Molecular mechanisms mediating vascular calcification: Role of matrix Gla protein (Review Article)
Abstract
SUMMARY: Patients with chronic kidney disease (CKD) have a higher incidence of vascular calcification and a greatly increased risk of cardiovascular death. The mechanisms involved in the accelerated vascular calcification observed in CKD have recently become clearer, leading to the hypothesis that a lack of natural inhibitors of calcification may trigger calcium deposition. One of these inhibitory factors, matrix Gla protein (MGP), is the focus of the present review. MGP, originally isolated from bone, is a vitamin K-dependent protein that is also highly expressed by vascular smooth muscle cells. MGP has been confirmed as a calcification-inhibitor in numerous studies; however, its mechanism of action is not completely understood. It potentially acts in several ways to regulate calcium deposition including: (i) binding calcium ions and crystals; (ii) antagonizing bone morphogenetic protein and altering cell differentiation; (iii) binding to extracellular matrix components; and (iv) regulating apoptosis. Its expression is regulated by several factors including retinoic acid, vitamin D and extracellular calcium ions, and a reduced form of vitamin K (KH2) is important in maintaining MGP in an active form. Therefore, strategies aimed at increasing its expression and activity may be beneficial in tipping the balance in favour of inhibition of calcification in CKD.
IMPORTANCE OF VASCULAR CALCIFICATION
Vascular calcification is an important pathology that is clearly associated with an increased risk of cardiovascular morbidity and mortality.1 In patients with chronic kidney disease (CKD) accelerated calcification occurs in the intima of the arterial vessel wall in association with atherosclerotic lesions and also the vessel media (where it is known as Mönckeberg’s sclerosis).2 Calcification also occurs commonly in heart valves and in rare cases in the arterioles of the skin, leading to the condition known as calciphylaxis, which is associated with extensive tissue gangrene and high morbidity and mortality. The type of calcium crystal that accumulates in the blood vessel wall exists mostly in the form of calcium apatite, which is the type of mineral found in bone. Indeed, vascular calcification has several similarities with bone formation including the presence of matrix vesicles, small membrane-bound vesicles that bud off from cells and are thought to be the nidus for calcification in bone. Vascular calcification had previously been thought of as a passive, degenerative process but the findings of bone-related factors in the vasculature and the vascular calcification observed in several gene-knockout mouse models imply that it is an actively regulated process that may be preventable or even reversed.3 The most striking of these mouse models is the matrix Gla protein (MGP) knockout mouse, indicating that this protein may be of primary importance in human vascular calcification. The present review focuses on how MGP achieves its calcification-inhibitory function and which factors regulate its expression and activity.
MGP INHIBITS VASCULAR CALCIFICATION IN MICE AND MEN
Matrix Gla protein was originally isolated from bone but it is known to be expressed in several tissues including kidney, lung, heart, cartilage and vascular smooth muscle cells (VSMC) of the blood vessel wall. It is an 84-amino-acid (approximately 12 kDa) protein that contains five γ-carboxyglutamic acid (Gla) residues4(Fig. 1). The Gla residues in MGP and all other vitamin K-dependent proteins are produced by γ-carboxylation of certain glutamic acid residues by γ-carboxylase, and require a reduced form of vitamin K as a cofactor. The only known function of Gla residues is to bind calcium ions or calcium crystals.5 In addition, MGP contains phosphorylated serines that may regulate its activity. The concentrations of calcium and phosphate ions in extracellular fluids are sufficiently high to sustain growth of a seed crystal, yet widespread tissue calcification does not usually occur in health. Protection against calcification is thought to be partly due to the complexing of calcium within tissues to inhibitors of calcification such as MGP.6
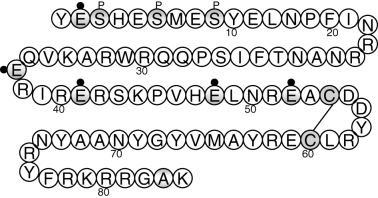
Amino acid sequence for matrix Gla protein (MGP). Glutamic acid residues (E) with a black dot indicate sites for γ-carboxylation. Serine residues (S) with a ‘P’ above indicate putative sites for phosphorylation. In the human MGP protein, a common polymorphism exists where either alanine (A) or threonine (T) is present at amino acid 83.
