Diagnosis of hospital-acquired pneumonia and methods of testing for pathogens
SUMMARY
• Hospital-acquired pneumonia is diagnosed in patients who, in addition to abnormal shadowing on chest radiography, have ≥2 of the following: fever, abnormal white blood cell count and purulent discharge.
• Treatment effect is judged from clinical symptoms and microorganism test results 2–3 days after the start of treatment, and reassessment is made with regard to change, addition or discontinuation of antimicrobial agents.
• Coordination with the microbiology laboratory is extremely important in diagnosing infectious diseases.
• Microorganisms isolated from tracheal aspirate at 106 cfu/mL (3+), from BAL at 104–105 cfu/mL (2+) and from a protected specimen brush at 103 cfu/mL (1+) have a high possibility of being the causative microorganisms.
• Pneumonia can almost be ruled out when no significant microbes are detected from the lower respiratory tract in patients with suspected ventilator-assisted pneumonia (when no change has been made to antimicrobial administration within 72 h).
• When MRSA or Pseudomonas aeruginosa are not detected in sputum tests, involvement of these drug-resistant bacteria may be considered unlikely, and the case treated accordingly.
• Involvement of aspiration is suspected when a number of pathogens are observed in lower respiratory tract specimens.
• When antimicrobials are administered with reference to breakpoint concentrations in Western countries, differences in dosage between these countries and Japan need to be considered.
INTRODUCTION
In addition to the causative microorganisms in community-acquired infection, opportunistic pathogens with weak pathogenicity are a problem in hospital-acquired pneumonia. A particular problem in hospital-acquired pneumonia is the high involvement of drug-resistant bacteria, such at MRSA, glucose non-fermenters, such as Pseudomonas aeruginosa, and enterobacteria, such as Escherichia coli, Klebsiella and Enterobacter. To treat hospital-acquired pneumonia patients effectively, identifying the causative microorganism is crucial. However, in actual practice, many patients must be treated while the cause remains unclear, as appropriate specimens cannot be obtained or limitations exist to the test methods themselves. Mortality rates from hospital-acquired pneumonia remain high, and obtaining specimens properly and quickly and efficiently identifying the pathogen is essential when pneumonia is suspected.
This report is based on the 2002 Guidelines for Respiratory Infections: Basic Concept for the Management of Hospital Acquired Pneumonia in Adults (Japanese Respiratory Society),1 and has been made with reference to the hospital-acquired pneumonia guidelines prepared jointly by ATS/IDSA in 2005.2 Together with additional evidence revealed since those reports, specific Japanese perspectives have also been incorporated, such as the importance of coordinating with the microbiology laboratory.
CLINICAL DIAGNOSIS OF HOSPITAL-ACQUIRED PNEUMONIA
Accurate clinical diagnosis of hospital-acquired pneumonia is often difficult. Hospital-acquired pneumonia is usually suspected based on the appearance of abnormal shadows on chest radiography, but this finding is caused by conditions other than infection in a fair number of cases. Important conditions that may be difficult to distinguish from hospital-acquired pneumonia include heart failure, atelectasis, pulmonary thromboembolism, drug-induced lung disease, pulmonary haemorrhage and ARDS (see Chapter VI). Methods of clinical diagnosis have been proposed to distinguish pneumonia from these conditions by including biological reactions induced by infection, in addition to abnormal infiltration on chest radiography.2,3 Hospital-acquired pneumonia is thus diagnosed if, in addition to the appearance of abnormal and worsening shadows on chest radiography, two of the following three criteria are fulfilled: (i) fever ≥38 °C; (ii) abnormal white blood cell count (increased or decreased); and (iii) purulent discharge. Based on the finding that delays in treatment are related to increased mortality rates, these diagnostic criteria were established with the aim of minimizing false negatives, even if that means accepting a small number of false positives. Meanwhile, in cases showing no abnormal shadows on chest radiography, bronchitis or tracheobronchitis are suspected even if symptoms (i)–(iii) are seen. These criteria were established with the aim of diagnosing hospital-acquired pneumonia soon after onset, and the validity of the diagnosis needs to be evaluated 2–3 days after the start of treatment based on trends in subsequent clinical symptoms and the results of laboratory tests (Fig. III-1). If improvements in clinical symptoms are seen at this time and no significant microbial growth is identified from laboratory cultures, antimicrobial treatment may be discontinued. In cases where symptoms improve but a pathogen that may be a causative microorganism is isolated, de-escalation of antimicrobials can be considered together with the results of drug susceptibility tests (see Chapter IV for details on de-escalation). When clinical symptoms worsen, on the other hand, the antimicrobial is changed based on culture results, or a reevaluation is made of pathogens that are difficult to culture, infections in different locations, or factors other than infection.
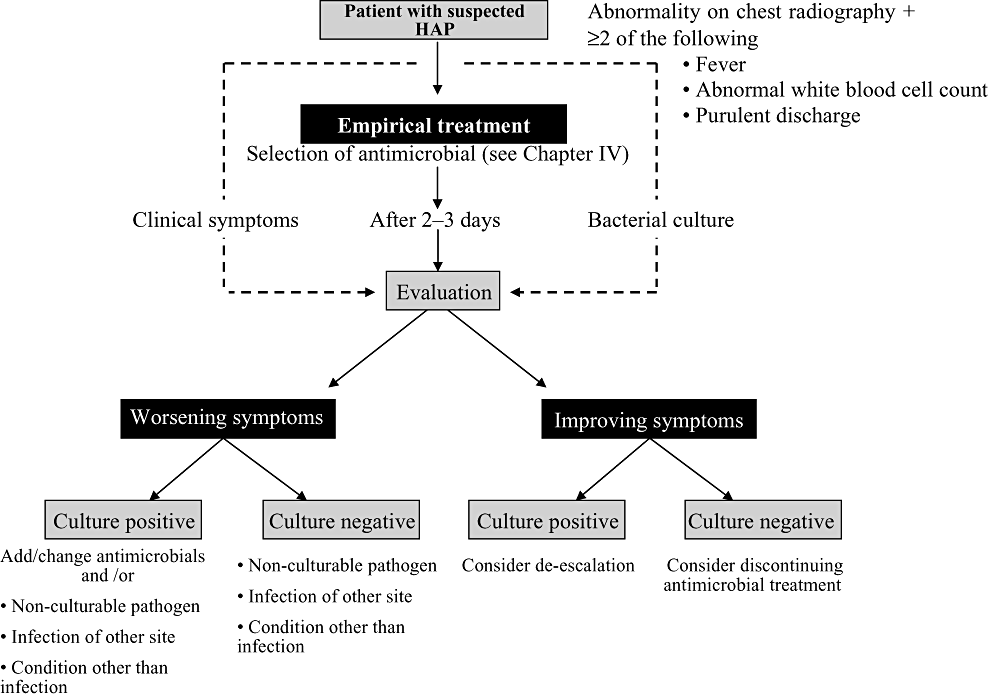
Flow of pathogen tests for cases of suspected hospital-acquired pneumonia (HAP).
