroprotection (Mizuno et al., 2004). Support for this assumption is shown by a recent report, showing expression of KCNN4 channel in activated microglia and beneficial effects of KCNN4 blockade on respiratory burst (Khanna et al., 2001). However, we did not perform a quantitative RT-PCR analysis, therefore we cannot conclude whether or not the different intensities observed in the different groups might reflect an increased expression of KCNN4 channels as it was reported for activated human T-lymphocytes (Logsdon et al., 1997).
(ii) Action via stabilization of cytoskeletal elements in brain vessel endothelium. Early changes after TBI include an initial rise in blood pressure, an early loss of the autoregulation of cerebral blood vessels and a transient breakdown of the BBB. In vitro studies reported a breakdown of cytoskeletal structures (mainly F-actin) caused by osmotic stress (Doukas et al., 1994), ischaemic periods (Hastie et al., 1997) or mechanical stimuli (Malek & Izumo, 1996), suggesting that alteration of the fine tuned cytoskeletal system plays a major role in vasogenic brain oedema development. Previous reports have demonstrated a functional expression of KCNN4 in blood vessel endothelial cells (Asbun et al., 1998) as well as a prominent role of KCNN4 channels in volume regulation, migration and [Ca2+]i homeostasis, indicating an involvement in the modulation of the state of the actin cytoskeleton (Schwab et al., 1999). Thus, maintenance of cell–cell interaction and organized cytoskeletal structure of brain vessel endothelial cells by blockade of KCNN4 channels might prevent or reduce water penetration into brain tissue.
(iii) Action via inhibition of migration and/or invasion of immune-competent cells. Block of KCNN4 channels on MDCK-F cells by charybdotoxin led to a dramatic reduced migration speed when compared with controls (Schwab & Oberleithner, 1996). This strongly supports the assumption that KCNN4 channels are involved in migration and might inhibit invasion of immune-competent cells into brain tissue and/or migration of microglia.
Regardless of the exact mechanism of action, we conclude that we have identified two new KCNN4 blockers from two different chemical classes with pronounced efficacy in a rat brain oedema model as well as strong neuroprotective efficacy. We therefore consider KCNN4 blockade as a new pharmacological approach for the treatment of acute brain damage following TBI.
Acknowledgements
The excellent technical assistance of K.-H. Augstein, M. Faßbender and J. Frielingsdorf is gratefully acknowledged.