The function of MGP as an inhibitor of calcification was clearly demonstrated in the MGP knockout mouse.7 These mice were normal at birth but within a few weeks developed abnormal cartilage calcification and extensive calcification of elastic arteries, which ruptured causing premature death. The calcification resulted from replacement of the medial VSMC by chondrocyte-like cells accompanied by deposition of a calcified matrix. More recently, it was demonstrated that restoration of MGP expression in MGP null mice specifically in the vasculature prevented arterial calcification.8 In that study, in vivo mutagenesis showed that the anticalcifying activity of MGP depended upon four of the five Gla-residues. Furthermore, in studies in rats, Price and colleagues found that extensive vascular calcification occurred when γ-carboxylation of MGP was inhibited using warfarin.9
In man, a rare human disorder, Keutel syndrome, characterized by abnormal cartilage calcification, is due to mutations in the MGP gene that predict truncated forms of MGP.10 The recent finding of extensive vascular calcification in these patients indicates that MGP is also an inhibitor of vascular calcification in man.11 However, unlike the mouse model, Keutel syndrome patients survive into adulthood, which implies that other factors in humans may function to inhibit vascular calcification. Alternatively, the small fragments of MGP generated in Keutel patients may still retain some anticalcifying effects in the vasculature.
HOW DOES MGP INHIBIT VASCULAR CALCIFICATION?
Site of action of MGP
Studies in MGP null mice by Murshed et al. showed that local production of MGP, specifically induced in VSMC, is essential in preventing vascular calcification.8 Expression of MGP in the liver of these animals and subsequent release into the circulation failed to have effects on rescuing the vascular calcification phenotype, even though the MGP produced by the liver was shown to have anticalcifying activity in vitro. These observations demonstrate that either circulating MGP is not taken up by artery wall cells or that the process of entering/travelling through the circulation renders the protein inactive, possibly via binding other serum components. Alternatively, MGP activity may depend on interaction with a matrix component upon release from VSMC. Furthermore, MGP may have an intracellular role in VSMC. To support this notion, MGP has been detected intracellularly in chondrocytes in vivo12 and also in VSMC induced to overexpress MGP adenovirally in human VSMC.13 Using MGP-specific antibodies, abundant endogenous MGP can also be detected within human VSMC in culture. As the Gla form of MGP can bind calcium ions, it is possible that MGP could have a role in intracellular calcium homeostasis.
Binding calcium ions or calcium crystals
The precise molecular mechanism of MGP function is not known although it was thought for some time to act by binding excess calcium ions or small crystals in tissues and clearing them to the circulation (Table 1). In support of this, MGP mRNA is expressed by many tissues but MGP protein only accumulates at sites of calcification and in plasma. Recent studies have also shown that MGP does indeed bind calcium ions and calcium crystals.5,14 The binding to calcium ions is thought to bring about a conformational change in MGP and other vitamin K-dependent proteins, allowing them to become ‘active’.
| MGP interaction | Effect | Reference |
|---|---|---|
| Binding calcium ions | Clears excess calcium to the circulation | 5 |
| Binding calcium crystals | Inhibits crystal growth | 14,15 |
| Binding BMP-2 | Inhibits osteoinductive effects of BMP-2 | 16,17 |
| Inhibits apoptosis? | 18 | |
| Binding extracellular matrix | ||
| Elastin fibres | Prevents nidus formation | 19 |
| Vitronectin | ? | 20 |
- BMP-2, bone morphogenetic protein-2; MGP, matrix Gla protein.
Matrix Gla protein was found to be a component of a serum complex consisting of hydroxyapatite, fetuin and other proteins.15 This complex occurs in the serum of etidronate-treated rats. In the same study it was interesting to observe that in control animals (where the hydroxyapatite complex was absent), a significant portion of MGP circulated as a >300 kDa component, whereas only a small portion circulated as a <300 kDa protein. This suggests that the majority of MGP circulates either in an aggregated form or bound to higher molecular weight chaperones.
Relatively few studies have analysed MGP at the protein level because it is poorly soluble in its pure form and it aggregates at neutral pH. MGP is soluble in physiological buffers only at very low concentrations.20 This could mean that when MGP accumulates, it binds to itself and no longer moves through tissues freely, unless it is bound to a chaperone that prevents aggregation/precipitation. At present, the mechanisms that regulate MGP transport from cells and tissues and factors that maintain MGP solubility are not known.