CRP, a marker of inflammatory response, is not a specific indicator of pathology, but is elevated in infectious diseases together with malignant tumour and other tissue injuries, including collagen disease, trauma and burns. CRP is a protein made in the liver that reacts with cytokines produced at the site of inflammation. Historically, CRP was discovered as a factor that is elevated in response to capsular polysaccharide (the ‘C’ in CRP) in pneumococci. Caution must therefore be exercised with regard to the fact that in patients with decreased ability to synthesize proteins in the liver, or in hosts with suppression of local cytokine production, elevations in CRP will remain slight regardless of the severity of the condition, even when a high level of tissue damage is present.
Reports in recent years have described potential new test methods for the diagnosis of pneumonia. Procalcitonin is a precursor of calcitonin, one of the thyroid hormones, and is produced throughout the body in cases of bacterial infection, in correlation with the severity of infection. In particular, higher levels are seen in patients with bacterial sepsis, in comparison with viral or fungal infections, and use of procalcitonin levels is reportedly effective in diagnosing bacterial pneumonia.4,5
Triggering receptor expressed on myeloid cells (TREM)-1 is a member of the immunoglobulin superfamily, and is expressed strongly in neutrophils and other inflammatory cells. Gibot et al. reported from a study of 148 patients with ventilator-assisted pneumonia that sensitivity was 98% and specificity was 90% when diagnosing fungal or bacterial pneumonia with elevated TREM-1 in BAL as a marker.6 In addition, pro-atrial natriuretic peptide7 and high-mobility group box 1 (HMGB1), which are recognized as late-stage mediators in sepsis, have been detected in the serum of pneumonia patients, and have been reported to indicate a high level of severity in pneumonia.8 These tests are not ready to be applied clinically any time soon, but indicate new possibilities for the diagnosis of hospital-acquired pneumonia. In the future, investigations in more detail with greater numbers of patients will be needed.
- •
Hospital-acquired pneumonia is diagnosed in patients who, in addition to abnormal shadowing on chest radiography, display ≥2 of the following: fever, abnormal white blood cell count and purulent discharge.
- •
Treatment effect is judged from clinical symptoms 2–3 days after the start of treatment and the results of laboratory tests.
- •
If improvement is seen in clinical symptoms 2–3 days after the start of treatment, and no predominant microbes are detected from lower respiratory tract specimens, discontinuation of antimicrobial treatment can be considered.
- •
De-escalation of antimicrobials is investigated based on the drug susceptibility results for the causative microorganism.
COLLECTING LOWER RESPIRATORY TRACT SPECIMENS: INVASIVE AND NON-INVASIVE TEST METHODS
As a rule, specimens obtained from the lower respiratory tract are tested to find the pathogen causing hospital-acquired pneumonia. However, in actual practice, only respiratory secretions or sputum collected non-invasively can be tested in many cases, for reasons such as the general condition of the patient. Obviously, the effects of contamination by resident microbes within the oral cavity must be considered when using these specimens. Sputum is often collected from intubated patients, but complete elimination of contamination by resident oral bacteria is impossible even with these samples. By considering the semiquantitative (quantitative) evaluation of isolated microorganisms or smear test findings (phagocytosis by white blood cells, presence of microbes corresponding to the site of leukocyte aggregation etc), the presence of the causative microorganism can also be inferred (described later).
Invasive methods that are often used include protected specimen brush (PSB) and BAL, but the indications for these methods must be judged carefully in consideration of effectiveness and risks. These methods of collecting specimens are often indicated in intubated patients, patients in whom suitable respiratory secretions cannot be obtained and in cases with the suspected involvement of drug-resistant bacteria, mycobacteria or fungus. They are also useful in distinguishing from non-infectious diseases that are difficult to discriminate in terms of clinical course. With the aim of decreasing contaminants, protected BAL using a catheter with a balloon-covered tip has been attempted, and 97% sensitivity and 92% specificity have been reported by combining protected BAL with quantitative culture.9 While some findings have shown the effectiveness of distinguishing between contaminating and causative microorganisms by assessing the volume of microbes in a specimen (semiquantitative and quantitative culture), problems remain in relation to the skills and efforts of laboratory personnel and time of onset, as well as in establishing the volume of microorganisms for judgment. Many reports have taken the results as clinically significant for bacterial counts ≥106 cfu/mL in tracheal aspirate, 104–105 cfu/mL in BAL and ≥103 cfu/mL in PSB, but these should not be considered absolute values10–12 (Table III-1).
| Test method | Microbial volume judged to be significant | Semiquantitative evaluation | Sensitivity/specificity (%) |
|---|---|---|---|
| Endotracheal aspiration | 106 cfu/mL | ≥3+ | 76 ± 9/75 ± 28 |
| BAL | 104–105 cfu/mL | 2+ | 73 ± 18/82 ± 19 |
| PSB | 103 cfu/mL | 1+ | 66 ± 19/90 ± 15 |
- From references 10–12. PSB, protected specimen brush.
