The current role of thoracic surgery in tuberculosis management
ABSTRACT
Although tuberculosis is mainly managed medically today, thoracic surgery continues to play a key role in its diagnosis and treatment in selected subgroups of patients. In certain scenarios such as multi-drug-resistant tuberculosis, advanced tuberculous empyema and symptomatic bronchial stenosis, modern thoracic surgery may represent the only effective means of management in selected patients. Advances in thoracic surgery in recent years, in particular the use of Video-Assisted Thoracic Surgery, not only reduce postoperative morbidity for individual patients, but may potentially allow a wider range of tuberculosis patients to benefit from surgery. Respiratory physicians and thoracic surgeons should continue to work together to ensure that tuberculosis patients who may benefit from surgery are identified for prompt and effective intervention.
TUBERCULOSIS AND THE THORACIC SURGEON
Although seldom considered a surgical disease today, tuberculosis (TB) is inextricably linked to the history of thoracic surgery. Indeed, it can be argued that the very first thoracic surgical procedure ever documented in history, open drainage of the pleura, was performed by Hippocrates himself for empyema thoracis resulting from TB.1 After the identification of Mycobacterium tuberculosis by Koch in the 1880s, the realization that this was an obligate aerobe led to the rapid growth of thoracic surgery as a great variety of collapse therapies were developed in the late 19th and early 20th Centuries to kill the organism through oxygen deprivation. These included thoracoplasty, induced pneumothorax, ball plombage, pneumoperitoneum and phrenic nerve crushing.2 Significantly, these are techniques still used by thoracic surgeons today for the treatment of many chest conditions. The basic skills and approaches still used in modern thoracic surgery were also honed at this time, such as the thoracotomy incision. Interestingly, as minimally invasive surgery is so important today, the practice of thoracoscopy was also first introduced around this time by Jacobeus as an approach for pleural biopsy and adhesiolysis in TB patients.3
After the Second World War, the advent of highly effective antimicrobial drug therapy for TB markedly reduced the role of surgery in managing this disease. The challenge this posed to the very existence of thoracic surgery as a specialty was considered as serious as the challenge of percutaneous coronary interventions poses to cardiac surgery today. However, the rapid increase in the incidence of lung cancer in the western world in recent decades meant that thoracic surgeons soon found another important application for their skills developed through years of treating TB surgically.
Today, we are witnessing a resurgence of the role of surgery in managing TB. This is due in large part to an overall increase in the global incidence of TB, the concomitant HIV epidemic, increasing survival of immunosuppressed patients, and the emergence of multi-drug-resistant TB (MDR-TB) since the mid-1980s.4 In 2005, it was estimated that the annual worldwide incidence of TB was 8.8 million new cases, and that TB accounted for about 2 million deaths per year.5 It is suggested that up to a third of the world's population may be infected with M. tuberculosis. Although most cases still occur in less developed countries, there has been a noted trend for an increasing incidence of disease even in the western world. Thoracic surgeons today are hence again increasingly involved in the management of TB.
Thankfully, thoracic surgery as a specialty has progressed significantly since the first heyday of surgical therapy for TB. Great strides have been made in not only improving the safety of thoracic surgery, but also in reducing perioperative morbidity. Significantly, the rise of modern minimally invasive thoracic surgical approaches has greatly enhanced operative management in the chest. Not only does the individual patient experience less pain and morbidity, but the less traumatic surgery means a wider range of patients can now be considered for operations that may help them. This article aims to provide a brief overview of what thoracic surgery can offer nowadays to patients with TB, and suggest how physicians can effectively involve the surgeon when managing such patients.
SURGERY FOR DIAGNOSIS OF TB
One of the first challenges in managing TB is to diagnose it. Because of the often paucibacillary nature of the disease, M. tuberculosis infection can often difficult to confirm by microbiological culture of sputum samples, bronchial aspirates and particularly pleural fluid. In patients who have clinical manifestations and radiological features compatible with TB and where less invasive investigative modalities have failed to yield a positive diagnosis, surgical biopsy may help confirm the diagnosis in selected patients.
For the surgeon, the primary consideration when called upon to diagnose TB is to identify a potential target site for biopsy. This is most commonly done using a thoracic CT scan. The most frequently identified sites for biopsy in suspected TB cases include lung parenchyma, mediastinal lymphadenopathy and pleura. Surgical approaches for each of these sites are discussed below. Which site is chosen to be biopsied is primarily determined by the likelihood of positive yield as suggested by the volume and accessibility of affected tissue seen on CT at the site. Secondary considerations include the patient's ability to tolerate one-lung ventilation and/or prolonged anaesthesia, and foreseeable obstacles to certain surgical approaches (such as tracheostomy obstructing a mediastinoscopy, or anticipated dense adhesions from previous thoracotomy or pleural infection complicating subsequent explorations in the ipsilateral chest). It is therefore essential whenever diagnostic surgery is contemplated for suspected TB to adopt a multidisciplinary team approach, involving the respiratory physician, the thoracic surgeon, the radiologist and the anaesthetist. In patients for whom surgery is potentially hazardous, alternative investigation modalities or even empirical anti-TB treatment may need to be considered.
Lung parenchyma
No radiological imaging can yet confirm a diagnosis of TB affecting the lung parenchyma. Even modern CT and PET have proven too non-specific. Various modes of non-surgical pulmonary sampling may also be insufficient for diagnosis. Percutaneous imaging-guided biopsy of suspicious lung lesions is said to yield a positive diagnosis in only 20–55% of cases.6–8 Fibre-optic bronchoscopy with BAL and/or transbronchial biopsy may give a positive diagnosis in only 30–58% of smear-negative TB cases.9,10 Surgical lung biopsy is therefore often required for diagnosis in a proportion of patients suspected to have TB.
Features that suggest a better chance of success with surgical lung biopsy are summarized in Table 1. The ideal case for surgical biopsy should be where one or more focal lung parenchymal target lesions (mass/nodules or focal opacification) are identified and which are situated at or close to a lung edge or inter-lobar fissure. Diffuse infiltrative lung areas can technically be biopsied also, but if TB is suspected there is the potential hazard of a staple or suture line running through inflamed or infected lung parenchyma, raising the risk of staple/suture line air leakage and poor healing. The requirement for a site near a lung edge of fissure is to enable a small, simple wedge to be cut out. A wedge resection removes only a small piece of lung (typically several cubic centimeters of tissue) and results in little respiratory impairment for the patient.
| Favourable | Unfavourable | |
|---|---|---|
| Findings on CT (or other imaging) | ||
| Extent of disease | Localized target lesion (e.g. discrete lung nodule) | Diffuse, hazy infiltrative changes only |
| Site of target for biopsy | Close to interlobar fissure or lung lobe edge | Deep in lung parenchyma and/or close to hilum |
| Bronchiectasis, fibrosis or old TB scars | No | Yes |
| Past history | ||
| Previous thoracic surgery, pleurodesis or radiotherapy | No | Yes—in ipsilateral chest |
| Previous episodes of TB or chest infections (pleural adhesions) | No | Yes |
| Lung function | Adequate for one-lung ventilation† | Dyspnoeic at rest or on mild exertion† |
- † Thoracic anaesthesiologist consultation mandatory in all patients.
