Proximal tubule endocytic apparatus as the specific renal uptake mechanism for vitamin D-binding protein/25-(OH)D3 complex (Review Article)
Abstract
SUMMARY: The renal proximal tubule exhibits a very extensive apical endocytic apparatus that is involved in the reabsorption of molecules filtered in the glomeruli. Several key receptors appear to be involved in this function, which serves not only to conserve protein but also to reabsorb different vitamins in complex with their binding proteins. Recent research has established megalin as probably the most important receptor in this endocytosis process. Cubilin is another receptor identified in the proximal tubule endocytic apparatus. Because cubilin lacks transmembrane or cytoplasmic domains required for endocytosis, this receptor associates with megalin to recycle and internalize its ligands. Recent studies have shown that vitamin D-binding protein (DBP)/25-(OH)D3 complex is one of the megalin/cubilin ligands. Megalin knockout mice develop vitamin D deficiency and bone disease owing to an inability of the proximal tubules to capture the DBP/25-(OH)D3 complexes from the glomerular filtrate. In the same way, kidney-specific megalin knockout mice have severe plasma vitamin D deficiency, hypocalcaemia and serious bone disease, like the complete megalin knockout mice. Anti-cubilin antibodies inhibit cellular uptake of DBP/25-(OH)D3 by up to 70%. Anti-megalin antibodies produced a similar reduction in DBP/25-(OH)D3 endocytosis. When both antibodies were applied, impairment of DBP/25-(OH)D3 was only slightly more impaired (around 80%), suggesting that cubilin and megalin function through the same endocytic pathway. Specific forms of renal Fanconi syndrome are associated with endocytic pathway dysfunction with disruption of megalin-mediated uptake DBP/25-(OH)D3 complex, producing metabolic bone disease in affected individuals as a prominent clinical finding.
It has long been known that the proximal tubules of the kidney are the primary site for the renal uptake and activation of 25 hydroxy vitamin D3 (25-(OH)D3). Steroid hormones as vitamin D3 metabolites are lipids. Rather than being transported by lipoproteins such as cholesterol, these cholesterol derivatives are bound to specific carrier proteins. These carrier proteins are thought to keep steroids in a biologically inactive form, and regulate the amount of free hormone that would enter the target cells by passive diffusion.1
Although the passive diffusion process can account for many of the biological effects of steroids, some experimental evidence suggests that there are alternative pathways by which steroid hormones can be directed to a specific target tissue where they are taken up actively.
Thus, tissues that are responsible for the metabolism of steroid hormones may use active uptake mechanisms to acquire large amounts of steroids independent of their circulating concentrations.2
As vitamin D has a complex activation pathway with large amounts of 1,25-dihydroxivitamin D3 (1,25-(OH)2D3) being produced daily, the dependence of the proximal tubule on a non-specific diffusion process to deliver adequate amounts of 25-(OH)D3 appears unlikely. Recent studies have shown that 25-(OH)D3 is specifically targeted to the proximal tubule through its carrier protein, the vitamin D-binding protein (DBP), and that this complex is actively internalized by the endocytic apparatus of the proximal tubule that is involved in the reabsorption of molecules filtered by the glomeruli.3
ENDOCYTIC APPARATUS OF THE PROXIMAL TUBULE
The renal proximal tubule exhibits a very extensive apical endocytic apparatus consisting of an elaborate network of coated pits and small coated and non-coated endosomes. In addition, the cells contain a large number of late endosomes/prelysosomes, lysosomes and so-called dense apical tubules involved in receptor recycling from the endosomes to the apical plasma membrane. This endocytic apparatus is involved in the reabsorption of molecules filtered in the glomeruli. The process is very effective as demonstrated by the fact that although several grams of protein are filtered daily in the human glomeruli, human urine is virtually devoid of proteins under physiological conditions. Several key receptors appear to be involved in this function, which serves not only to conserve protein as such for the organism but also to reabsorb vital substances such as different vitamins in complex with their binding proteins.4 Recent research has established megalin, a 600 kDa protein belonging to the low-density lipoprotein (LDL) receptor family, as probably the most important receptor in this process in the proximal tubule mediating endocytosis of a large variety of ligands and therefore classifying it as a scavenger receptor. There are also more specific receptors like the folate receptor, IGF-II/Man-6-P receptor and gp280/IFR, identical to the intrinsic factor receptor, functioning in the apical endocytic pathway of renal proximal tubules.