Lower respiratory tract specimens are often contaminated with resident bacteria from the upper respiratory tract, and if even a few bacteria are present at the time of the laboratory test, the number can easily increase with changes in patient condition. This is part of the difficulty in dealing with infectious diseases in immunocompromised hosts. Once a microbe is detected, completely ruling out pneumonia is difficult, and antimicrobials need to be administered in a large number of patients just to be on the safe side. Hayon et al. conducted a prospective study of 125 patients with ventilator-assisted pneumonia to examine the effectiveness of bacterial culture before the appearance of symptoms, and noted the limited effectiveness of rutin culture of lower respiratory tract specimens.13 The significance of surveillance culture in intubated patients, and how to deal with microbes isolated as a result of surveillance culture, are difficult problems. In contrast, in cases where pneumonia is suspected and tracheal aspirate is collected in patients who use a mechanical ventilator, and significant numbers of bacteria or inflammatory cells cannot be observed, the possibility of complication with pneumonia is low. In cases where changes or additions have not been made to antimicrobials within 72 h and no microbes have been cultured from lower respiratory tract specimens, pneumonia can be essentially ruled out.14 In addition, as indicated in the guidelines for community-acquired pneumonia in the USA (2007), when Staphylococcus aureus or Gram-negative bacteria are not detected in sputum tests, the recommendation is that cases be treated on the assumption that involvement of these pathogens is unlikely.15 Similarly, when MRSA or Pseudomonas aeruginosa are not detected in sputum tests in cases of suspected hospital-acquired pneumonia, involvement of these drug-resistant bacteria is thought to be unlikely and narrow-spectrum antimicrobials are selected or de-escalation of antimicrobials is considered.
- •
In searching for causative pathogens of hospital-acquired pneumonia, the general rule is to conduct laboratory culture using lower respiratory tract specimens.
- •
Causative microorganisms can be predicted from semiquantitative (quantitative) laboratory culture and smear test findings.
- •
Collection of lower respiratory tract specimens by PSB or BAL must be judged carefully in consideration of their effectiveness and risks.
- •
Invasive test methods are indicated when appropriate respiratory secretions cannot be obtained, involvement of drug-resistant bacteria, mycobacteria or fungi is suspected, or non-infectious diseases cannot be ruled out.
- •
Microorganisms are likely to be the causative microorganisms when isolated from tracheal aspirate at ≥106 cfu/mL, from BAL at 104–105 cfu/mL, and from PSB at ≥103 cfu/mL.
- •
Pneumonia can nearly be ruled out when no significant microbes are detected from the lower respiratory tract in patients with suspected ventilator-assisted pneumonia (if no changes have been made to antimicrobial administration within 72 h).
- •
When MRSA or Pseudomonas aeruginosa are not detected in sputum tests, involvement of these drug-resistant bacteria is thought to be unlikely and narrow-spectrum antimicrobials can be selected or de-escalation of antimicrobials considered.
CHARACTERISTICS OF MICROORGANISM TEST METHODS AND ESTIMATION OF CAUSATIVE PATHOGENS
Similar to bacterial tests for other infections, (i) smear microscopy; (ii) isolation culture; (iii) detection of pathogen antigens; (iv) gene diagnosis; and (v) measurement of serum antibody titre are conducted to diagnose hospital-acquired pneumonia. Selection and performance of appropriate tests with reference to the clinical characteristics and test findings in each case is important, while considering the frequency of causative pathogens for hospital-acquired pneumonia in the community or within hospitals (Fig. III-2).
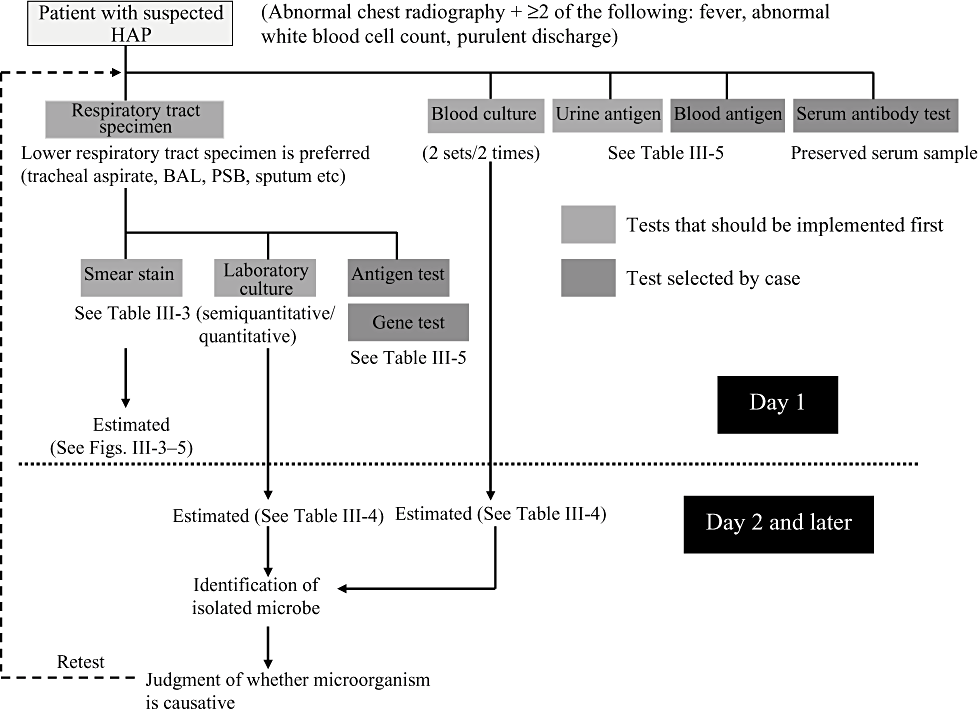
Search for causative pathogen in cases of suspected hospital-acquired pneumonia (HAP). PSB, protected specimen brush.