- TB, tuberculosis.
Deep-sited lesions pose a surgical challenge in most cases. If the target lesion is very deep in the lung parenchyma, it is often not possible to remove it without depriving the subtended distal parts of the lung lobe of ventilation and perfusion. To remove such deep lesions, a very large wedge resection (commonly referred to as a ‘hemi-lobectomy’) or even an anatomical lobectomy may be required. An alternative is an enucleation procedure, which is not unlike removing a grape embedded deep inside a fruity birthday cake. This is an effective procedure in selected patients, but not without risk of significant parenchymal trauma and subsequent air leakage or bleeding.11 Furthermore, small, deep lesions may often prove to be technically difficult to locate during surgery because they are not visible from the outside of the lung lobe, and may be impalpable. Biopsy of deep parenchymal lesions should therefore be carefully considered and planned before being undertaken.
If all other factors are equal in bilateral disease, some surgeons prefer the right lung for biopsy as the extra fissure and lobes edges give more possibilities for an easy biopsy at a lung lobe edge. It was previously reported that the lingula and right middle lobe made poor biopsy sites, given their alleged propensity for inflammation, scarring and congestion that can affect histological diagnosis.12 However, others have since found this not to be the case,13 and most surgeons nowadays do not deliberately avoid biopsy at these sites.
Patient selection criteria for lung biopsy are essentially the same as for any thoracic surgical procedure. The primary consideration after a patient is deemed fit for general anaesthesia, is whether or not he or she can tolerate one-lung ventilation during surgery. Generally, it is a pre-requisite for thoracic surgery that the lung on the operation site can be deflated during surgery to allow for complete exploration of the pleural cavity. This is most commonly assessed by spirometry and by exclusion of carbon dioxide (CO2) retention, but a detailed discussion of the criteria for one lung ventilation is beyond the scope of this article. A thoracic anaesthesiologist's assessment prior to offering surgery is obligatory in all patients. In selected patients with poor lung function (and where the anticipated biopsy is simple), it may be possible for the surgeon to perform the required biopsy quickly during brief periods of intermittent apnoea after general anaesthesia is induced. Limited biopsies via mini-incisions performed under local anaesthesia have been reported for patients who cannot tolerate general anaesthesia, but these should be considered only in exceptional circumstances.
The traditional approach for lung biopsy is via a thoracotomy. A full postero-lateral thoracotomy is one of the most painful of all surgical incisions,14 and is often unnecessary. If a target lung lesion is identified preoperatively, it is often sufficient to use a limited thoracotomy—such as an anterior thoracotomy or muscle-sparing lateral thoracotomy—sited over the target. A visual and digital exploration is performed to locate the site of biopsy, and a representative wedge of lung tissue is excised. This is usually done nowadays using a surgical staple resection device. The resected lung wedge is routinely sent for histology, standard microbiological culture and acid-fast bacillus smear and culture tests. It is important for the respiratory physician and thoracic surgeon to communicate preoperatively to consider if any other investigations are required.
Over the past 15 years or so, there has been an inexorable trend towards performing lung biopsies using a video-assisted thoracic surgery (VATS) approach.15 This is minimally invasive or ‘keyhole’ surgery in the chest. The standard VATS procedure uses three surgical ports of 5–15 mm diameter, through which a video thoracoscope and two surgical instruments can be placed. Ports are sited on the lateral chest wall, although the precise positions may vary depending on the site of the target lesion. Open thoracotomy is especially painful because of the need to forcibly spread apart the ribs during surgery to allow the surgeon to have visual and manual access into the chest. By using a video thoracoscope to visualize inside the thorax, VATS completely foregoes the hurtful rib spreading and thereby minimizes surgical access trauma. The established advantages of VATS over open thoracotomy include reduced pain and morbidity, faster recovery, shorter hospital stays and less postoperative compromise of the patient's respiratory and immune function.16–21 Importantly, the use of the video thoracoscope provides a 360° complete exploration of the ipsilateral pleural cavity that is potentially superior to that via a limited thoracotomy. With a finger placed via one instrument port and the lung delivered to that finger using an instrument through another port, the experienced VATS surgeon can accomplish a digital palpation of the entire lung virtually as thoroughly as via an open thoracotomy in most cases. Lung biopsy is performed again as a wedge resection using an endoscopic staple resection device.
For many thoracic surgeons experienced with minimally invasive techniques, the VATS approach is now preferred for most lung biopsies. Few absolute contraindications now exist for the VATS approach. In the past, pleural adhesions, very common in TB patients, were considered an indication to convert from a VATS to an open approach. However, experienced VATS surgeons find that most adhesions can be divided readily using VATS and conversion to an open procedure is now an infrequent event.
Following surgery, it is customary to place one chest drain. Provided there is no significant air leak or drainage, this drain is usually removed within 24 h of surgery. Most patients are discharged within 1–2 days of VATS surgery, although some centers are now reporting lung biopsies being performed as day surgery procedures. When used for diagnosis of solitary lung lesions, many studies have consistently confirmed that in the hands of experienced VATS surgeons the diagnostic accuracy of VATS can be as high as 95–100% with complication rates of 3–6%.22,23
Pleural effusion
When TB manifests as a pleural effusion, microbiological analysis of the fluid itself often cannot confirm the diagnosis. Percutaneous biopsy of the parietal pleura is a common strategy used in such patients, but the problem is that this is essentially a blind procedure. There is no effective way of determining whether the pleura at the site of percutaneous puncture is a good site for biopsy. This method reportedly gives positive diagnosis in only 50–75% of cases.24,25
For cases where diagnosis cannot be achieved by blind biopsy or pleural fluid analysis, the thoracic surgeon can offer a surgical exploration. Although a limited open thoracotomy incision can be used, increasingly today such exploration is performed using a minimally invasive VATS approach. The patient selection criteria and standard three-port VATS procedure as described above can be used. More recently, the use of a single-port VATS strategy or even ‘needlescopic’ VATS using instruments only 2–3 mm wide has been described.26,27 These offer potentially even less pain and morbidity than ‘conventional’ VATS. When a pleural effusion exists, there is usually sufficient lung–chest wall separation in situ to allow thorough video thoracoscope exploration. Furthermore, using a 30° angled video thoracoscope, the surgeon has potentially better visualization of the lateral chest wall pleura than even through an open thoracotomy.