MEGALIN AND CUBILIN
Megalin was initially identified as an autoantigen that induces Heymann Nephritis, a rat model for human membranous glomerulonephritis.5 This nephritis is induced by intravenous administration of rat renal cortex extract or crude membranes from proximal tubules. Megalin is also called gp330, because it was initially estimated to have a molecular seize of 330 kDa. However, subsequent studies showed that the protein has a much higher molecular size, about 550–600 kDa.6,7 Megalin belongs to the super gene family of the LDL-receptor-related receptors (LDLR) and therefore is also referred to as LDL-receptor-related protein-2 (LRP-2).2,8 The sequence of the complete cDNA of megalin was identified from a rat kidney cDNA library and it encodes a 4660-amino acid protein.9 The amino acid sequence of human megalin has also been clarified and it was found that it has a 77% sequence identity with that of the rat.10 Megalin has three domains: a 4400-aa extracellular amino-terminal domain, a 22-aa transmembrane domain and a 213-aa carboxy-terminal cytoplasmic tail, indicating that it is a type I cell-surface receptor. The extracellular domain contains four cysteine rich clusters of LDLR type A (complement-type) repeats. The complement type repeat consists of approximately 40 amino acids containing six cysteine residues and the SDE (Ser-Asp-Glu) motif responsible for high-affinity binding of positively charged sequences in ligands for LDLR.11 The four cysteine-rich clusters are flanked by epidermal growth factor (EGF)-type repeats and spacer regions containing YWTD (Tyr-Trp-Thr-Asp) motif which are responsible for pH-dependent dissociation of ligands in endosomal compartments.12 In contrast to the extracellular domain, the cytoplasmic domain of megalin has little similarity to that of other members of the LDLR gene family except for the tetra-amino-acid NPXY motif, which is essential for rapid endocytosis of the ligand–receptor complex via clathrin-coated pits. The ctroplasmic tail of megalin contains three of these NPXY motifs which mediate the clustering in coated pits.13 It has been recently demonstrated that the first and third NPXY motifs are responsible for efficient endocytosis whereas the second NPXY motif is essential for the apical sorting of megalin.14
Megalin is present in many epithelial cells (type II pneumocytes, thyroid and parathyroid cells, the choroid plexus, labyrinthic cells of the inner ear, the endometrium, the oviduct epididymis and ependymal cells and in some strain of rats, in glomerular podocytes), but it is most abundantly expressed in the renal proximal tubule.4,15In contrast to lipoprotein-receptor-related protein 1 (LRP-1), megalin is generally expressed in the apical membranes which do not directly contact with the capillaries and bloodstream. In the renal proximal tubular (PT) cells, megalin is localized to the endocytic apparatus such as brush-border membrane, clathrin-coated pits, and large endocytic vesicles and dense apical tubules which represent the recycling compartment for membrane proteins. The expression of megalin in the endocytic apparatus is much more abundant in segments 1 and 2 of the proximal tubule than segment 3.16 Megalin was originally shown to bind to a broad spectrum of proteins having regions rich in positively charged amino acids.17 It is now recognized that megalin is an endocytic receptor that binds diverse substances. These ligands have been categorized in several groups: vitamin-binding proteins and other binding proteins, apolipoproteins, hormones and hormone precursors, drugs and toxins, enzyme and enzyme inhibitors, immune- and stress-response-related proteins, and others including calcium.17
Cubilin was first identified as a receptor for intrinsic factor-B12 (IF-B12) complex in the terminal ileum.18 It is a 460 kDa receptor with no transmembrane domain and no signals for endocytosis. The complete cDNA sequences have been identified for the rat, human and canine cubilin. Cubilin contains 27 CUB domains responsible for the ligand binding and eight EGF-type repeats preceded by a stretch of 110 amino acids, where the N-terminal region appears essential for membrane anchoring. A direct association between cubilin and megalin has been demonstrated;19 it was suggested that molecular cooperation provides the basis for internalization of ligands bound to cubilin. Thus, cubilin with bound ligand may undergo megalin-mediated endocytosis, unload its cargo in lysosomes and recycle back to the plasma membrane together with megalin.19,20 Functional defects in humans (Imerslund–Grasbeck syndrome) and in dog results in intestinal malabsorption of