Significance of prostate-specific antigen-doubling time on survival of patients with hormone refractory prostate cancer and bone metastasis: Analysis on 56 cases of cancer-specific death
Abstract
Objective: Most of the metastatic diseases initially respond to maximum androgen blockade, but then relapse and lose response, and finally die. After relapse, the disease progresses in various courses. The present study was aimed to establish the predicting factors influencing the survival period of patients at prostate-specific antigen (PSA) relapse (entering the hormone refractory state).
Materials and Methods: Fifty-six patients with prostate cancer and bone metastasis, who were treated during the entire disease period at the same hospital and died were studied. To calculate PSA-doubling time, assay of PSA was carried out every 3 months or less.
Results: The period between PSA relapse and death was related with PSA-doubling time at relapse, nadir PSA and the period between the start of treatment and PSA relapse. The PSA-doubling time of 2 months or less at relapse was suggestive of a poor outcome. Final PSA-doubling time was not correlated with the survival period after PSA relapse.
Conclusion: The PSA-doubling time at relapse is one of the relevant factors for predicting the survival period after PSA relapse.
Introduction
Hormone therapy is a reliable treatment for advanced prostate cancer with bone metastasis, but most of these patients relapsed after a certain period from the start of treatment. After entering the hormone refractory state, there is scarcely an effective strategy, although several methods using chemotherapy and gene therapy are being developed. Progression after relapse can be variable, and the response to second-line treatment is hard to predict, therefore it seems important to find the predicting factors influencing the outcome after prostate-specific antigen (PSA) relapse, for treating the patients with advanced prostate cancer and bone metastasis. Although many reports have been issued concerning the factors that are closely related to outcome after relapse from radical prostatectomy or radiation therapy, there are relatively few reports on the predicting factors of PSA relapse after hormone therapy. To establish the predicting factors at PSA relapse, the present study was undertaken to examine the items influencing the outcome of patients with bone metastasis after hormone therapy, with special interest in the significance of PSA-doubling time.1
Methods
Fifty-six patients with M1b cancer,2 who did not receive any previous treatment for prostate cancer, were chosen for the study. Initially, their performance status was grade 0–1 with no or little lumbar pains. The patients began treatment with luteinizing hormone-releasing hormone (LH-RH) agonist or castration accompanying anti-androgen (maximum androgen blockade [MAB]) at Asahi General Hospital between 1992 and 2002, and all died of a cancer-specific cause. Anti-androgens, chlormadinone acetate, flutamide and bicaltamide were adequately used. All patients were treated during the entire course of the disease at the same hospital and the complete records from initial treatment until death were provided. Prostate-specific antigen (as total PSA) was assayed serially every 3 months or less, and was checked more frequently when progression was evident. All patients showed an increase in PSA after relapse. The time of relapse was defined as an exponentially increasing PSA at three or more points, and the date of relapse was determined to be the initial increase. Determination of PSA relapse was not referred to changes in other subjective and objective signs. After PSA relapse was confirmed, LH-RH agonist was continued for few months, then patients were examined for anti-androgen withdrawal syndrome for 1–3 months. After that time period, patients were mostly treated with second-line hormone therapy such as other anti-androgens, estrogens (estramustine phosphate with/without etoposide, diethylstilbestrol diphosphate, etc.) and dexamethasone by turns. Other chemotherapy was carried out for some cases.
For PSA-doubling time (PSA-DT), the slope was obtained from the values taken at three or more points by the least square test and doubling time was calculated from loge 2/slope. Relapse PSA-DT and final PSA-DT were defined as the values at PSA relapse and during the last 6 months before death, respectively. Both of the doubling times from one case are shown in Figure 1. Kits used for assaying PSA were IMAX PA (Abbott, Tokyo, Japan) (before 1994), AxSYM PA Dainapack (Abbott) (1994–1998), and AxSYM PSA Dainapack (after 1999), and the correlation of PSA values by different kits was confirmed.
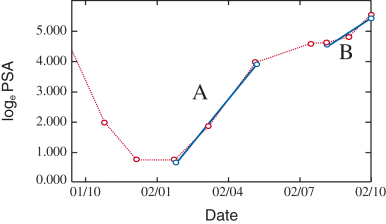
Prostate-specific antigen-doubling time. Horizontal axis indicates year and month (01/10 = 2001/10). (A) 1.7 months (57 days), (B) 2.3 months (69 days).
Statistical analysis was calculated by Mann–Whitney U-test, Student’s t-test and simple regression analysis. Survival analysis was calculated using Kaplan–Meier method and Cox proportional hazard model, and the significance was checked with a log–rank test. A P-value equal to or below 0.05 was considered to be significant. All calculations were carried out using the StatView program.