Regardless of the surgical approach used, the aim of surgery is to break down loculations or septations in the pleural space to facilitate full drainage of all the pleural fluid, and then to explore the parietal pleura to identify target sites for biopsy. Samples of pleural fluid are routinely sent for cytological and microbiological tests. In addition, TB typically produces inflammatory exudates and later fibrous peel on the parietal pleura, which are readily seen by video thoracoscope. These solid exudates, peel and any other suspicious areas on the parietal pleura should also be generously biopsied by endoscopic biopsy forceps. Worldwide experience over the past two decades has shown VATS to be a safe and quick procedure for pleural diagnosis in most patients.15,28 VATS consistently achieves positive diagnosis for indeterminate pleural effusions in 95–100% of cases. One meta-analysis of 1500 cases of indeterminate pleural effusions worldwide confirmed that VATS gave 90% diagnostic accuracy with only 3% morbidity.29 A key advantage of surgery is that if the pleural effusion turns out to be an established stage II–III empyema thoracis on exploration, a surgical drainage and decortication procedure can be performed in the same sitting for selected patients (see later).
Postoperative course for most patients is as described above for lung biopsy. Typically, patients can be safely discharged home within 1–2 days of surgery.
One emerging alternative to VATS for indeterminate pleural effusions is ‘medical’ thoracoscopy under local anaesthesia.30,31 Thoracoscopy is in fact a variation of the original procedure pioneered over a century ago by Jacobeus, and which is considered the ancestor of modern VATS. Through a single incision in the chest wall, either a rigid thoracoscope tube or a fibre-optic pleuroscope is advanced into the pleural space. Fluid can be drained from the pleural space and parietal pleural biopsies can be taken. The potential advantages are the foregoing of general anaesthesia, making it more suitable than VATS as a day-case procedure and making it a viable option for patients deemed unsuitable for general anaesthesia. Thoracoscopy may also be potentially cheaper to perform than surgery. On the downside, the lack of one-lung ventilation using local anaesthesia means that an inflated or semi-inflated lung may obstruct complete exploration of the entire ipsilateral chest. Furthermore, the anxiety and discomfort of the patient (who may have to endure some degree of discomfort from pneumothorax during the procedure), plus the stress on the operator and nursing staff working on an awake patient, are often cited as notable disadvantages. At present, further global experience is awaited to establish the diagnostic efficacy of this procedure.
Mediastinal lymphadenopathy
In many TB patients, the only radiologically identifiable lesions may be enlarged intrathoracic lymph nodes. In such patients, if a diagnosis of TB cannot otherwise be made, it is possible to biopsy these nodes surgically.
For the surgeon, a distinction must be made between hilar nodes and mediastinal nodes in these cases. Hilar lymph nodes correspond to nodal stations 10–14 on the standard map for lung cancer lymph node staging adopted by the American Joint Committee on Cancer (AJCC) and the Union Internationale Contre le Cancer (UICC).32 In practice, these nodes are only reachable via thoracotomy or VATS. Where such nodes are enlarged on CT, they also tend to be associated with post-inflammatory adhesions in the interlobar fissures, and between nodes and adjacent pulmonary vessels. The result is often technically challenging and relatively invasive surgery. Careful consideration should be taken before embarking on biopsies of hilar nodes alone in the absence of other target lesions.
Mediastinal lymph nodes correspond to nodal stations 1–9 on the AJCC/UICC map. Stations 2, 3, 4, 7, 8 and 9 on the right side, and stations 2, 4, 5, 6, 7, 8 and 9 on the left side can be accessed using VATS or thoracotomy. Either approach allows a complete exploration of the ipsilateral pleural cavity as well as enabling complete removal of an entire mediastinal node in most cases for histological and/or microbiological analysis. Postoperative recovery is the same as after VATS lung or pleural biopsy. The main limitation of surgery is that normally only lesions on one side of the chest can be biopsied in one sitting (bilateral VATS is possible but rarely indicated).
An alternative surgical approach is the traditional cervical mediastinoscopy procedure. Under general anaesthesia, a small 2–3 cm incision is made in the anterior neck midline and a rigid mediastinoscope tube can be placed along the pre-tracheal plane to access nodal stations 2 and 4 on both sides, and often station 7 as well. If only these nodal stations contain target lesions and there is no need to explore the whole pleural space, mediastinoscopy is often preferable VATS. This is because mediastinscopy does not require one-lung ventilation during anaesthesia and can be better tolerated by patients with poor lung function.
Anterior mediastinotomy (often called a Chamberlain's procedure) is an older surgical approach used most commonly on the left side to gain access to the nodal stations 5 and 6, which are not reachable via standard mediastinoscopy. A parasternal, intercostal incision is made and often the internal mammary artery underneath is ligated and divided. In some cases, one or two costal cartilages at the anterior end of the rib, or even a short segment of rib itself, are removed to allow better access. Although mediastinotomy has been largely supplanted by VATS nowadays, it still has a role to play in selected patients who may not tolerate the one-lung ventilation required for VATS.
Traditionally, a proven technique for biopsy of nodal stations 2, 4, 7 and often 10 and 11 on both sides of the chest has been transbronchial fine needle aspiration (FNA) via fibre-optic bronchoscopy. This can performed in a similar fashion as conventional fibre-optic bronchoscopy under local or general anaesthesia, and as an endoscopic procedure it is less invasive than any of the above surgical approaches.33 In experienced hands, traditional transbronchial FNA remains a safe and effective choice for biopsy of certain mediastinal and even hilar lymph nodes. More recently, the latest development in bronchoscopic technology is endobronchial ultrasonography (EBUS). The more detailed imaging of lymph nodes by EBUS may be used to distinguish between benign or malignant lymph nodes, and to guide transbronchial FNA with potentially greater safety and accuracy. Nonetheless, as a relatively new technique, the role of EBUS in the investigation of TB remains to be confirmed by future experience. It should be borne in mind, however, that the total volume of tissue sampled by transbronchial FNA (traditional or EBUS-guided) is substantially smaller than with any of the above surgical procedures. Where transbronchial FNA fails to yield a positive diagnosis, surgery may be considered.
Figure 1 shows a suggested algorithm for selecting an appropriate surgical investigative approach in suspected TB involvement of intrathoracic lymph nodes.