vitamin B12 and in megaloblastic anaemia and urinary loss of several specific low-molecular weight proteins.21,22 Because cubilin lacks transmembrane or cytoplasmic domains required for endocytosis, the receptor associates with megalin to recycle through the endocytic compartment of cells. There is another protein called amnionless that constitutes with cubilin another endocytic receptor called Cubam. Mutation in either of these genes also causes autosomal recessive Imerslund–Grasbeck syndrome in humans. The cubilin–amnionless complex is essential for the renal tubular uptake of albumin, one of the carrier proteins of another vitamin, folate. Mutations that abrogate amnionless expression block cubilin processing and targeting to the apical membrane.23
VITAMIN D3 METABOLISM
Vitamin D3 is a steroid hormone with a complex activation pathway involving several tissues.24 The principal source of vitamin D3 is the skin, where this steroid is produced from 7-dehydrocholesterol by non-enzymatic processes. Some vitamin D3 is also taken with the diet and is absorbed through the intestine. Vitamin D3 is an inactive precursor that requires several hydroxylations to become an active compound. First, it is taken up by the liver to be hydroxylated to 25-(OH)D3. Afterwards it is taken by the kidney, where it is converted to 1,25-(OH)2D3, the active form of vitamin D, in the primal tubular cells by the mitochondrial enzyme 1 alfa hydroxylase (CYP27B1), with actions in several target organs such as intestine bone and kidney. Although 1 alfa hydroxylase is present in various tubular segments, only the proximal tubule produces large amounts of 1,25-(OH)2D3.
IMPORTANCE OF THE CARRIER PROTEIN: VITAMIN D-BINDING PROTEIN
Vitamin D-binding protein represents the main plasma carrier for vitamin D metabolites. This 50 kDa protein has the highest affinity for 25-(OH)D3,25 and as it is present in 100-fold molar excess in plasma, virtually all 25-(OH)D3 is bound to its carrier protein. It was originally thought that DBP regulated the bioavailability of vitamin D metabolites, protecting the organism against excessive amounts of the free vitamin.26 This concept was challenged by the findings in DBP knockout mice. These animals have significantly reduced plasma levels of vitamin D metabolites (25-(OH)D3 and 1,25-(OH)2D3). On a vitamin D depleted diet, the animals suffered from vitamin D deficiency and bone formation defects. In these animals, 25-(OH)D3 was inappropriately delivered to the liver, where it was catabolized, or it was lost in the urine because of lack of uptake by the kidney.27 The crucial role of this carrier molecule for targeting the activation of 25-(OH)D3 was supported by the observation that DBP knockout mice are protected rather than sensitized to vitamin D intoxication.27
INTERACTIONS BETWEEN DBP, MEGALIN AND CUBILIN
As mentioned before, the urinary loss of 25-(OH)D3 in DBP-deficient mice suggested that the carrier protein played a crucial role in the delivery of the steroid to the proximal tubule. The role of the endocytic apparatus as the retrieval mechanism for the DBP/25-(0H)D3 complex by PT cells was suggested in mice carrying a targeted disruption of megalin gene. Megalin knockout mice developed vitamin D deficiency and bone disease owing to an inability of the proximal tubules to capture the DBP/25-(OH)D3 complexes from the glomerular filtrate, which are lost in the urine (Fig. 1). These animals had a decrease of more than 70% in plasma levels of 25-(OH)D3 and 1,25-(OH)2D3.28 Almost all of megalin-deficient mice died perinatally from developmental defects of the forebrain and/or from respiratory failure; only one out of 50 of these megalin knockout mice survived to adulthood.29 The surviving mice excreted low-molecular weight proteins without change in urinary excretions of glucose, electrolytes, urea uric acid or amino acids.30 Recently, Muller et al. found a child who displayed a phenotype highly reminiscent of that observed in mice genetically deficient for megalin: holoprosencephaly, pulmonary insufficiency, absent circulating vitamin D metabolites, mild albuminuria and urinary excretion of DBP, although they were not able to implicate megalin gene locus directly.31 A mouse model with a kidney-specific megalin gene defect has recently been produced, generated using the Cre/loxP system.32 The kidney-specific megalin knockout mice were viable and fertile, and the renal expression of megalin was decreased by as much as 90%. In those kidney-specific megalin knockout mice, severe plasma vitamin D deficiency, hypocalcaemia and serious bone disease were observed, like in the complete megalin knockout mice.