Binding to bone morphogenetic protein-2 (BMP-2) and alteration of VSMC phenotype
An additional theory for the mechanism of action of MGP is that it is involved in VSMC differentiation. The basis for this hypothesis is that: (i) the arteries that calcified in the MGP null mice contained chondrocyte-like cells in the medial layer rather than VSMC and expression of the osteoblast-specific transcription factor cbfa1/Runx2 was upregulated;21 (ii) MGP has been shown to bind BMP-2 and inhibit its effects on differentiation in multipotent mesenchymal cells and marrow stromal cells;16,17 (iii) overexpression of MGP in chondrocytes delays their maturation;22 and (iv) human calcified blood vessels globally express lower levels of MGP mRNA and upregulate expression of both chondrocytic and osteoblastic markers, such as collagen type II and osteocalcin, respectively.23,24 From these observations it can be postulated that MGP is required for VSMC to maintain their normal VSMC contractile phenotype and prevent their differentiation towards an osteogenic phenotype. Thus in the absence of MGP, cells are driven along a different mesenchymal differentiation pathway and VSMC are able to take on the properties of both chondrocytes and osteoblasts and produce a matrix that favours calcium crystal deposition.
The finding that MGP binds to BMP-2 and antagonizes its actions is an important step in understanding how MGP functions. BMP-2 is a potent bone morphogen and its expression may trigger the induction of an osteogenic gene expression profile in VSMC.18 BMP-2 is expressed by cells in atherosclerotic lesions25 and its expression may be induced by oxidative stress, inflammation or hyperglycaemia.26–28 The antagonistic activity of MGP on BMP-2 is therefore expected to prevent or reduce the osteo-inductive effects of BMP-2 in the vessel wall. The interaction of BMP-2 with MGP appears to be dependent on calcium ions and the Gla domain of MGP.29 Therefore, undercarboxylated forms of MGP may not be as effective in inhibiting BMP-2 actions.
Binding to extracellular matrix components
A recent investigation into the binding interactions of MGP with matrix components demonstrated that MGP binds specifically to vitronectin.20 In contrast to the binding of MGP to BMP, the binding to vitronectin was not Ca-dependent and involved binding to the C-terminal region of MGP, an interaction independent of the carboxylation status of MGP. The relevance of MGP binding to vitronectin in the vasculature is not yet known but it was proposed that this interaction may alter its effects on BMP-2 activity.
Elastin is another potential extracellular matrix component that MGP may bind. In immunohistochemical studies, MGP (particularly the Gla-form) was shown to be present in healthy human arteries at the site of elastin fibres.30 The location of MGP at these sites may be a mechanism to prevent calcification as elastin is a potential substrate for initiation of calcium crystal formation.19 Elastic fibres consist of an elastin core surrounded by micofibrils and perturbation of the structure of these fibres can enhance calcification. The major protein component of microfibrils is fibrillin-1, the defective gene product in Marfans syndrome. In mice that underexpress fibrillin-1, medial calcification of the aorta is the first pathological sign detected.31 Fibrillin-1 is therefore crucial in maintaining a calcification-free media. Uncovering the precise interaction of MGP with elastin fibre components will no doubt increase the understanding of how MGP achieves its function.
Role of MGP in apoptosis
Apoptosis is an important mechanism in the initiation of vascular calcification and apoptosis precedes calcification in VSMC multicellular nodule cultures.32 Apoptotic bodies derived from VSMC can act as a nidus for calcium crystal formation32 and these structures have been detected in both atherosclerotic lesions and Monckberg’s sclerosis.33 VSMC nodules contain a relatively high number of apoptotic cells and apoptotic bodies and MGP expression is highest when the apoptotic index peaks in nodules,32 thereby implicating an association between MGP and apoptosis. MGP has also been detected in apoptotic bodies/matrix vesicles produced by VSMC in vitro and may be present in these vesicles to limit their calcification-potential.34 Other studies also suggest that MGP expression is upregulated in response to apoptosis. MGP mRNA expression was increased when apoptosis was induced in rat glioma cells or rat ventral prostate epithelial cells.35,36 In mouse chondrocytes in culture, MGP over- or underexpression at specific stages of maturation leads to apoptosis.37 Additionally, the extracellular matrix and its constituent proteins are well known to have effects on cell survival.38 Futhermore, the observation that BMP-2 induces apoptosis in VSMC and MGP antagonizes BMP-2 action is consistent with the potential role for MGP as an anti-apoptotic factor. However, although several studies have associated MGP with apoptosis, it remains to be tested whether high/adequate levels of MGP expression are required to protect VSMC from apoptosis.