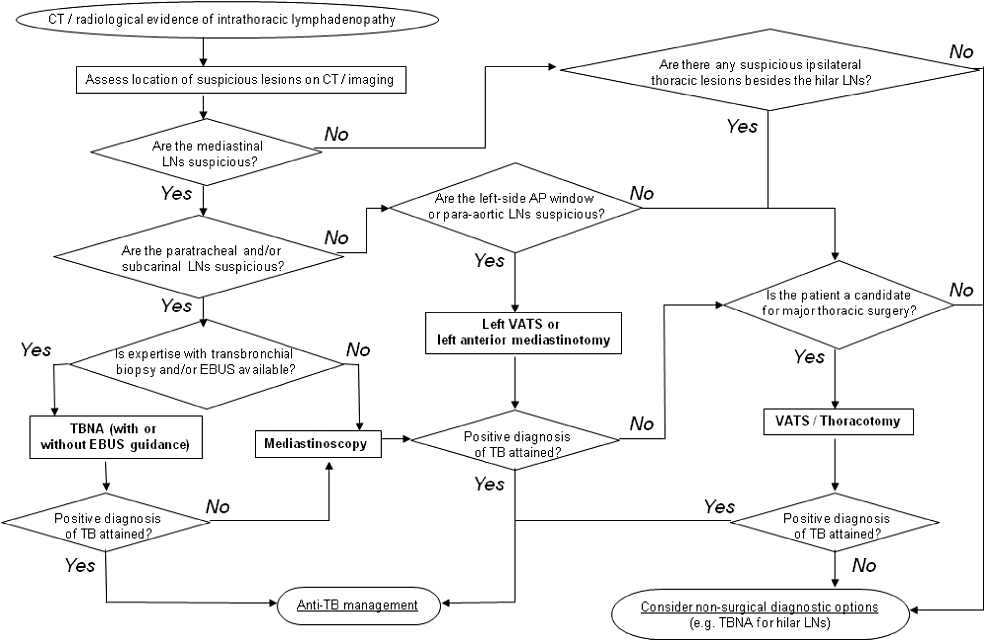
Suggested algorithm for selection of diagnostic surgical modality in investigation of intrathoracic lymphadenopathy suspected to be TB-related. AP, aorto-pulmonary; EBUS, endobronchial ultrasound; LN, lymph node; TBNA, transbronchial needle aspiration; VATS, video-assisted thoracic surgery.
SURGICAL LUNG RESECTION FOR PULMONARY TB
Surgery as adjunctive treatment of pulmonary TB
It cannot be overemphasized that the primary management of TB today is essentially medical, and that anti-TB drugs are highly effective in the vast majority of cases. The various forms of collapse therapy mentioned earlier are often considered obsolete in modern medical practice in the developed world. Nonetheless, there remains a role for surgical lung resection as a therapeutic modality in a small, selected proportion of patients with TB. The most commonly reported indications for surgical therapy include the treatment of MDR-TB, persistent smear- and/or culture-positive TB despite adequate drug therapy; localized disease such as a focally destroyed lung or a cavitary lesion that has developed complications (such as haemoptysis or abscess formation); and infections by mycobacteria other than TB (MOTT) that are symptomatic and unresponsive to antimicrobial therapy.
The problem of MDR-TB deserves special mention. Currently, MDR-TB is recognized as a serious and growing health problem worldwide.34 Because anti-TB chemotherapy occasionally proves inadequate to cure MDR-TB, adjunctive surgical resection has been advocated in selected patients who have relatively localized disease, such as a cavitary lesion or a single destroyed lobe.35,36 The recent emergence of extensively drug-resistant tuberculosis (XDR-TB) denoting MDR-TB with additional bacillary resistance to fluoroquinolones and second-line injectable drugs likely enhances the demand for adjunctive lung resections in patients with this formidable condition.37
Although there has been no randomized study to address the comparative efficacy of chemotherapy versus combined chemotherapy and surgery in the management of MDR-TB, one recent small series reported significant and durable improvement with surgery and postoperative first-line chemotherapy in MDR-TB and XDR-TB patients.38 This suggests the possibility of an independent role of lung resection in the management of these difficult drug-resistant scenarios.
The purpose of surgical treatment is to drastically reduce the mycobacterial burden harboured in the predominant lesion(s).39,40 In recent years, there have been a number of reports of achieving bacteriological cure or improvement in MDR-TB patients using a strategy of pulmonary resection combined with the best available chemotherapy. The results from a selection of the most recent series are shown in Table 2.40–44 It has been suggested that factors predictive of poor outcome in surgical resection for MDR-TB include poor lung function, preoperative haemoptysis, low BMI, primary resistance, resistance to ofloxacin and cavitary lesions that are not amenable to complete resection.41,42
| Case series | n | % Male | Average age (years) | Indications for surgery | % bacteriologically positive at time of surgery | % Pneumonectomy | Bronchial stump management | % Mortality | % Complications | % BPF | % Prolonged air leak | TB treatment outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Shiraishi et al. (2004)40 | 30 | 70 | 48 (median) | Refractory to drug TxHigh risk of relapse | 33 (smear) | 40 | Latissimus dorsi muscle flap | 0 | 30 | 6 | 6 | 100% patients sputum negative postoperatively10% relapse after median follow up for 24 months |
| Kim et al. (2006)41 | 79 | 61 | 29 (median) | Refractory to drug TxLocalized disease | 98 (culture) | 22 | n/a | 1 | 23 | 5 | 9 | 72% culture negative for final 12 months of Tx |
| Somocurcio et al. (2007)42 | 121 | 66 | 27 (median) | Refractory to drug TxProgressive symptomsLocalized disease | 79 (culture) | 22 | n/a | 5 | 23 | 7 | 4 | 73% of patients who were culture positive converted to culture negative postoperatively63% still ‘cured’ at median follow up of 36 months |
| Mohsen et al. (2007)43 | 23 | 87 | 24 (mean) | Refractory to drug TxHigh risk of relapse | 35 (smear) | 48 | No flap used | 4 | 35 | 0 | 33 | 100% patients sputum negative postoperatively4% relapse at 12 months |
| Wang et al. (2008)44 | 56 | 59 | 39 (mean) | Progressive symptomsLocalized disease | 100 (smear) | 45 | 75% with pleural or pericardial patch | 0 | 25 | 16 | n/a | 91% patients sputum negative postoperatively4% relapse at 30 months |
- BPF, broncho-pleural fistula; MDR-TB, multi-drug-resistant tuberculosis.
In selecting any patient with drug sensitive TB or MDR-TB for major lung resection, several principles should be observed. First, there should be a sufficiently localized target area of TB-affected lung (such as a single lobe only) identified by radiological imaging for surgical resection. This is to ensure that there is a reasonable chance of complete surgical clearance of the most diseased lung parenchyma. On the other hand, it has been reported that the morbidity of surgery is associated with the anatomic extent of disease as suggested by preoperative CT.45 The selection of patients for surgery should therefore also take into account the predicted extent of resection based on radiological findings. Second, surgery should not be regarded as curative in the absence of appropriate anti-TB medical therapy. It must be ensured that safe and effective anti-microbial drug therapy has been selected for patients receiving surgery, and which will be available for use before and after surgery. Infection with a strain of TB with resistance to multiple drugs (such as in MDR-TB) is an indication for surgery, yet ideally there should still be enough drug activity to minimize the impact of TB on bronchial stump healing.39 Third, careful assessment of lung function with special precautions is also mandatory. Not only must the patient face the irreversible loss of part of a lung, but the risks of postoperative respiratory complications (see later) are considerable in many TB patients. Such stresses may potentially overwhelm the limited respiratory reserves of some patients, emphasizing the need for caution in patient selection based on preoperative lung function testing.
Once the decision is made to proceed for surgery, it is important to realize that this is an elective and not an emergency procedure. Sufficient time should be spent to fully optimize the patient before surgery. Preferably, patients should receive at least 2–3 months of anti-TB drug therapy before operation.39,40 Ideally, the patient should be rendered culture-negative at least transiently. This not only reduces the risk of postoperative complications and of transmission of TB to operating theatre staff, but also reduces the mycobacterial burden impairing bronchial stump healing. The nutritional status of many TB patients is also often poor, and it is advisable to optimize their nutrition in conjunction with clinical dieticians prior to major surgery. Poor nutrition not only contributes to postoperative complications, but also reduces the bulk of chest wall muscles that may be required for use as flaps during surgery.