1. Smear tests
The most important point in smear tests is a qualitative evaluation of whether the specimen that has been collected is worth testing. With sputum, in particular, specimens are judged to be suitable for microorganism tests based on macroscopic assessment (Miller and Jones’ classification) and microscopic assessment (Geckler's classification) (Table III-2). Collection of appropriate specimens is fundamental to microorganism tests, and if the sputum is not of satisfactory quality further measures will be required, including the collection of another specimen. Appropriately collected specimens from the lower respiratory tract, such as aspiration sputum, BAL and PSB, are useful and reliable. Macro- and microscopic assessments as well as stains of representative pathogens that can be inferred from smear tests are shown in III-3-III-5. The presence of pathogens that are major causative agents of hospital-acquired pneumonia, such as Pseudomonas aeruginosa (mucoid strains), Klebsiella and staphylococci, can be identified quickly and with a high degree of accuracy. Involvement of aspiration can also be estimated from the presence of multiple pathogens.
| Gross evaluation (Miller & Jones classification) | |
|---|---|
| Classification | Properties of sputum |
| M1 | Saliva, completely viscous sputum |
| M2 | Small amount of purulent sputum included in viscous sputum |
| P1 | Purulent portion comprising <one-third of all sputum |
| P2 | Purulent portion comprising one-third–two-thirds of all sputum |
| P3 | Purulent portion comprising >two-thirds of all sputum |
| Microscopic evaluation (Geckler's classification) | ||
|---|---|---|
| Classification (group) | Cell number/field (microscopic test ×100) | |
| Leukocytes (neutrophils) | Squamous cells | |
| 1 | <10 | >25 |
| 2 | 10–25 | >25 |
| 3 | >25 | >25 |
| 4 | >25 | 10–25 |
| 5 | >25 | <10 |
| 6 | <25 | <25 |
- Specimen confirmed grossly to contain purulent portion, and bacterial examination requested.
- Geckler's classification groups 4 and 5 (numerous neutrophils, few epithelial cells) considered to be suitable for tests. However, some patients with few leukocytes are also seen in group 6.
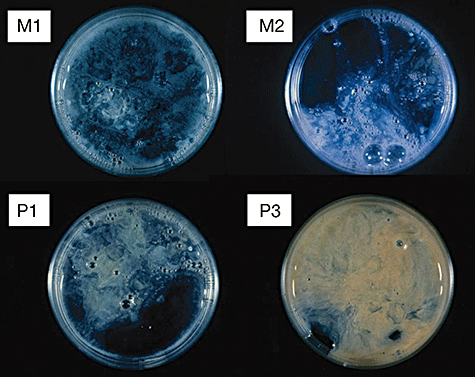
Gross evaluation (Miller & Jones classification).
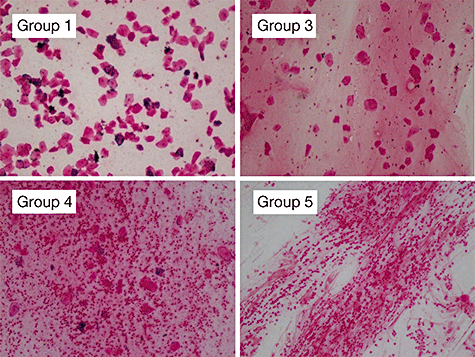
Microscopic evaluation (Geckler's classification).
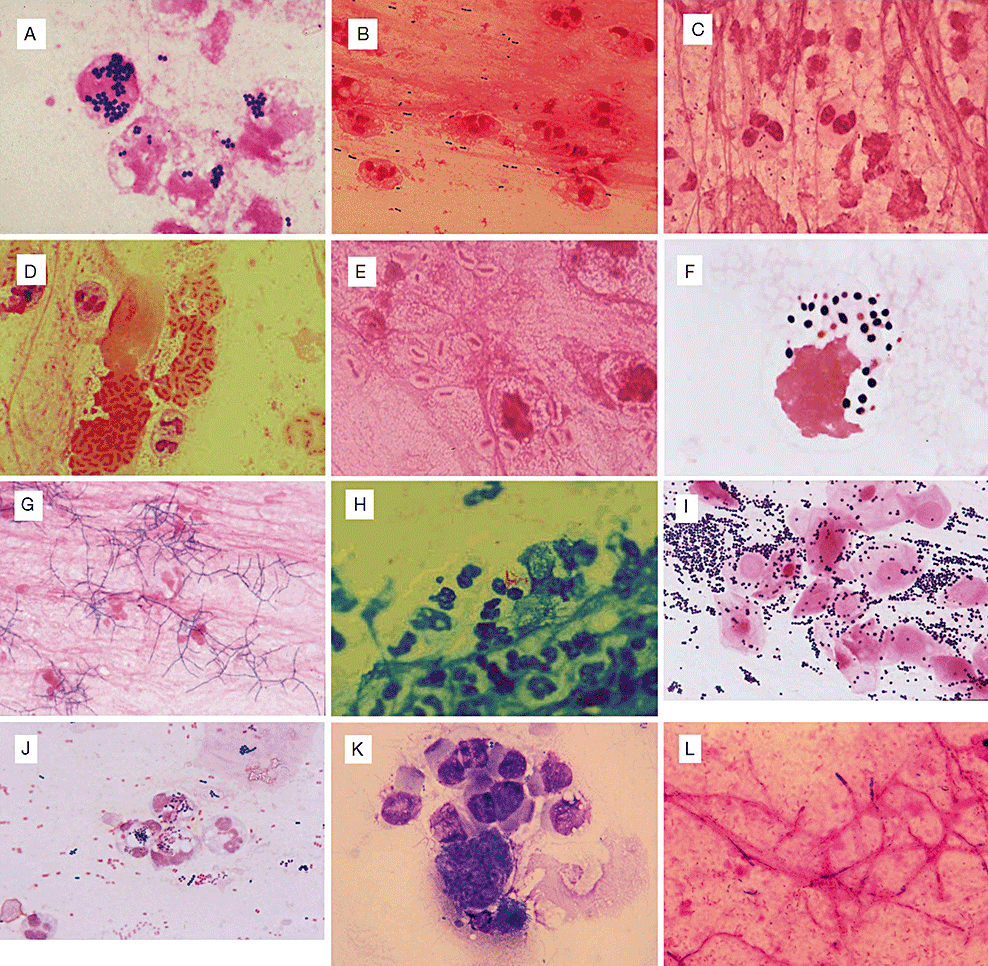
Pathogens that can be inferred from smear tests. (A) Staphylococcus aureus phagocytosed by leukocytes (several days later Staphylococcus aureus was identified). (B) Streptococcus pneumoniae pneumonia (numerous Gram-positive Diplococci are observed. Capsules are seen as transparent layers around bacterial cells). (C) Acute exacerbation caused by Haemophilus influenzae seen in a patient with chronic pulmonary occlusive disease (numerous Gram-negative short rods are seen). (D) Mucoid Pseudomonas aeruginosa (weakly Gram-negative stained mucoid substances are seen around Gram-negative rods). (E) Klebsiella pneumonia (large Gram-negative rods are seen, surrounded by thick, transparent capsules). (F) Cryptococcus infection (bacterial cells are stained Gram-positive, and surrounding capsules are observed as transparent layers). (G) Gram-positive rods, with characteristic branching observed (several days later, Nocardia was cultured aerobically). (H) Mycobacteria observed within cells (Mycobacterium tuberculosis identified several days later). (I) Staphylococcus is observed in many epithelial cells. Identified as adhesive bacteria. (J) Aspiration pneumonia (Escherichia coli, Staphylococcus aureus, streptococcus, and many other species of bacteria cultured). (K) Pneumocystis jirovecii (modified Giemsa stain). (L) Aspergillus (Gram-negative staining hyphae observed).