FACTORS REGULATING MGP ACTIVITY
γ-Carboxylation
Factors that induce or increase MGP expression would be expected to protect against calcification. However, any upregulation of MGP will require an increase in availability of a reduced form of vitamin K to act as a cofactor for γ-carboxylation. The form in which vitamin K occurs in the diet (vitamin K quinone) is the non-reduced form (inactive) and requires reduction to vitamin KH2 by the vitamin K epoxide reductase (VKOR) system. Vitamin KH2 is used as the active factor in the carboxylation reaction. Oxidation of KH2 then drives the addition of the carboxyl-group to glutamic acid (Glu) residues in MGP (to create γ-carboxyglutamic acid, Gla) and the oxidized vitamin K (KO) can be reduced again in a cycle known as the VKOR cycle. Coumarin derivatives such as warfarin can block the VKOR cycle.
Many studies have shown that the Gla residues are necessary for MGP anticalcifying activity. The importance of γ-carboxylation of MGP in human calcified arteries was demonstrated recently by Schurgers et al. who developed conformation-specific antibodies to the Gla- and undercarboxylated forms of MGP.30 They found that the Gla-form of MGP located to the healthy media of the vessel wall, whereas the Glu-MGP (undercarboxylated form) located to calcified areas of atherosclerotic plaques. These studies indicated that in atherosclerotic plaques, γ-carboxylation of MGP is insufficient or ineffective and that upregulation of MGP seen at these sites is likely to be inactive. The reasons for this may be that γ-carboxylase is downregulated in plaques or that the environment of the plaque does not favour efficient vitamin K reduction. To support this, an endogenous inhibitor of γ-carboxylation has been reported, calumenin.39 Calumenin is a calcium-binding protein that was first identified in mouse hearts and shown to be expressed in the endoplasmic reticulum and Golgi.40 Importantly, it has been shown to bind to VKOR and decreases its activity leading to a less effective vitamin K-dependent carboxylation system.39 Calumenin is also thought to interfere with warfarin binding to VKOR. Interestingly, calumenin is a secretory product of activated platelets and has been detected in human atherosclerotic lesions.41 Calumenin may therefore be an important factor in contributing to the undercarboxylated (and inactive) accumulation of MGP in atherosclerotic plaques and further studies are required to investigate if it has a role in VSMC calcification.
It was recently reported that a polymorphism in the VKOR complex (VKORC1) gene (CC genotype, +2255) was associated with a twofold increased risk of stroke, aortic dissection and coronary heart disease.42 This association may, in part, be due to an altered MGP carboxylation status43 and it would be interesting to determine the calcification status in these patients.
A diet rich in vitamin K (particularly K2) has, in one study, been shown to correlate with a healthy vessel wall.44 Few studies have investigated the effects of the commonly prescribed anticoagulant drug, warfarin. However, two reports showed that warfarin treatment correlated with an increase in aortic valve calcification.45,46 It has also been noted that warfarin may exacerbate calcification in patients with kidney failure.47
Phosphorylation
It has been postulated that phosphorylation of serine residues is important in secretion/activity of several secreted proteins including MGP.48 Phosphorylation of MGP is thought to occur in the Golgi apparatus by a casein kinase (Fig. 1). However, the importance of these groups in MGP is not yet known.
FACTORS REGULATING MGP EXPRESSION
In addition to post-translational modifications, levels of expression of MGP at the mRNA level are also important. For example, as mentioned above, MGP mRNA is globally expressed at lower levels in calcified blood vessels compared with non-calcified vessels.23 A reduction in the production of MGP may therefore trigger calcification. Several factors have been shown to affect MGP expression (Table 2). The MGP promoter contains a number of putative regulatory sequences including possible binding sites for retinoic acid and vitamin D.52
Retinoic acid
Retinoic acid is a major regulator of chondrocyte maturation and mineralization and a high intake of retinoids can lead to vascular calcification.53 Retinoic acid increases MGP mRNA expression in cultured human fibroblasts, chondrocytes, osteoblasts, osteosarcoma cell lines and rat type II pneumocytes.54,55 However, it downregulated MGP expression in rat kidney cells, human breast cancer cells and rat VSMC in culture.49,56 Therefore, retinoic acid increases MGP expression in chondrocytes but has the opposite effects in VSMC.
