Renal involvement in bone marrow transplantation
Abstract
SUMMARY: Bone marrow transplantation (BMT) is an effective therapeutic strategy for leukaemic malignancies and depressed bone marrow following cancer. However, its side effects on kidneys have been reported. Some drugs and irradiation are also suggested to be nephrotoxic. It is well known that haemolytic uraemic syndrome (HUS) after BMT develops as late-onset BMT nephropathy. Cyclosporine A (CsA) is a possible cause. Radiation nephropathy shows changes that are similar to the histology of HUS. These findings suggest that endothelial damage is closely associated with the pathogenesis of post-BMT nephropathy. Recently, some patients have developed glomerulonephritis accompanied by graft-versus-host disease (GVHD) after BMT. In these patients immune deposits are found mainly in subepithelium and mesangium equal to those of secondary membranous glomerulonephritis. A murine experimental model of GVHD manifests similar symptoms and histological changes to those of actual patients and may suggest the pathogenesis of glomerulonephritis.
Currently, 15 000 patients worldwide receive bone marrow transplantation (BMT) every year. Autograft BMT is done after resection of solid neoplasm and solid organ transplantation to avoid bone marrow depression by chemotherapy or irradiation. Allograft BMT is performed principally for leukaemic or lymphoid malignancies.
Acute renal failure occurring within 3 months post-BMT is mainly related to sepsis, nephrotic antibiotics, other therapeutic agents and hepatorenal syndrome. The rate of renal insufficiency after 3 months post-BMT is estimated at 26%.1 Late-onset chronic renal failure occurs in up to 20% of survivors of BMT.2 Haemolytic uraemic syndrome (HUS) and radiation nephropathy are well-known side effects of BMT. Bergstein et al. stated that the morphological similarities between HUS and acute radiation nephropathy suggest the existence of a common pathogenetic mechanism of endothelial cell damage.3 Moreover, the sensitivity of the endothelial cell to radiation effects is thought to be enhanced by chemotherapeutic agents.
In allograft BMT, graft-versus-host disease (GVHD) could be the main problem, for donor T cells are included in transplanted bone marrow. The main organs involved are usually the skin and liver; however, with the increased number of bone marrow transplants being performed, renal dysfunction after BMT has been reported and there are some recent reports of GVHD-related glomerulonephritis.4–18 In this review we mainly discuss glomerulonephritis relating to GVHD after BMT and look at its pathogenesis compared to the experimental murine graft-versus-host reaction (GVHR).
EXPERIMENTAL MURINE GRAFT-VERSUS-HOST REACTION
Background
Gleichmann et al. introduced the chronic allogeneic disease mechanism of murine GVHR in 1972.19 Murine GVHR is classified into two entities (Table 1). When major histocompatibility complex (MHC) class I and II are both different between the donor and the recipient, acute GVHR can be induced. This type of GVHR manifests in thymic degeneration and haematopoietic hypoplasia following immune suppression. Recently, Allen et al. reported that murine chromosome 1 and chromosome 4 loci possibly influence the occurrence of acute GVHR.20
| Acute GVHR |
| Immunodeficiency |
| Lymphoid atrophy |
| Hypogammaglobulinaemia |
| Dermatitis |
| Diarrhoea |
| Anaemia |
| Chronic GVHR |
| Lymphoid hyperplasia |
| Autoantibody production |
| Immune complex glomerulonephritis |
| Connective tissue disease |
Chronic GVHR showing an immunostimulatory state is a result of abnormal cooperation between donor T cells and recipient B cells.21 In this state, autoantibodies including anti-DNA antibody are produced, resulting in immune complex glomerulonephritis, resembling human lupus nephritis.22–24 Cytokines such as IL-4, TGF-β, IL-1, IL-6, TNF and PDGF are considered to be closely related to T– B cell interaction in murine GVHR and to promote deposition of the extracellular matrix, which leads to hyperfiltration.25–28
Autoantibodies in murine chronic GVHR