The surgery for TB typically involves an anatomical lobectomy, removing the most severely affected lobe of lung. In some situations, bi-lobectomies, pneumonectomies or sublobar resections may be considered.43 The surgical approach of choice is traditionally a full postero-lateral thoracotomy. This is a 20- to 30-cm-long incision, which involves prolonged, forcible rib spreading and often partial resection of one or more ribs. However, increasingly it is possible to perform lung resections via a VATS approach in selected patients. For a VATS lobectomy, a three-port strategy is typically used with one port extended to a 4-cm-long utility incision to allow extraction of the resected lobe. The technique of one of the authors (A.D.L.S.) has been previously described.46 By eschewing rib spreading altogether, the same operation can be performed with potentially less pain and morbidity than open thoracotomy. Figure 2 illustrates the typical incisions used for major lung resections with the open thoracotomy and VATS approaches. It was previously noted by some surgeons that post-inflammatory adhesions between the chest wall and lung necessitated conversion from a VATS to an open approach. However, for experienced VATS surgeons division of pleural adhesions and even extrapleural dissection are technically feasible. Such cases have therefore become a relative rather than an absolute contraindication for VATS. Nonetheless, very dense adhesion is often found around the pulmonary vessels in some TB patients (especially where TB-affected lymph nodes are present), and surgeons should have little hesitation in converting to an open procedure should the risk of vascular injury be high.
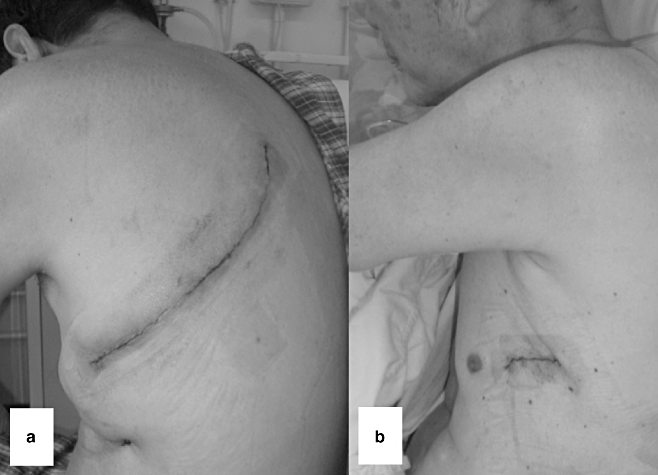
Surgical incisions used for major lung resection for pulmonary tuberculosis. (a) The open thoracotomy approach remains the ‘gold standard’ and provides good access into the chest in technically complex operations. (b) The VATS approach provides equally effective therapeutic results with reduced morbidity in selected patients. The main wound required for extracting the resected lobe is typically around 4 cm long.
Although it is now established that VATS reduces pain and complication rates, and better preserves postoperative respiratory function, immune function, quality of life and shoulder mobility,16–20,46 one of its key advantage is commonly overlooked. With these benefits, VATS may potentially lower thresholds for candidacy for major surgery, so that some patients deemed unfit for surgery in the past may now be eligible for curative lung resection surgery. Recent studies indeed suggest that VATS lobectomy can be safely performed in patients with relatively poor lung function or advanced age.47 Selected TB patients previously regarded as marginal candidate for open surgery may therefore benefit from a multidisciplinary assessment involving respiratory physicians, thoracic surgeons and thoracic anaesthesiologists to consider if VATS surgery be feasible.
Regardless of surgical approach, the operative mortality rate for lung resection in TB in general is typically quoted as less than 5%, and the postoperative complication rate at between 9–26%.40,43,48,49 This compares with a typical mortality rate of less than 3% for lobectomies for lung cancer. The higher risk for lung resections in TB patients reflects that greater technical difficulty in dissecting through dense adhesions and scarring so commonly seen in TB cases. Of the complications, postoperative persistent air leakage resulting from release of pleural adhesions is by far the most common, occurring in up to 40% of cases.50 Because of reduced lung compliance in many patients with TB, residual air space is another complication that may occur due to the residual lung inadequately expanding to fill the pleural cavity. Surgeons routinely endeavour to minimize this problem using an array of surgical techniques, such as pulmonary release procedures, induced phrenic palsy, pleural tent creation, pneumoperitoneum induction and so forth. It is recognized that in MDR-TB cases, recurrent infections may arise in any residual postoperative air space and hence the need to prevent the occurrence of this situation is particularly great in these patients. Other complications include empyema thoracis, pneumonia, wound breakdown and fistulation (notoriously associated with TB), respiratory failure, recurrent laryngeal nerve palsy and others. Acute exacerbations of TB pneumonitis may also occur.
However, the most feared complication after TB surgery is broncho-pleural fistula (BPF). For the thoracic surgeon, a BPF specifically refers to a breakdown of the bronchial stump after surgery. The incidence of postoperative BPF is thankfully low,49 but its significance is that it can quickly lead to a pleural space infection with a high rate of mortality. Once a BPF occurs it is difficult to treat, and may require very radical, debilitating thoracoplasty, muscle flap procedures or prolonged pleural space decontamination strategies.51 TB patients are at particular risk for BPF given their often poor nutritional status at the time of surgery and the usually already inflamed/infected bronchi. Endobronchial TB, in particular, has been identified as a risk factor for development of BPF.44 There have therefore been calls for careful preoperative bronchoscopic assessments or even on-table frozen section analysis of bronchial margins to exclude endobronchial TB, especially in cases of MDR-TB. Most surgeons would attempt to reduce the risk of BPF by covering the bronchial stump with a flap. Such flaps may be fashioned from pericardial fat, from pleura or pericardium, or from pedicled muscles, including pedicled intercostal muscle flaps or latissimus dorsi flaps. This highlights the need for good nutrition prior to surgery in order to maintain healthy viable muscles should muscle flaps become necessary.
Postoperatively, patients who are otherwise free of complications have chest drains removed in 2 days and can usually be discharged home in about 7–8 days (4–5 days if the VATS approach is used). Good nutrition and early mobilization are essential for recovery in TB patients after surgery. If necessary, dietary supplements and even parenteral feeding may be considered. The use of parenteral opiates, which may impair appetite and mobility, are minimized through the use of perioperative regional anaesthesia, regular non-opiate oral analgesics and early drain removal. Anti-TB drug therapy is typically continued for 12–24 months following surgery,39 with perhaps a longer duration for patients with MDR-TB.