Vitamin D
High levels of vitamin D3 can cause vascular calcification in vivo.57 This effect may be only partly due to its effects on serum calcium as vitamin D3 also induces calcification of VSMC in culture.58 Vitamin D3 has been shown to increase MGP mRNA expression in human osteoclasts and rat chondrocytes, osteoblasts and osteosarcoma cells59–61 but has no effect on human fibroblasts, chondrocytes and osteoblasts. At physiological levels, vitamin D3 increased MGP transcription in VSMC.49
Extracellular calcium ions
In a rat model of hypercalcaemia plasma MGP levels increased dramatically.15 This may be explained by the demonstration that VSMC can ‘sense’ changes in the concentration of extracellular calcium ions via a calcium-sensing mechanism related to the calcium-sensing receptor and respond by increasing expression of MGP mRNA.50 MGP is known to associate with calcification in human arteries and in the developing skeleton it accumulates in mineralized cartilage and bone. MGP may be upregulated in these areas to regulate/inhibit pathological calcification. Extracellular fluids in bone or at sites of inflammation can contain high extracellular levels of ionic calcium and would be expected to increase the potential for calcium crystallization. It is therefore possible that VSMC or chondrocytes expressing the calcium-sensing receptor sense these changes in extracellular calcium and respond by increasing MGP expression and secretion. Therefore, although extracellular calcium is a potential inducer of calcium crystal formation,34,62 it may also serve as a signal to regulate calcification via stimulation of MGP synthesis.
Cytokines and other factors
In chondrocytes in culture, IGF-1 (a known stimulator of chondrocyte differentiation) caused a reduction in MGP mRNA expression.63 Conversely, FGF2 (known to inhibit chondrocyte differentiation) increased MGP mRNA expression. These growth factors are therefore thought to act via MGP to alter chondrocyte differentiation. Interestingly, IGF-1 is also a potent anti-apoptotic factor and in addition to its downstream signalling via Akt to protect against apoptosis, its effects on increasing MGP expression may also contribute to cell survival. TGFβ has been reported to increase MGP mRNA expression in embryonic lung cells.64 In contrast, using MGP promoter constructs transiently transfected into rat VSMC, TGFβ leads to a downregulation of MGP transcription.49 In non-dividing, confluent rat kidney cells in culture, MGP expression was vastly reduced in the presence of EGF.65 Recently, the thyroid hormone triiodothyronine (T3) was shown to increase MGP transcription and mRNA expression in rat and human VSMC.51 It was also reported that in a rat hypothyroid model, whereas MGP mRNA was reduced, aortic calcium deposition increased.51
Therefore, a variety of factors can affect MGP expression levels, albeit with differing effects on different cell types. It should be noted, however, that the effect of altering MGP expression is not always predictable because an increase in MGP expression has been associated with sites of calcification.30,66,67 In these cases, an increase in MGP mRNA expression may be an attempt by the cells to respond to and inhibit further calcification in a positive feedback-like manner.
RELEVANCE TO CKD
As described earlier there is now overwhelming evidence that MGP is an important and potent inhibitor of vascular calcification in man. These studies also highlight that MGP expression and function may be suboptimal in patients with CKD. For example, as vitamin D increases MGP expression in VSMC and low levels of vitamin D are seen early in renal failure, this may exacerbate calcification in the long term in these patients. In addition, calcium binding to MGP is weakened in the presence of phosphate ions.14 This may be due to competition between phosphate and calcium ions for binding. In CKD where phosphate levels can be supraphysiological, one can envisage that calcium binding to MGP may be hindered, encouraging the development of calcification. Two other important modifiers of MGP function, vitamin K and warfarin, are also likely to be critical. Many CKD patients have poor nutrition; however, increasing vitamin K levels might have beneficial effects on their vascular health. Warfarin, a commonly used anticoagulant is also likely to compromise MGP function. Therefore, the use of warfarin in already heavily calcified patients who are most likely to have inefficient inhibitory capacity may lead to further calcification. The association of warfarin treatment with calciphlaxis lends weight to this possibility. Genetic factors may also be important. Polymorphisms that could potentially alter MGP function exist within the human MGP gene.68,69 A polymorphism in the coding region at amino acid 83 that predicts either Thr or Ala at this position could potentially affect proteolytic processing of MGP70 (Fig. 1). Several correlative studies have focused on common polymorphisms in the promoter region of MGP. One of these, the T-138C polymorphism altered binding of an AP-1 transcription factor complex and was associated with altered MGP serum levels in man.68 Interestingly, CKD and haemodialysis patients were found to have a different distribution of T-138C or G-7A polymorphisms when compared with a normal population.71 This raises the possibility that altered MGP promoter polymorphisms may be a negative prognostic factor for CKD. Finally, although to date specific therapeutic strategies have not been developed to increase MGP expression and function the possibility exists that the new class of calcimimetics may increase MGP expression in the artery wall as it has previously been shown that MGP expression is upregulated by calcium via the calcium-sensing receptor.50,72 Clearly MGP is an important target if the problem of vascular calcification is to be resolved in CKD patients.