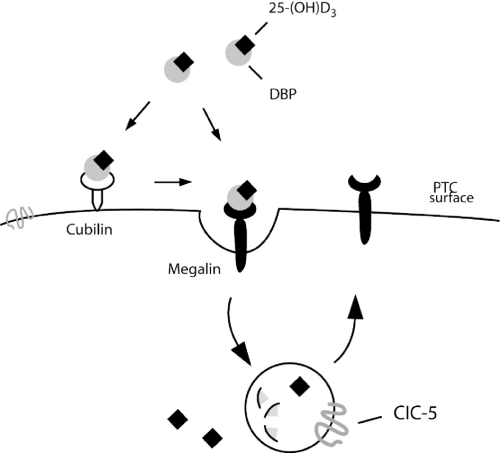
Model of megalin/cubilin function in renal uptake of vitamin D-binding protein (DBP)/25-(OH)D3 complex. DBP/25-(OH)D3 complexes are filtered through the glomerulus and endocytosed into the proximal tubular cell (PTC) by megalin/cubilin. The complexes are delivered to the lysosomal compartment, where DBP is degraded and vitamin D is released into the cytosol. Megalin sorting to the apical PTC surface. Chloride channel 5 (ClC-5) is present at the lysosomal membrane and at the PTC surface. (Modified from Willnow and Nykjaer,3 with permission.)
The importance of megalin as the capture molecule for DBP was further confirmed in other animal models of reduced receptor function. Rats treated with maleate, a substance that causes shedding of megalin, produce an increased excretion of DBP.33 The chaperone receptor-associated protein (RAP) is important for normal processing of megalin in kidney proximal tubules.34 In RAP knockout mice, expression of megalin is reduced by 50%.35 This reduction is correlated with increased urinary excretion of DBP.
As we mentioned before, the other protein that is part of the apical endocytic apparatus is cubilin. As cubilin lacks the transmembrane or cytoplasmic domains required for endocytosis, the receptor has to associate with megalin to recycle through the endocytic apparatus36 (Fig. 1). The role of cubilin in the renal uptake of DBP was identified when DBP affinity chromatography was applied to identify membrane proteins in the kidney that could be involved in the uptake of the DBP/25-(OH)D3 complexes.37 Apart from megalin, the other protein identified was cubilin. Interestingly, while RAP, the endoplasmic reticulum-resident chaperone for megalin blocked BDP binding to megalin, binding to cubilin remained unperturbed. To investigate the relative contribution of cubilin and megalin in the uptake process, the internalization of DBP/25-(OH)D3 complexes by BN/MSV cells in the presence of antibodies specific for each of the two receptors was studied. The addition of anti-cubilin antibodies inhibited the cellular uptake of DBP/25-(OH)D3 by up to 70% while control IgG had no effect. Anti-megalin antibodies produced a similar reduction in DBP/25-(OH)D3 endocytosis. When both antibodies were applied, DBP/25-(OH)D3 was only slightly more impaired (around 80%), suggesting that cubilin and megalin function through the same endocytic pathway.37
Other forms of endocytic pathway dysfunction with disruption of megalin-mediated uptake of DBP/25-(OH)D3
In renal Fanconi syndrome, the entire array of proximal tubule transport functions is impaired, resulting in glycosuria, generalized aminoaciduria, proximal renal tubular acidosis, phosphaturia and uricosiduria. All forms of the renal Fanconi syndrome are also associated with tubular proteinuria due toan inability of the proximal tubule to reabsorb macromolecules. The most prominent clinical finding in affected individuals is metabolic bone disease, either rickets in children or osteomalacia in adults, which is the result of the complex disorder of mineral metabolism and plasma vitamin D deficiency. Fanconi syndrome is present in various genetic and acquired diseases. It can be produced by cadmium intoxication as seen in Itai-Itai disease, where DBP is one of the proteins excreted in the urine.38
Dent’s disease is a rare hereditary tubular disorder characterized by low-molecular-weight proteinuria, hypercalciuria and nephrolithiasis due to inactivating mutations in the X-linked renal-specific chloride channel 5 (CLC-5). CLC-5 belongs to the family of voltage-gated chloride channels that is expressed in endosomes of PT cells.39–41 CLC-5 is believed to be responsible for sustaining chloride conductance required for import of protons to produce acidification of endosomes. CLC-5 defects result in endocytic dysfunction and the inability to reabsorb filtered proteins from the PT lumen.