Parent-to-F1 hybrid murine strain combinations are useful for the induction of GVHR (Table 2). A single cell suspension of donor spleen cells of DBA/2 (H-2d) injected intravenously into recipient (C57BL/10xDBA/2)F1(BDF1; H-2b/d) mice can induce severe chronic GVHR with a rapid increase of autoantibodies bypassing the immunosuppression phase of acute GVHR. In this model, alloactivated donor Ts/Tk cells disappear early from the host in spite of longevity of alloactivated donor Th cells.21 It has been reported that polyclonal IgM- to IgG-class switch occurs in murine chronic GVHR as well as in spontaneous autoimmune animal models, leading to autoimmunity.29,30 IgG-class antibody production in BDF1 mice includes antibodies to autoantigens (DNA, natural thymocytotoxic autoantibody and mouse red blood cells), and to conventional antigens (ovalbumin and TNP-KLH).31 Other investigators have reported that the antibodies include antinuclear antigens (ANA), glomerular basement membrane (GBM) and renal tubular epithelial antigens (RTE).32,33 A significant increase in the number of IgM- and IgG-secreting cells by the ELIspot assay has also been reported in this model.34 Anti-IgM-secreting B cells in BDF1 mice increased during the second week after transfer of DBA/2 spleen donor cells, and anti-IgG-secreting B cells showed a higher titre 1 week later than that of anti-IgM-secreting cells. However, their antigenic specificity of B-cell activation during GVHR is restricted.35,36 Anti-RTE antibody binds directly to the glomerular enzyme dipeptidyl peptidase type IV, and the resulting 160 kDa RTE component is followed by abnormal accumulation of extracellular matrix molecules such as fibronectin, laminin and collagen types I III, IV and VI, promoting glomerulosclerosis.37,38 When DBA/2 and BALB/c donor lymphocytes carrying an H-2d haplotype and differing only at the non-MHC loci were injected into H-2b/d F1 hybrids of BL10 mice, all recipients developed proteinuria with massive mesangial deposits by week 10. However, only the strain combination using DBA/2 mice as donors can produce anti-GBM antibody.39 Via et al. described how this state responds to the ratio of the controlling gene CD4/CD8 in DBA/2 mice.40 Their study demonstrates that non-MHC genes govern the pathogenesis of immune complex nephritis in this model by influencing the autoantibody profile.39 A similar mechanism is considered in human GVHD after BMT in spite of MHC-matched donors and recipients, indicating the importance of non-MHC loci.
| GVHR | Recipient (haplotype) | Donor (haplotype) |
|---|---|---|
| Acute | (C57BL/10xDBA/2)F1 (H-2b/d) | B10.D2 (H-2d) |
| Chronic | (C57BL/10xDBA/2)F1 (H-2b/d) | DBA/2 (H-2d) |
Glomerular lesions in murine chronic GVHR
Three weeks after inoculation of donor DBA/2 spleen cells in F1 recipients, serum anti-DNA antibody is raised to its peak level.41 In this period, mesangial cell proliferation is beginning in glomeruli without significant proteinuria, which develops later. By immunofluorescent study, both IgG and IgM are found segmentally from week 1 and gradually expand in distribution. At week 10, the capillary lumina are filled with hyaline-like materials and/or a foamy substance, and mesangiolysis is seen without irradiation or drugs (Fig. 1). Other glomeruli show spike formation by PAM staining (Fig. 2). Electron microscopy examination reveals massive deposits in subepithelium, mesangium (Fig. 3) and occasionally in subendothelium. Both direct mesangial damage and massive immune complex deposition might be involved and followed by the release of cytokines in this model.41,42
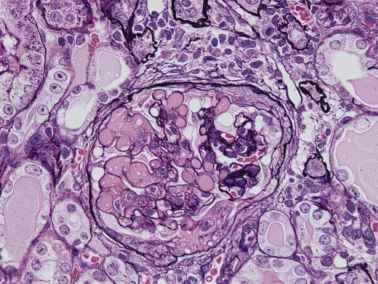
Murine graft-versus-host disease at week 10. The capillary lumina contain hyaline-like materials accompanied by mesangiolysis. The mesangial matrix is focally increased and reticulated. Fibrocellular crescent with adhesion is formed. HE stain; original magnification, ×40.
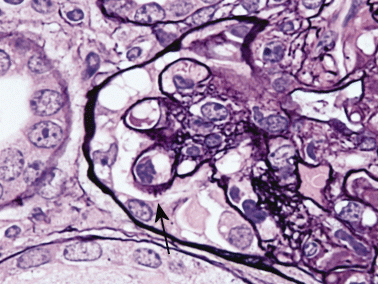
Murine graft-versus-host disease at week 10. There is focal spike formation of the glomerular basement membrane (arrow). PAM stain; original magnification, ×100.