Results
Regarding histological grade, 57% of the cases showed poorly differentiated cancer, followed by moderately differentiated cancer (Table 1). Initially all cases had bone metastasis confirmed by bone scan but the extent of disease (EOD) for bone metastasis was examined in 42 cases because the other 14 cases had some record loss (indicated as unknown).
| Number of patients (n = 56) | |
|---|---|
| Age (years)† | 71.8 (71.0, 58–87) |
| Histologic grade‡ | |
| Well (2–4) | 3 (5%) |
| Moderately (5–7) | 21 (38%) |
| Poorly (8–10) | 32 (57%) |
| Extent of disease for bone metastasis | |
| 2 | 28 (50%) |
| 3 | 10 (18%) |
| Superbone | 4 (7%) |
| Unknown | 14 (25%) |
- † Average (median, range).
- ‡ ‡Number in parenthesis shows Gleason score.
Of the 56 cases in the study, 55 cases (98%) showed a certain decrease in PSA to nadir (lowest value) after the start of hormonal therapy. The average survival period between the start of treatment and the cancer-specific death was 33.8 ± 18.4 months, and one-third of the entire survival period occupied the period between the start of treatment and PSA relapse (11.1 ± 7.0 months). The survival period after PSA relapse, which is in the hormone refractory state, was the other two-thirds of the entire survival period, therefore this term markedly affected the prognosis, and factors influencing this term were arbitrarily selected (Table 2). The survival period after PSA relapse was not influenced by EOD, initial histologic grade, initial PSA and final PSA-DT, however, the period between the start of treatment and time at nadir PSA (start–nadir), the period between the start of treatment and PSA relapse (start–relapse), nadir PSA and relapse PSA-DT markedly affected the survival (Table 3). The period of start–relapse significantly correlated with the period of start–nadir (P < 0.0001).
| Average | Median | Range | |
|---|---|---|---|
| Start–death (months) | 33.8 | 30.5 | 5–78 |
| PSA relapse–death (months) | 23.1 | 21.5 | 1–64 |
| Start–nadir† (months) | 6.0 | 5.1 | 1–24 |
| Start–PSA relapse (months) | 11.1 | 10.0 | 3–34 |
| Initial PSA (ng/mL) | 652.8 | 278.5 | 5.7–4818.8 |
| Nadir PSA‡ (ng/mL) | 35 | 2.4 | 0.02–191.8 |
| Relapse PSA-DT§ (months) | 2.7 | 2.0 | 0.5–10.5 |
| Final PSA-DT (months) | 3 | 2.2 | 0.5–10.3 |
- † Period between start of treatment and time at nadir PSA.
- ‡ One extraordinary high case was omitted (PSA of start and nadir; 4443 and 2711 ng/mL).
- § Relapse and final PSA-doubling time (PSA-DT) calculated at PSA relapse and 6 months before death.
| Survival (months) | P-value | |||
|---|---|---|---|---|
| EOD, grade | 2 versus 3 + 4 | 24.2 ± 14.9 | 27.4 ± 16.8 | 0.55 |
| Histologic grade | Well + moderate vs poor | 27.3 ± 14.0 | 19.9 ± 15.1 | 0.06 |
| Start–nadir† | >5.1 months versus ≤5.1 months | 30.1 ± 17.1 | 20.3 ± 14.8 | 0.05 |
| Start–relapse‡ | >10 months versus ≤10 months | 28.1 ± 16.0 | 19.4 ± 13.2 | 0.03 |
| Initial PSA | ≤279 ng/mL versus >279 ng/mL | 23.0 ± 15.6 | 23.3 ± 14.3 | 0.93 |
| Nadir PSA§ | ≤2.4 ng/mL versus >2.4 ng/mL | 29.1 ± 14.8 | 16.7 ± 12.5 | 0.001 |
| Relapse PSA-DT | >2 months versus ≤2 months | 27.9 ± 15.5 | 17.9 ± 12.6 | 0.01 |
| >3 months versus ≤3 months | 28.8 ± 14.2 | 21.0 ± 14.9 | 0.08 | |
| Final PSA-DT | >2.2 months versus ≤2.2 months | 24.6 ± 14.2 | 21.6 ± 15.9 | 0.46 |
- †Period between start of treatment and time at nadir of PSA. ‡Period between start of treatment and PSA relapse. §One extraordinary high case was omitted (Table 2). Data are shown as mean ± SD. EOD, extent of disease; PSA, prostate-specific antigen; PSA-DT, prostate-specific antigen-doubling time.