Smear tests also provide important information not only for identifying the presence of microbes in a specimen, but also in determining whether those microbes are causative organisms or contaminants. In particular, findings of phagocytosis by leukocytes and the presence of bacteria corresponding to the site of leukocyte aggregation may be considered findings that suggest that the observed microbe is a causative agent. In cases of suspected infection from special microorganisms (mycobacteria, Legionella, fungi), corresponding staining methods are employed (Table III-3). In addition, special staining (Grocott's methenamine silver stain, fluorescent antibody staining) of tissue obtained by transbronchial lung biopsy may lead to a definitive diagnosis.
| Causative microorganism | Specimen | Staining method | Laboratory culture and methods of diagnosis |
|---|---|---|---|
| Aerobes/facultative anaerobes | Respiratory tract specimen | Gram stain | Medium using rutin |
| Anaerobes | Respiratory tract specimen | Gram stain | Anaerobic culture (note transport method) |
| Legionella | Respiratory tract specimen | Gimenez stainAcridine orange stain | BCYE-α medium etc, urine antigenGene test, serum antibody titre |
| Nocardia, Actinomyces | Respiratory tract specimen | Gram stainModified acid fast bacteria stain (Nocardia) | Long-term culture (Actinomyces is an anaerobe) |
| Mycobacteria | Lower respiratory tract specimen | Acid-fast bacteria stain | Ogawa medium, MGIT |
| Induced sputum, gastric juice | Auramine-rhodamine stain | Gene diagnosis, QuantiFERON test | |
| Fungi | Lower respiratory tract specimen | HE stain | Sabouraud's medium etc |
| Lung biopsy tissue | Grocott's methenamine silver stain | Blood antigen detection on Sabouraud's medium (mannan, galactomannan, etc.) | |
| Pneumocystis | Lower respiratory tract specimen | Giemsa stain, Diff-Quik stainGrocott's methenamine silver stain, fluorescent antibody staining | Blood antigen detection (β-D-glucan) |
2. Isolation culture methods
Several days are required until the results of isolation cultures are known, so these cultures are of little use from the perspective of selecting an antimicrobial for initial treatment. However, in cases of hospital-acquired pneumonia, the causative microorganism is often an opportunistic pathogen that shows resistance to antimicrobials. To avoid complicating the treatment, isolation cultures are important for identifying the causative microorganism and clarifying drug susceptibility. In cases of suspected hospital-acquired pneumonia, quantitative culture tests are best. In practice, however, many hospitals use semiquantitative tests instead, and assessment must therefore be made as to whether a microorganism is the causative agent while estimating bacterial number from the test results (Table III-1). In addition, final determination of the causative agent and clarification of drug susceptibility are important for a proper understanding of the epidemiology of the infection, and at the same time can be used as a valuable source of information for empiric treatment.
The relation between culture test results and the clinical approach is shown in Figure III-6. Whether a microbe isolated from the lower respiratory tract is the causative microorganism, a contaminant from the upper respiratory tract or a colonizing bacteria is often difficult to judge. In smear tests, infection may be suspected from findings of phagocytosis, presence of microorganisms corresponding to the site of neutrophil aggregation or the bacterial counts, whereas non-infectious conditions (colonization, contamination) are presumed from findings such as epithelial cell predominance, small number of neutrophils, and microbes resident in the oral cavity. In cases of suspected infection, antimicrobial treatment is started (aggressive therapy). However in actual practice, determination is difficult in many cases, and situations arise in which there is no choice but to start administration of antimicrobials, including cases in this borderline region. Repeated tests, including laboratory cultures, need to be conducted in cases when distinguishing between infection, contamination, and colonization is difficult, and minimizing unnecessary administration of antimicrobials is important. Conversely, when laboratory culture of sputum is negative, the involvement of MRSA, Pseudomonas aeruginosa or other drug-resistant bacteria is thought to be unlikely, and de-escalation of antimicrobials or a change to narrow-spectrum antimicrobials is warranted. When cultures of lower respiratory tract specimens yield negative results, infection is thought to be unlikely and discontinuation of antimicrobials can be considered.