Chloride channel 5 is typically regarded as an intracellular Cl– channel and thus the defect in this receptor-mediated uptake pathway was initially attributed to the failure of the early endosomes to acidify correctly. Investigations conducted in a CLC-5 knockout mouse model harbouring all the characteristic renal tubular defects of Dent’s disease showed a severe impairment of endocytosis by PT cells, such that the endocytic tracer peroxidase was poorly transferred into early endocytic vesicles.42 These data demonstrate that an impairment of receptor-mediated endocytosis in PT cells is the basis for the defective uptake of low molecular weight (LMW) proteins in patients with Dent’s disease. As we mentioned before, endocytosis and processing of LMW proteins involve the multiligand tandem receptors, megalin and cubilin, which are abundantly expressed at the brush border of PT cells. Using analytical subcellular fractionation and quantitative immunogold labelling, a selective disappearance of megalin and cubilin at the brush border of PT cells was demonstrated in CLC-5 knockout mice, suggesting that this was the endocytic defect present in these animals.43 Thus, the low levels of expression of ClC-5 at the apical cell surface appears to be a key component in the assembly of the macromolecular complex of megalin–cubilin involved in protein endocytosis.44 Among the ligands affected by the endocytic malfunction was DBP/25-(OH)D3 complexes, with urinary loss of these metabolites and a threefold decrease in plasma levels of 25-(OH)D3 and 1,25-(OH)2D3. A striking deficiency of urinary megalin, compared with normal individuals, was also observed in eight of nine families with Dent’s disease.45
Lowe syndrome (the oculocerebrorenal syndrome of Lowe, OCRL) is an uncommon, X-linked disorder characterized by anomalies affecting the eye, the nervous system and the kidney.44 Bilateral cataracts and severe hypotonia are present at birth, and they subsequently develop a proximal renal tubulopathy of the Fanconi type in weeks or months. OCRL was first described in 1952, and exactly four decades later, the gene responsible was identified and found to encode a protein (OCRL1 protein) highly homologous to inositol polyphosphate 5-phosphatase. This suggested that Lowe syndrome represented an inborn error of inositol phosphate metabolism. Although subsequent studies confirmed that such metabolism is indeed perturbed in Lowe syndrome cells, the mechanism by which loss of function of the OCRL1 protein brought about Lowe syndrome remained ill defined. Suchy et al. demonstrated that OCRL patients’ fibroblasts show no abnormality in inositol polyphosphate 5-phosphatase activity but are deficient in a phosphatidylinositol 4,5-biphosphate (PtdIns(4,5)P(2)) 5-phosphatase activity localized to the Golgi apparatus. Subsequently, it was found that OCRL1 was a Golgi-localized protein in kidney and lens epithelial cells and was further identified as a protein of the trans-Golgi network (TGN). The TGN is a major sorting site and has the specialized function in epithelial cells of directing proteins to the apical or basolateral domains. The epithelial cell phenotype in Lowe syndrome and the localization of OCRL1 to the TGN imply that this PtdIns(4,5)P(2) 5-phosphatase plays a role in trafficking.46,47 The disruption of the TGN in OCRL could impair megalin to recycle through the endocytic apparatus producing a proximal renal tubulopathy of the Fanconi type. In support of this theory is the recent finding in two families with Lowe’s syndrome of a striking deficiency of urinary megalin with normal urinary levels of cubilin.45
CONCLUSION
Recent studies have shown the importance of the proximal tubule endocytic apparatus in the specific renal uptake of 25-(OH)D3. This vitamin D metabolite is directed to the kidney through the association with DBP, which is internalized from the glomerular filtrate by megalin and cubilin, two key receptors of the endocytic apparatus. Disruption of megalin/cubilin-mediated uptake of DBP/25-(OH)D3 complexes results in urinary loss of these metabolites, decrease in plasma levels of 25-(OH)D3 and 1,25-(OH)2D3, and associated bone disease.