Several prognostic factors were analyzed with Cox proportional hazard model (Table 4). Nadir PSA and relapse PSA-DT significantly influenced the survival period after PSA relapse. The relationship between relapse PSA-DT and the survival period after PSA relapse was shown in Figure 2 by simple regression analysis, and correlation coefficient test showed weak association (r = 0.42. P = 0.02). Because the median of relapse PSA-DT was found to be 2 months, the survival period after PSA relapse was divided by this value (Fig. 3). The survival period was longer for patients with PSA-DT of more than 2 months than for patients with PSA-DT of 2 months or less.
| Hazard ratio | 95% CI | P-value | ||
|---|---|---|---|---|
| Start-PSA relapse | >10 months versus ≤10 months | 1.14 | 0.62–2.10 | 0.68 |
| Initial PSA | ≤279 ng/mL versus >279 ng/mL | 0.85 | 0.48–1.53 | 0.59 |
| Nadir PSA | ≤2.4 ng/mL versus >2.4 ng/mL | 2.67 | 1.44–4.97 | 0.002 |
| Relapse PSA-DT | >2 months versus ≤2 months | 2.30 | 1.21–4.39 | 0.01 |
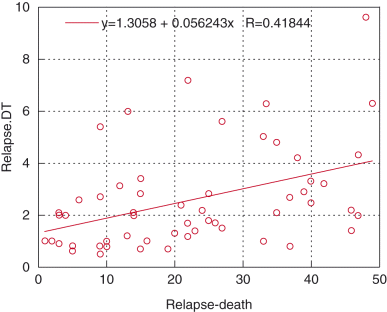
Correlation between prostate-specific antigen (PSA)-doubling time at PSA relapse after hormone therapy and survival period after PSA relapse.
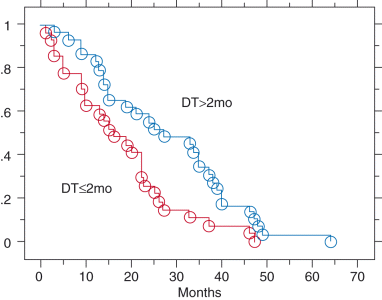
Kaplan–Meier survival curve after prostate-specific antigen (PSA) relapse following hormone therapy for prostate cancer patients with bone metastasis. Divided by PSA-doubling time (DT) at PSA relapse: PSA-DT of 2 months or less (27 cases) and more than 2 months (29 cases). P = 0.01.
To compare relapse PSA-DT with final PSA-DT, patients were divided into three groups (Table 5). Patients with relapse PSA-DT longer than their final PSA-DT (group I) showed more prolonged survival period after PSA relapse compared with patients in the other two groups (relapse PSA-DT equal to or less than final DT). This means that growth just after PSA relapse significantly affected the survival period after PSA relapse, and that the influence of final PSA-DT seems to be little, if any, on the survival period after PSA relapse.
| PSA-DT | No of cases | Relapse DT† (months) | Final DT‡ (months) | Survival after relapse§ (months) |
|---|---|---|---|---|
| I Relapse > final | 16 | 5.0 ± 2.8 | 2.3 ± 1.7 | 30.9 ± 14.1 |
| II Relapse < final | 22 | 1.6 ± 0.8 | 4.1 ± 2.3 | 21.7 ± 13.5 |
| III Relapse = final | 18 | 2.1 ± 1.6 | 2.1 ± 1.7 | 18.0 ± 15.4 |
- † I vs II and I vs III, P < 0.01.
- ‡ I vs II and II vs III, P < 0.01.
- § I vs II, P = 0.052, I vs III P = 0.015. Data are shown as mean ± SE.
Discussion
Prognostic factors on hormone therapy at the start of treatment are well discussed and the most relevant factors are T-stage, histologic grade, PSA, EOD, performance status, pain and anemia, etc.3 After PSA relapse entering the hormone refractory state, the correlation of the initial tumor properties such as histologic grade, PSA and EOD with the subsequent course is changed, as is shown in the present study. Patients with M1b cancer and a 90% or greater decline in PSA after initial hormone therapy, show prolonged progression-free survival.4 The duration of PSA response is the other prognostic factor for the hormone refractory prostate cancer,5 and the same tendency was obtained in the present study (nadir PSA and the period of start–relapse).