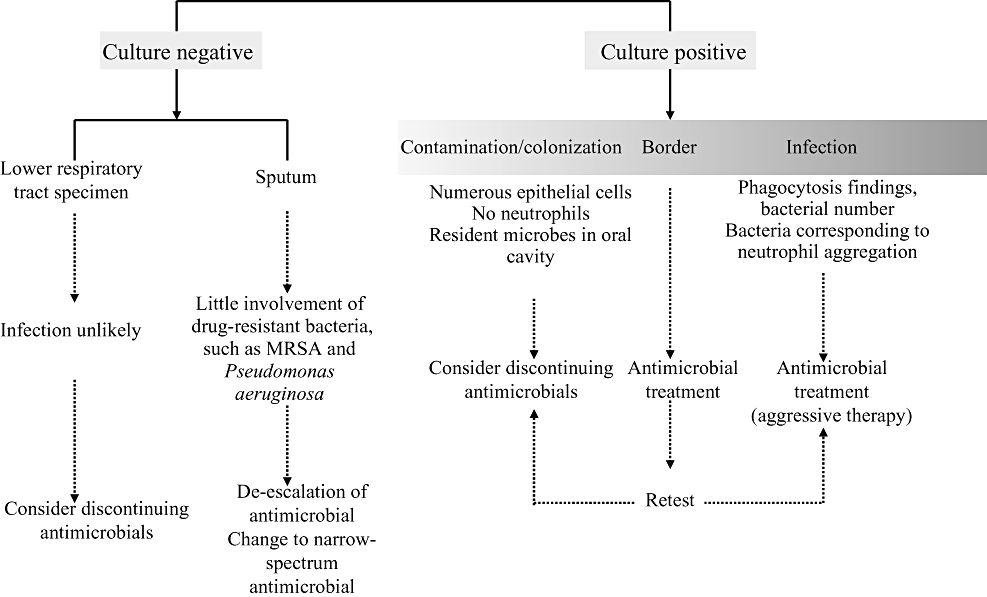
Correlations between laboratory culture results and clinical approach. MRSA, Methicillin-resistant Staphylococcus aureus.
In diagnosing infectious diseases, coordination with the microbiology laboratory is extremely important. Particularly when anaerobic bacteria or pathogens that are difficult to grow are suspected, the microbiology laboratory needs to be consulted with regard to methods of collecting, preserving, and transporting specimens and other matters. In addition, depending on the bacterial species, isolated microorganism can be inferred with a high degree of certainty from the results of quick kits and simple identification techniques in some cases, even while still working to identify the microorganism definitively. Such ‘results before the final report’ can provide very useful information derived through close cooperation between clinicians and the microbiology laboratory (Table III-4).
| Target species | Bases for presumption |
|---|---|
| Glucose non-fermenter | Presumed glucose non-fermenter from oxidase test positivity |
| Pseudomonas aeruginosa | Oxidase test positive and pigmentation (blue-green–yellow) |
| Acinetobacter | Oxidase test negative and Gram-negative short rod formation |
| Stenotrophomonas | Oxidase test negative and characteristic yellow colonies |
| Staphylococcus aureus | Pale yellow or white, weak β haemolysis, coagulase test positive |
| Pneumococcus | Central hollow, α haemolysis |
| Klebsiella | Characteristic mucoid colony |
| Moraxella | Characteristic colony |
| Legionella | Grows only on special media, such as BCYE-α |
| Nocardia | Gram-positive rods showing aerobic branched growth |
| Actinomyces | Gram-positive rods showing anaerobic branched growth |
Opinions are divided with regard to the usefulness of blood cultures in diagnosing hospital-acquired pneumonia. In many reports, the positive rate for blood culture is <20% in patients with hospital-acquired pneumonia. Moreover, judging whether a microorganism is derived from pneumonia or from another infection site is difficult even when blood cultures are positive.16 Microorganisms isolated from lower respiratory tract specimens and also cultured from blood culture can be considered likely to be the causative microorganism of pneumonia.
3. Detection of pathogen-derived antibodies
In recent years, a method that can quickly and with high sensitivity detect pathogen antibodies or their metabolites in patient specimens has been applied clinically. This method is not only fast, but also has the characteristic that positive results can be obtained even in patients who are already receiving antimicrobials. This method is often used with aseptic specimens such as blood, pleural effusion, ascites and cerebrospinal fluid. Table III-5 shows representative methods of detecting pathogen antigens and target species for pneumonia. Tests that target group A haemolytic streptococcus, influenza virus, pneumococcus, and Legionella in particular are widely used and their utility has been confirmed. However, in tests of respiratory tract specimens, such as pharynx or nasal wipe materials, the possibility obviously arises of false-negative results depending on collection site and time. Caution also needs to be exercised for false-positive results in tests of blood antigens such as endotoxin, and β-D-glucan. Meanwhile, antigens in urine that are detected in patients with pneumococcus or Legionella are known to continue to be positive several weeks after first detection.17 Elsewhere, pneumococcal urine antigen shows a high false-positive rate in children, and clinicians should understand in using the test for Legionella urine antigen that this is basically a test for L. pneumophila serogroup 1. Recently, a method has been developed to detect pneumococcus antigen quickly using respiratory tract specimens, and further study is needed for problems including that of distinguishing from microbes resident in the oral cavity.
| Specimen used | Target microorganism/antigen | Characteristics | Test time | Test method |
|---|---|---|---|---|
| Respiratory tract specimen | Influenza virus | Rapid test using nasal wipe material and lavage fluid T | 15 min | Immunochromatography |
| Respiratory syncytial virus | Rapid test using nasal wipe material and lavage fluid | 15 min | Immunochromatography | |
| Adenovirus | Rapid test using pharyngeal wipe material | 10–15 min | Immunochromatography | |
| Pneumococcus (under development) | Rapid test using respiratory tract specimen | 25 min | Immunochromatography | |
| Blood | Aspergillus | Detection of galactomannan antibody | 30–150 min | Latex agglutination/EIA |
| Cryptococcus | Detection of capsular polysaccharide antigen | 40 min | Latex agglutination | |
| Candida | Detection of mannan antibody | 15 min–4 h | Latex agglutination/EIA | |
| Cytomegalovirus | Detection of specific antigen in stained cells | 6–8 h | Immunostaining | |
| Endotoxin | Surface layer structure of Gram-negative bacteria | 6–8 h | Colourimetry using the limulus reaction | |
| β-D-glucan | Somatic antigen of fungus | 1 h | Colourimetry using the limulus reaction | |
| Urine | Pneumococcus | Detection of capsular serotype of 23 varieties | 15 min | Immunochromatography |
| Legionella | For L. pneumophila serotype 1 | 15 min | Immunochromatography |
4. Genetic diagnosis
Gene diagnosis has been developed as a method that is fast and offers high specificity for antigens that require long culture times (Mycobacterium tuberculosis, Legionella, mycoplasma etc) and for antigens that are difficult to culture on artificial media (virus, Rickettsia, chlamydophila (chlamydia) etc). Advances in gene diagnosis techniques have been rapid, and specific genes for many pathogen antigens, genes for etiological agents and genes for drug resistance have been found, enabling identification and diagnosis at the gene level. In theory, PCR can be used to amplify and confirm the presence of a target gene if just one copy of the gene exists in a sample. In practice, however, problems arise from the effect of substances in samples that can prevent reaction, or from the extraction efficiency of the gene. Detection sensitivity is often around 103 cfu/mL. Problems remain in gene diagnosis, including false-positive results due to contamination in the handling process and the inability to distinguish between live and dead microbes.