Overall, the rate of eventual treatment success (as defined by discontinuation of drug therapy) is quoted to be 75–98%.39,40,43,48 Nonetheless, it remains difficult to gauge the true benefit for surgery given that the sickest patients who most require surgery are paradoxically often the ones who are least physically fit to endure major surgery.36 The fact that many patients receiving surgical treatment often die of other causes means that long-term follow-up data, such as 5-year survival, are often lacking. Consequently, firm guidelines defining the role of surgery have yet to be established. The risks and benefits of surgical therapy for each potential surgical candidate should therefore always be carefully assessed on a case-by-case basis by a multidisciplinary panel involving experienced microbiologists, internists, anaesthesiologists, radiologists, physiotherapists, social workers and dieticians as well as thoracic surgeons.
Surgery for bronchiectasis
One of the corollaries of highly effective anti-TB drug therapy today is that most patients survive the initial TB episode, and hence more patients remain with complications resulting from their disease. The development of bronchiectasis following TB remains a complication still seen in many Asian countries (including China). In some patients, the condition may progress and predispose to significant haemoptysis and/or mycetoma formation. Conventionally, management is medical with symptomatic control of exacerbations and complications as they arise. However, by definition bronchiectasis is irreversible and there is no medical means to halt the progression. Surgery is therefore occasionally indicated in those patients with severe manifestations, such as haemoptysis and recurrent infections.
Emergency surgical management of massive haemoptysis is dealt with separately below. Elective surgery is warranted where the haemoptysis affects the patient's quality of life, or where increasing frequency and/or volume of haemoptysis may be heralding the onset of massive haemoptysis. In such cases, resectional surgery may be considered once the site of the bleeding can be identified by bronchoscopy and/or bronchial angiography. Although CT can be used to highlight sites of maximal bronchiectatic change, such changes alone are insufficiently specific to confirm the site of bleeding when major irreversible surgery is being contemplated. Once a bleeding site is confirmed, alternative approaches such as bronchial arterial embolization (BAE) should always be considered prior to embarking on surgery. BAE is proven to be effective even for severe haemoptysis and is relatively less invasive than surgery.52,53 Surgery more reliably halts the bleeding but, given its traumatic and irreversible nature, elective surgery is typically reserved only for patients in whom BAE is ineffective or unavailable.
Recurrent or recalcitrant lung infections are not uncommon in patients with bronchiectasis. Should frequent admissions be required for this, or if the chronic purulent sputum production causes significant impairment to the patient's quality of life, surgery may be considered. Again, it is important to identify a target for resection. Focal bronchiectasis localized to one lobe, a lung abscess, or a mycetoma represent clear-cut and valid targets for resection. In a patient with a lung abscess who is considered to have high risk for surgery, percutaneous insertion of a drainage catheter remains a less traumatic alternative to surgery.54 For mycetomas, intracavitatary instillation of an anti-fungal agent (such as amphotericin B) has also emerged as a viable alternative to major lung resection in selected patients with poor lung function.55
Whenever surgery is considered for a bronchiectasis patient, it is crucial to fully counsel him or her on the implications of surgery. First, this is technically difficult surgery with a relatively high rate of morbidity due to the extremely dense pleural adhesions associated with bronchiectasis. Second, surgery cannot prevent the progress of bronchiectasis in other parts of the lungs and hence recurrent symptoms after surgery are entirely possible. Third, because surgery results in the irreversible loss of a major part of a lung, it is usually most unlikely that the patient can receive further lung surgery in future should such symptoms recur. Suitably high thresholds for surgery should therefore be maintained for most bronchiectasis patients, especially young ones who may have many years ahead of them during which the disease may progress.
Once decided upon, surgery is performed as described above for primary treatment of TB. Typically, a lobectomy is performed. However, surgery for bronchiectasis is often technically demanding because the chronicity of the disease and the recurrent infections associated with bronchiectasis usually result in extreme degrees of pleural and hilar adhesions. Prolonged surgery, relatively greater blood loss and persistent postoperative air leakage are relatively common. Also, the remaining unresected lung is usually diseased with loss of compliance. Failure of the stiff lung to re-expand and fill the pleural cavity may result in residual air spaces, which may in turn compromise respiration or become infected.
Massive haemoptysis
Massive haemoptysis can occur in patients with active TB, but is more commonly associated with post-TB bronchiectasis. While some degree of blood-stained sputum production is common in many TB or bronchiectasis patients, true massive haemoptysis is thankfully infrequent. Massive haemoptysis can be defined as blood loss into the tracheobronchial tree that causes cardiorespiratory compromise or is life-threatening.56 Massive haemoptysis is a definite medical emergency that carries a mortality of up to 75% if not promptly treated.
In the acute setting, the patient who is coughing up massive volume of blood must first receive haemodynamic resuscitation and be managed in the Intensive Care Unit (ICU). Most patients who die from massive haemoptysis do so because of asphyxiation. Therefore, should any suspicion of respiratory compromise be noted, the patient must be promptly intubated. Ideally, a double-lumen endotracheal tube should be placed by an experienced anaesthesiologist. This provides isolation of the left and right lungs, reducing the risk of blood from the haemorrhaging lung flooding the other lung.
Once the patient is adequately stabilized, the most important consideration is to locate the site of bleeding. Considerable controversy surrounds the relative merits of CT scanning, rigid bronchoscopy and fibre-optic bronchoscopy as the first-line investigation. Regardless of which method is used, determining laterality of the bleeding is vital. First, it allows the patient to be laid in bed with the bleeding side down to protect the non-bleeding lung. Second, should a thoracotomy be required, the surgeon will know which side to incise. Third, should urgent BAE be required, the interventional radiologist will know which side to focus on to find the bleeding source, saving precious time.
The options for haemostasis include rigid bronchoscopy, BAE and emergency surgery. Rigid bronchoscopy under general anaesthesia can be offered by the thoracic surgeon. This aims to remove blood and clots from the airways and then achieve haemostasis through localized therapy, such as application of topical adrenaline, surgical sealants or intrabronchial balloon catheters for tamponade. The advantage of rigid bronchoscopy is that immediate airway control is gained if a patient with active haemoptysis has not already been intubated. However, haemostasis via rigid bronchoscopy is often suboptimal given that the scope only reaches the proximal airways, but the site of bleeding may be very peripheral in the lung. BAE is highly effective in the acute cessation of bleeding, even in severe cases of haemoptysis.52,53 It is less invasive than emergency surgery, and can be repeated if necessary for recurrent episodes. However, the patient must usually be transported to the radiology suite for the procedure, and the process itself may be slow as the interventional radiologist must carefully explore the bronchial arterial vasculature to locate and adequately embolize the bleeding site. Therefore, BAE may not always be suitable for haemodynamically unstable patients who are losing a large amount of blood.