Production of PSA might presumably relate to the volume of cancer cells, therefore the serum PSA is a good indicator for estimating the volume of cancer tissues. It is very difficult to measure the volume of cancer tissues in metastatic foci directly. Prostate-specific antigen is one of the most reliable markers for conjecturing cancer volume. Prostate-specific antigen velocity is an index correlating with the growth of cancer tissues,6,7 and from this view PSA-DT has been proposed.8
Prostate-specific antigen-doubling time is applied to patients with prostate cancer at an appropriate timeframe.9–13 In cases of low-risk prostate cancer, in which watchful waiting is indicated, PSA-DT was found to be approximately 24–36 months and in some cases was up to 120 months. Untreated cases of stage B2-C cancer showed PSA-DT of 27.9 ± 11.6 months.14 Before radiation therapy, patients with T1-3 cancer showing PSA-DT of 60 months or more, resulted in only 3% of relapse by 3 years.15 Therefore, untreated cancer of low- to intermediate-risk with PSA-DT of more than 12 months indicates slow progression.
At PSA relapse after radical prostatectomy, prediction about the appearance of local or distant metastatic diseases is discussed by means of PSA-DT and Gleason score of surgical specimens.16–19 The median survival of patients with PSA-DT of less than 3 months was 6 years.20 Cases with PSA-DT of less than 12 months more frequently developed into metastatic disease when compared to those with PSA-DT of more than 12 months, who might have local relapse.21,22 Progression after radiation therapy was also considered relative to relapse PSA-DT and that of less than 3 months showed a worse outcome.23 From these reports PSA-DT of 3–6 months at PSA relapse might be a turning point to conjecture further courses after prostatectomy or radiation therapy.
At PSA relapse after castration of patients with T2-3 cancer, the subsequent outcomes were classified into low-, intermediate- and high-risk groups. The high-risk group was defined by PSA-DT of less than 6 months, the period of less than 7 months between the start of treatment and PSA relapse, and more than 0.5 ng/mL of nadir PSA. This group showed a 14-month survival period, shorter than those of the other two groups.24
After intensive hormone therapy (such as MAB) for metastatic disease, patients showed a much shorter PSA-DT after relapse. Patients in the hormone refractory state showed PSA-DT of 2.1 ± 1.6 months.14 A similar trend was reported for patients with metastatic disease; PSA-DT was 9.4 ± 1.1 months before androgen ablation and 3.9 ± 0.4 months in the hormone refractory state.25 The patients with metastatic hormone refractory cancer unresponsive to chemotherapy (idarubicin) showed PSA-DT of 2.1 months.26 Regarding the response to chemotherapy, average PSA-DT in partial remission was 7.9 months, that in stable disease was 7.4 months, and that in progressive disease was 3.8 months.27 The hormone refractory state in the present study showed median PSA-DT at PSA relapse of 2.0 months and median survival of 21.5 months. Some reports noticed the difference in survival period after PSA relapse by separating PSA-DT of more than 3 months from that of 3 months or less and a similar tendency was obtained in the present study (Table 3). Together with these results, PSA-DT at PSA relapse following hormone therapy for patients with advanced stage shows a cut-off of 2–3 months for prediction of a survival period, and patients with longer value of PSA-DT might have rather slow growing tumors with a prolonged survival period.
Some of the patients with relapse after surgery or radiation therapy revealed similarly short PSA-DT, such as approximately 2–3 months, and this value is relative to patients in the hormone refractory state, but the former experienced much longer survival period than the latter. Although PSA-DT of 2–3 months seems to indicate a rapid growth of tumors, the difference in survival periods might be, at least partly, because of the tumor volume and environmental status, such as tumors in the prostate or bone. Tumors in patients with advanced cancer might be a great burden in the bone and other metastatic foci, in the contrary, residual tumors after operation or radiation are a small amount of volume. Moreover, the environmental condition in the bone might be favorable for growth of tumors by abundant growth-stimulating factors.
Final PSA-DT did not correlate with relapse PSA-DT and did not influence the survival period after PSA relapse. One explanation is that the period of final stage might be markedly affected by various effects including second-line treatment, nutrition, and general conditions, etc. These effects modify the growth of tumors affecting final PSA-DT and the survival period. It might indicate that subsequent course after PSA relapse seems to be markedly swayed by the tumor growth potency at relapse, indicated by PSA-DT.
Elevation of PSA is a definitive evidence of relapse, but progression to the neuroendocrine tumor might be accompanied with no, or a little elevation of PSA.28 Attention is needed on the shift to the neuroendocrine tumor, because PSA-DT of this tumor is, if any, a very long value but the tumor growth is rapid. In this context, the determination of chromogranin-A for detection of the neuroendocrine tumor has been discussed.29
Acknowledgments
This work was approved by the ethical committee of the Asahi General Hospital.