5. Determination of serum antibody titres
Serum antibody titres are normally determined by comparing two points in the acute and recovery phases (after 2–4 weeks). As a result, the value as a method for making diagnoses in the early stages of a disease is low. However, when tests of samples collected in the acute phase are all negative, definitive diagnosis can sometimes be made from elevated serum antibody titres in the recovery stage. Important pathogens targeted in determining serum antibody titres include various types of viruses and Legionella. Since serum antibody titres are tests that observe the biological response to the antigen, caution must be exercised with regard to the possibility that a normal antibody production response does not occur in immunocompromised hosts or patients that are receiving steroids.
- •
Quality of specimens is assessed by macro- and microscopic observation of sputum.
- •
Presence of Pseudomonas aeruginosa (mucoid type), Klebsiella, Staphylococcus and other bacteria can be quickly inferred with a high degree of certainty from smear tests.
- •
Involvement of aspiration is suspected when a number of pathogens are observed in lower respiratory tract specimens.
- •
Causative microorganisms can be strongly inferred from findings of phagocytosis by leukocytes and the presence of bacteria corresponding to the site of leukocyte aggregation.
- •
Coordination with the microbiology laboratory is extremely important in diagnosing infectious diseases.
- •
Depending on the bacteria, bacterial species can be inferred with a high degree of certainty at the point when colonies are formed.
- •
In urine antigen diagnoses (Pneumococcus, Legionella), problems remain with false-positive results in Pneumococcus tests and false-negative results for L. pneumophila serogroups other than serogroup 1.
ANTIMICROBIAL SUSCEPTIBILITY TESTS
Antimicrobial susceptibility tests are normally conducted using disk diffusion assays, agar dilution or broth microdilution. In disk diffusion assays, the minimum inhibitory concentration (MIC) cannot be calculated in the strict sense, but the antimicrobial susceptibility of isolated bacteria can be determined from the broad criteria of S (sensitivity), I (intermediate resistance) and R (resistance). With agar dilution and broth microdilution, meanwhile, MIC can be accurately measured, and the minimal bactericidal concentration (MBC) can also be investigated with broth microdilution. Ultimately, however, these susceptibility tests look at the growth inhibition effect of the antimicrobial in a test tube, and do not necessarily reflect clinical effects in the body. Points to be noted in interpreting the results of antimicrobial susceptibility tests are outlined as follows.
1. Relation between break point and antimicrobial dose
The Clinical and Laboratory Standards Institute (CLSI) in the USA has established break point concentrations for each species and antimicrobials as indicators of the clinical efficacy of antimicrobials. Table III-6 shows break point concentrations for Pseudomonas aeruginosa by way of example. A clinical effect can thus be expected when the MIC value for the isolated microorganism is lower than the given S value, but cannot be expected when the MIC value is larger than the R value. However, these break point concentrations were established on the basis of antimicrobial doses in Western countries, and large differences exist in maximum antimicrobial doses between Japan and Western countries. The largest difference is the 3.5-fold difference seen in gentamicin, followed by 3-fold differences in piperacillin, cefotaxime, ceftizoxime, and meropenem. Therefore, when reference is made to CLSI break point concentrations, such differences must be kept in mind while selecting antimicrobials and setting doses.
| Antimicrobial | S | I | R | Dose in Western countries | Dose in Japan | Maximum dose ratio (Western countries/Japan) |
|---|---|---|---|---|---|---|
| Piperacillin | ≤64 | ≥128 | 12–24 g | 2–8 g | 3 | |
| Ceftazidime | ≤8 | 16 | ≥32 | 2–6 g | 1–4 g | 1.5 |
| Cefepime | ≤8 | 16 | ≥32 | 2–4 g | 1–4 g | 1 |
| Cefozopran | ≤16 | 32 | ≥64 | 2–12 g | 1–6 g | 2 |
| Cefotaxime | ≤8 | 16–32 | ≥64 | 2–12 g | 1–4 g | 3 |
| Ceftizoxime | ≤8 | 16–32 | ≥64 | 2–12 g | 1–4 g | 3 |
| Aztreonam | ≤8 | 16 | ≥32 | 3–8 g | 1–4 g | 2 |
| Imipenem | ≤4 | 8 | ≥16 | 2–4 g | 1–2 g | 2 |
| Meropenem | ≤4 | 8 | ≥16 | 1.5–6 g | 0.5–2 g | 3 |
| Gentamicin | ≤4 | 8 | ≥16 | 420 mg | 80–120 mg | 3.5 |
| Amikacin | ≤16 | 32 | ≥64 | 900 mg | 200–400 mg | 2.25 |
| Tobramycin | ≤4 | 8 | ≥16 | 420 mg | 120–180 mg | 2.3 |
| Ciprofloxacin | ≤1 | 2 | ≥4 | 400–1200 mg | 200–600 mg | 2 |
| Levofloxacin | ≤2 | 4 | ≥8 | 250–750 mg | 200–600 mg | 1.25 |
- From reference 18.
2. Antibacterial agents that cannot be assessed from the results of susceptibility tests
Major reasons that assessment is not made with antimicrobial susceptibility tests in vitro are: (i) migration of antimicrobials within tissues and cells; (ii) absorption/discharge, pharmacokinetics and protein binding rate; (iii) side-effects and interactions between agents; and (iv) antimicrobial effects including bacteriostatic and bacteriocidal actions. Legionnaire's disease is an example of an infectious disease in which migration, of antimicrobials within cells plays a significant role. This bacteria is an intracellular parasite that proliferates within host cells (mainly macrophages) after infection. Drugs, such as macrolides and quinolones that have good intracellular migration, are thus the drugs of choice. Conversely, good clinical efficacy cannot be expected from β-lactams and aminoglycosides, even if good susceptibility test results are achieved. Moreover, with regard to antimicrobial migration to the lungs, β-lactams, aminoglycosides and glycopeptides are generally poor, while quinolones (particularly respiratory quinolones), macrolides (clarithromycin, azithromycin etc) and oxazolidinones (linezolid) are good.