Emergency surgery is the most invasive option. In the emergency setting, there is no role for a VATS or limited thoracotomy approach. Instead, a full postero-lateral thoracotomy is used. In the operating theatre, haemodynamic control is established and the patient is intubated and anaesthetized. Further fibre-optic bronchoscopy can be performed to re-confirm the site of the bleeding. The lung lobe containing the bleeding site is promptly dissected and resected. However, expeditious surgery is often hindered by the inevitable post-TB adhesions, haemodynamically instability during general anaesthesia, and coagulopathy if the patient has had significant blood loss already. Another critical consideration is that in many acutely haemoptysizing patients, lung function tests have usually not been done and it may be impossible to determine if the patient can tolerate major lung resection. For all these reasons, many surgeons may prefer to achieve acute control of blood loss with BAE first. One can then consider if surgery is indicated later as an early elective procedure after BAE has bought time for more thorough patient assessment to be conducted.52
PLEURAL SPACE COMPLICATIONS FROM TB
Empyema thoracis and fibrothorax
The pleural space can be involved in TB. In Hong Kong, around a third of all indeterminate pleural effusions are ultimately found to be secondary to TB.57 As with any parapneumonic effusion, pleural involvement with TB can progress through phases of an exudative effusion, fibrinopurulent empyema thoracis and organized fibrothorax.58 Each phase is managed differently, with most early exudative effusions resolving through control of the underlying infection, with or without simple pleural drainage.
The thoracic surgeon is involved usually for purulent empyema thoracis.59 In such situations, antimicrobial management is often inadequate because of poor drug penetration to the pleural collection. Drainage is also often ineffective because the pus is thick and the collection may be multi-loculated with fibrous septations. Failure to manage the condition can result in persistent sepsis, fistulation of the empyema through the skin or into other viscera, and the development of restrictive fibrothorax. In such cases, the only effective management is surgical drainage and decortication. The first step of the operation is to mechanically break down all the pleural septations, rendering a multi-loculated collection into a unilocular pleural space amenable to complete drainage by one or more chest drains. Next, all the empyema is fully drained and the pleural space lavaged with antibiotic or antiseptic solution. Finally, fibrous exudates on the chest wall and, most importantly, the lung surface are removed as completely as possible by a process of decortication. The objective is to completely free the lung of tethering by the fibrous deposits, allowing it to fully re-expand and fill the pleural cavity.60 Surgery should only be considered complete if all these have been done. It is important that no significant space, which may harbour infection, is left.
For patients with organized fibrothorax, there is often little or no pus to drain. Instead, the fibrous peel that has formed on the lung surface is very thick and often extremely adherent. The decortication required to remove the peel and restore full lung re-expansion can be technically difficult, and has been likened to stripping wallpaper from a wall. Inevitably, the stripped lung surface is raw and prone to air leakage, while there may be considerable blood loss from stripping of the chest wall. Decortication should always be potentially regarded as a highly traumatic operation in such patients. Because of this, it is always preferable to operate on an earlier phase purulent empyema. It must be stressed that the respiratory physician and the thoracic surgeon should work together to identify patients with early evolving empyema for prompt surgery and reduce the incidence of delayed surgery for the sake of patients.59 In patients who present late to the surgeon, it may paradoxically be advisable to delay surgery further unless the patient is septic and requires prompt surgery. This is because over time tissue planes may become better defined between fibrous peel and lung surface, allowing slightly easier decortication. Generally, some thoracic surgeons would recommend that surgery should be performed within 1–2 weeks of onset of a parapneumonic effusion if it does not resolve on medical therapy alone. If the presentation is late, one should probably wait until 6 weeks or more after onset before operating to allow better delineation of the dissection planes. However, these times have not been substantiated by formal studies and should only be regarded as crude ‘ballpark’ figures only.
In terms of preoperative preparation, a CT scan is mandatory for all patients referred for surgery. This is necessary to plan the site of the surgical incision(s) and to locate the major pleural collections to be drained. In addition, patients with TB-related empyema are often diagnosed as having TB only after presenting with the empyema. They may, therefore, not have received the 2–3 months of anti-TB treatment prior to surgery, which is recommended for lung resections for TB. It is usually preferable for the patient to have received a few weeks of anti-TB medication prior to pleural surgery to reduce TB-related complications after surgery.
When performing a drainage and decortication procedure, the traditional approach is via open thoracotomy. In recent years, however, VATS has as a viable alternative to open surgery, even for tuberculous empyema.60 Potential advantages of VATS include better clearance of peel at the lateral chest wall, reduced pain, better patient satisfaction and recovery, and lower rates of wound complications. With growing experience, some thoracic surgeons now find that even extremely thick and dense organized pleural peel can be fully decorticated using VATS, achieving lung re-expansion as good as with open surgery. For individual surgeons, VATS has now become the standard approach used for both purulent empyema and organized fibrothorax, and conversion to open thoracotomy is increasingly rarely required.
Persistent pleural space
Despite much progress in thoracic surgery, not every TB patient with pleural space involvement can achieve full lung re-expansion and sometimes a significant pleural space remains after treatment. In some patients, the underlying pneumonitis caused by TB may have caused interstitial fibrosis or damage of lung parenchyma, so that lung compliance is reduced and lung volume is irreversibly reduced. Some patients experience no further complications or symptoms related to the residual space and can be managed conservatively. In some others, however, there may be persistent or recurrent infection in the pleural space that demands surgical intervention.
Historically, the earliest described intervention for such residual spaces, which become infected, is open drainage. This can range from applying a stoma bag over a pleuro-cutaneous fistula, through open drainage with a corrugated drain, to creation of a window thoracostomy (an Eloesser procedure).61 Popular in the heyday of TB surgery in the mid-20th Century, they are much less commonly performed nowadays. They do, however, retain a role for patients who may not tolerate more radical procedures.
In addition to drainage alone, attempts can be made to clean or sterilize an infected space. Several strategies have been described to do this, such as repeated lavage and/or packing of the pleural space with antiseptics.62 Antiseptic instillations for lavage may be given via an open thoracostomy or via an indwelling chest tube. In some patients, the cleansing of the pleural space is an intermediate step towards subsequent thoracoplasty and/or muscle flap placement. In other patients, the pleural cleansing may represent a definitive procedure, especially if the patient is unfit for more radical surgery and is no longer septic or symptomatic after pleural cleansing.
Should imaging suggest that the persistent space is a result of formation of organized, post-inflammatory pleural peel, surgical decortication may be considered as described above. The technical difficulty and traumatic nature of decortication in such cases should be key considerations in such patients. Typically, such surgery is therefore only indicated if the persistent space gives rise to significant clinical morbidity, such as recurrent infection and/or compromised lung function.
Where a space persists despite the above measures, surgical obliteration of the pleural space defect can be offered to provide a more definitive ‘cure’. This can be done by a thoracoplasty procedure, by filling the space, or by a combination of both.51,61 With thoracoplasty, the principle is that if the lung cannot expand out to meet the chest wall, then the chest wall can be pushed in the meet the lung. Many variations exist on how to do this, but the most common strategy involves multiple rib resections to remove the bony ‘scaffold’ propping up the muscular chest wall. The latter can then be pressed inwards onto the lung surface, obliterating the space. This procedure is disfiguring and can result in respiratory compromise, but it is often highly effective in managing the persistent space. The alternative to thoracoplasty is to fill the persistent space with a solid. Several decades ago, plombage with foreign objects was popular. Today, persistent spaces are most commonly filled by applying a muscle flap (such as latissmus dorsi) into the space in a myoplasty procedure. Again, many variations exist.
AIRWAY STENOSIS FOLLOWING ENDOBRONCHIAL TB
In Hong Kong, it has been estimated that up to 18% of patients with pulmonary TB also have central airway involvement by the disease.9 The involvement of major airways by TB may result in their fibrosis and consequent stricture. This can result in significant airways obstruction with impairment of pulmonary function and exercise capacity and also possibly recurrent infections. In parts of the world where TB is endemic, many people may be unaware that they have previously contracted TB. It is an unfortunate but not uncommon occurrence in such regions for patients presenting with obstructive airways disease and recurrent chest infections to be managed for many years as having ‘asthma’ before it is realized that they actually suffered from post-TB airway stenosis. Clinicians should remain vigilant when facing patients from TB-endemic countries with asthma-like symptoms that do not respond to standard asthma therapy.
Patients with significant impairment of quality of life or with recurrent lung infections resulting from post-TB airway strictures can be considered for surgical intervention. Investigations for potential surgical candidates should include fibre-optic bronchoscopy and CT scanning. Modern high-resolution CT scanners can produce 3D images of the airways or ‘virtual bronchoscopy’ images reconstructed from the digital scan data. These high quality images have proved an invaluable asset in airways assessment, but should nonetheless not be regarded as a complete substitute for fibre-optic bronchoscopy. The object of the imaging is not only to locate the stricture and the degree of narrowing, but to assess the airways and lung distal to the stricture. If all the distal airways are significantly narrowed, for example, dilatation of a more proximal stenosis alone will hardly improve ventilation into that part of the lung. Similarly, if the lung parenchyma in the part of the lung distal to the stricture is grossly destroyed by TB and is essentially non-functional, it may be more effective to resect that part of the lung to prevent recurrent infections instead of merely relieving the stenosis. The ideal candidate for intervention should have a short segment stenosis of the trachea, main bronchi or lobar bronchi, with relatively patent airways and relatively healthy lung tissue distal to the stenosis. Patients with very long stenotic segments (several centimeters or more) or with stenotic airways extending to the segmental bronchi or beyond are usually not good candidates.
The ‘gold standard’ for managing short-segment benign strictures of proximal airways (trachea and main bronchi) remains surgical resection of the stricture with re-anastomosis, or more complex tracheobronchoplasty operations. Although effective, these operations are technically demanding and best results are usually seen only with high-volume specialist centers highly experienced in airways surgery. Furthermore, re-stenosis at anastomotic sites has been reported.
In regions where expert airway surgery is not so readily available, airway dilatation and stenting has emerged as an effective and less traumatic alternative.63,64 The procedure is performed via rigid bronchoscopy with the patient under general anaesthesia. Through the rigid bronchoscope tube, the stenotic segment can be progressively dilated by the surgeon using gradated bougie or balloon dilators. Once an adequate airway diameter is achieved, an airway stent can be placed to maintain the airway patency. A well-placed stent will not only maintain airway patency, but may also help ‘mould’ the airway to the dilated diameter and probably prevent subsequent re-stenosis even after eventual stent removal. For technical reasons, it is usually not feasible to dilate and stent airways more distal than the lobar bronchi level. It is also technically more difficult to dilate the upper lobe bronchi. This emphasizes the need for accurate CT or bronchoscopy to assess the stenosis.
Airway stents generally fall into the categories of either the self-expandable metallic stents (SEMS) or the silicone stents. Although effective, SEMS are regarded by many surgeons as less user-friendly because they are difficult to reposition or remove after placement. There are also concerns over their potential for triggering airway inflammation and other complications.65 Silicone stents are not as rigidly fixed as SEMS in the airway lumen, and rarely has it been reported that they may be coughed out by patients. However, their advantages are that they are easy to place, position and remove should they be suspected of causing any complications. Tracheobronchial dilatation plus stenting with silicone stents has already been shown to provide effective and lasting treatment for airway stenosis secondary to TB.63 After placement, controversy exists over if and when the stent(s) should be removed. Empirically, early pioneers suggested removal after 6–12 months. However, more recent reports have shown that silicone stents are well tolerated in patients for up to 5 years.66 Some surgeons now choose to only remove stents should they show dislodgement or complications (such as local mucosal inflammation or plugging by sputum).
Patients receiving rigid bronchoscopy, dilatation and stenting can generally be discharged home the morning after the procedure. Regular surveillance bronchoscopy may be offered as an outpatient procedure for stented patients following discharge.
OTHER SURGICAL ISSUES IN TB PATIENTS
The above sections have outlined the main areas where the thoracic surgeon is most commonly involved in the management of TB. However, given the capacity of M. tuberculosis to affect almost any part of the human body, other clinical manifestations may also require the attention of the thoracic surgeon. Secondary spontaneous pneumothorax from the diseased lungs of TB patients is sometimes encountered, but is managed in the same fashion as other secondary pneumothoraces.67 Mediastinal lymphadenopathy from TB has been reported on rare occasions to cause significant airway compression and compromise in young children. These can be removed or decompressed using a VATS or open surgical approach.68 Cold abscesses in the chest wall are infrequently encountered, but can be readily excised or surgically debrided in most cases without undue difficulty.69
It is perhaps worth mentioning that TB and lung cancer can coexist in the same patient, especially in TB-endemic regions. This can pose clinical challenges for the respiratory physician and thoracic surgeon alike in several ways.70 First, when investigating suspicious lung lesions, a diagnosis of TB does not automatically exclude the presence of lung cancer. In particular, lung cancer has been reported to occasionally arise within pre-existing lung opacities that have resulted from previous TB infection (the so-called ‘scar cancers’).71 Second, TB can confound the accuracy of non-invasive staging modalities for lung cancer. It is recognized that enlarged lymph nodes on CT or hypermetabolic lesions on PET can just as easily represent TB as metastatic deposits.72 This emphasizes the need to involve the surgeon in invasive staging for suspicious lesions in TB-endemic regions. Third, as mentioned above, previous TB can give rise to significant pleural and hilar adhesions, which can considerably increase the technical difficulty of major lung resections. In Hong Kong almost half of all patients receiving lobectomy for lung cancer have histories or radiological evidence of previous pulmonary TB. How this may affect the results of lung cancer surgery in TB-endemic countries compared with countries where TB is rare has never been addressed.
CONCLUSIONS
Although the management of broncho-pulmonary TB remains mostly medical, for selected subgroups of TB patients surgery provides unique diagnostic and therapeutic options. Today, modern surgery not only offers highly effective treatment of TB and its sequelae, but promises to deliver it with less trauma and morbidity than ever before. The advantage of minimally invasive thoracic surgery is not only its capacity to reduce morbidity for individual patients, but its potential to allow a wider range of TB patients to be considered for effective surgical management. It behooves respiratory physicians and thoracic surgeons to continue working in partnership to identify those TB patients who can benefit from surgery.