With regard to absorption, vancomycin, aminoglycosides and other drugs are absorbed very little in the intestinal tract, whereas quinolones, macrolides and linezolid show superior absorption from the intestinal tract.
3. Precautions in reading the results of antimicrobial susceptibility tests
Antimicrobials are not selected based on susceptibility results of MIC or S/I/R alone. Drugs with low MIC obviously have a strong antimicrobial effect in a test tube, but when selecting antimicrobials for clinical use, factors (i)–(iv) above must be considered in addition to MIC. When drug susceptibility results are known, the antimicrobial with the narrowest spectrum should be selected, and the best antimicrobial therapy is selected with consideration of the PK/PD of each drug (see Chapter IV). A good understanding of the meaning of MIC and its limitations in antimicrobial susceptibility tests, and using this knowledge skilfully, is also demanded from the perspective of effective and proper use of antimicrobials.
- •
Antimicrobial susceptibility tests look only at the suppression of antimicrobial growth in test tubes, and do not necessarily reflect clinical effects in the body.
- •
Break point concentrations (CLSI) are determined based on antimicrobial dosages in Western countries, and when considering doses in Japan it must be remembered that these dosages are set relatively high.
SPECIES OF CAUSATIVE MICROORGANISMS IN HOSPITAL-ACQUIRED PNEUMONIA AND THEIR INCIDENCE
Major routes of infection for hospital-acquired pneumonia are upper respiratory tract bacterial flora that fall into the lower respiratory tract, aspiration, aerial infection (Legionella, Mycobacterium tuberculosis, fungi, viruses), haematogenous infection and infections that accompany medical procedures, such as endotracheal intubation. Bacteria in the oral cavity or upper respiratory tract are normally aspirated into the lower respiratory tract with high frequency, and after admission to hospital, microorganisms in the mouth, nose and throat of the patient represent a main cause of hospital-acquired pneumonia. As general condition of the host deteriorates with long-term hospitalization, the rate of detection of Gram-negative Enterobacteriaceae (Klebsiella, Enterobacter, Escherichia coli) in the nose and throat increases. Table III-7 summarizes reports of microorganisms isolated from sputum of hospitalized patients, and the incidence of bacteria isolated as a causative pathogen of hospital-acquired pneumonia.1,19–22 In all reports, the highest incidences are of Staphylococcus aureus and Pseudomonas aeruginosa, followed by enterobacteria. Of note is the finding that Acinetobacter increases in Western countries as a causative organism of ventilator-assisted pneumonia. Pneumococcus and Haemophilus influenzae often seen in community-acquired pneumonia are isolated from patients in early hospitalization who have not received any antimicrobials, but from few patients to whom antimicrobials have already been administered. However, incidences of causative microorganisms and drug-resistant bacteria vary considerably according to regions and facilities, and will also change over time. In hospitals that conduct their own microorganism tests, results of monthly or yearly counts of isolated organisms and isolation frequency of drug-resistant bacteria can be useful when inferring causative microorganisms and selecting antimicrobials.
| Bacterial species | Sputum of hospitalized patients (20?823 strains) | Watanabe A+ (812 strains) | Beardsley JR++ (194 strains) | Chastre J§ (2490 strains) | Kollef MH+ (835 strains) | Range (%) |
|---|---|---|---|---|---|---|
| Staphylococcus aureus | 26.1 | 25.6 | 22.7 | 20.4 | 49.1 | 20.4–49.1 |
| Pseudomonas aeruginosa | 21.6 | 18.1 | 11.3 | 24.4 | 18.4 | 11.3–24.4 |
| Klebsiella | 7.6 | 8.3 | 5.7 | 2.2 | 7.1 | 2.2–8.3 |
| Enterobacter | 4.0 | 2.1 | 11.3 | 2.7 | 4.3 | 2.1–11.3 |
| Stenotrophomonas | 4.0 | 1.6 | 1.0 | 1.7 | 1.0–4.0 | |
| Serratia | 3.9 | 3.0 | 6.7 | 1.7 | 1.7–6.7 | |
| Haemophilus influenzae | 1.9 | 3.6 | 8.2 | 9.8 | 5.6 | 1.9–9.8 |
| Pneumococcus | 1.7 | 5.0 | 3.6 | 4.1 | 3.1 | 1.7–5.0 |
| Acinetobacter | 2.7 | 0.7 | 14.9 | 7.9 | 2.0 | 0.7–14.9 |
| Escherichia coli | 2.1 | 2.7 | 2.6 | 3.4 | 4.7 | 2.1–4.7 |
| Other streptococci | 6.7 | 1.0 | 8.0 | 13.9 | 1.0–13.9 |
- + Incidence of microorganisms isolated from all patients in whom hospital-acquired pneumonia has occurred.
- ++ Survey conducted at one hospital with the purpose of investigating differences in isolated organisms between hospitals. Analysis of hospital-acquired pneumonia overall.
- § Incidence of microorganisms isolated from sputum of hospitalized patients.
- ¶Incidence of microorganisms isolated from patients with ventilator-assisted pneumonia (total results from 24 studies).
- ++ Incidence of microorganisms isolated from cases of hospital-acquired pneumonia in patients who did not use a mechanical ventilator.
- From references 1, 19–22.
- •
In addition to MRSA and Pseudomonas aeruginosa, enterobacteria are frequently the causative microorganism in hospital-acquired pneumonia.
- •
If the incidence of isolated microorganisms and trends in drug-resistant bacteria in a hospital are tracked, the results can be useful in inferring causative microorganisms and selecting antimicrobials.
CONFLICT OF INTEREST
No conflict of interest has been declared by The Committee for the Japanese Respiratory Society guidelines for the management of respiratory infections.