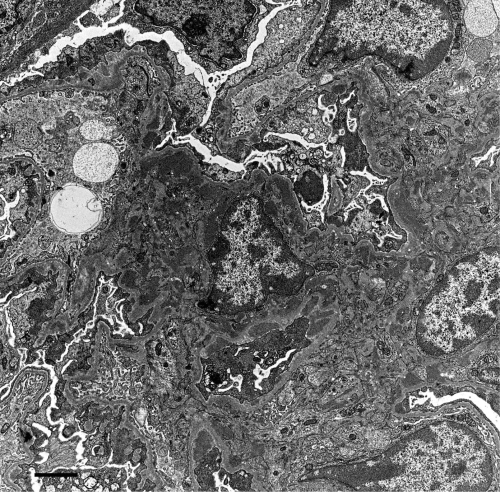
Murine graft-versus-host disease at week 10. Electron-dense deposits are noticed in the subepithelium and mesangium. Bar, 2 µm.
HUMAN GRAFT-VERSUS-HOST DISEASE
Seventeen cases of human glomerulonephritis associated with GVHD after therapeutic allogeneic BMT have been reported since 19884–18 (Table 3). All patients developed nephrotic syndrome or nephrotic range proteinuria and six patients also manifested increased serum levels of antinuclear antigen (ANA) and/or anti-DNA antibodies. Renal manifestations appeared during the tapering off of immunosuppressants. Sixteen cases received treatments such as steroids and immunosuppressants, which proved to be effective. Attention should be paid to renal manifestations, especially during the tapering off of the use of immunosuppressants. Renal biopsies of patients showed that three had minimal change; 11 had membranous glomerulonephritis, with or without mesangial and subendothelial deposits; one had IgA nephropathy; and one had diffuse proliferative glomerulonephritis. One case showed minimal change disease at the first biopsy; however, focal segmental glomerulonephritis showed up at the second biopsy. In another case, mesangiolysis was noticed.10 Mesangiolysis has also been reported in the radiation nephropathy and HUS after BMT. Yabana et al. concluded that mesangiolytic changes resulting in glomerulosclerosis were associated with irradiation or chemotherapy.10 However, mesangiolysis was seen in the murine model of GVHR without irradiation or any drugs, as we reported previously;41 therefore, we presume that this event does not always depend only on therapeutic agents or irradiation.
| Author | Gender | Age (year) | Period after BMT (month) | Renal pathology | Detected autoantibodies | TBI | Treatment |
|---|---|---|---|---|---|---|---|
| Gomez-Garcia | M | 15 | 7 | MCD | ANA | + | Pren |
| Gomez-Garcia | F | 12 | 6 | MCD | NM | + | Pren, azathioprine |
| Hiesse | M | 44 | 12 | MN | – | + | CsA |
| Muller | M | 20 | 7 | MN | ANA | – | CsA |
| Barbara | M | 43 | 24 | MN | ANA | NM | Prel, CsA |
| Walker | F | 10 | 10 | MCD | ANA | – | CsA |
| Sato | M | 20 | 13 | MN | ANA | + | CsA, Pren |
| Yabana | M | 27 | 13 | MN | – | + | Prel, M-prel |
| Yorioka | M | 34 | 11 | MN | NM | NM | CsA |
| Oliveria | M | 26 | 13 | MCD→FSGS | ANA | – | CsA, Pren |
| Nergizoglu | F | 30 | 12 | MN | Anti-DNA | – | CsA, corticosteroid |
| Ohsawa | M | 44 | 9 | MN | – | + | – |
| Lin J | F | 56 | 24 | MN | – | + | Prel, CsA |
| Lin J | M | 23 | 24 | MN | – | + | Pren |
| Rossi | M | 54 | 22 | MN | – | + | CsA, Pren |
| Suehiro | M | 15 | 48 | DPGN | – | + | M-prel, Prel, Cy |
| Kimura | M | 34 | 9 | IgA nephropathy | ANA | + | CsA |
- ANA, antinuclear antigen; CsA, cyclosporine A; Cy, cyclophosphamide; DPGN, diffuse proliferative glomerulonephritis; F, female; FSGS, focal segmental glomerulosclerosis; M, male; MCD, minimal change disease; MN, membranous nephropathy; M-prel, methylpredonisolone; NM, not mentioned; Prel, prednisolone; Pren, prednisone; TBI, total body irradiation.
Figure 4 shows glomerular lesion after a patient developed renal dysfunction following BMT. This patient had an episode of acute GVHD that had been controlled by steroids and CsA. Renal biopsy revealed segmental spike formation along the capillary walls with adhesion of the glomerular tufts to the Bowman's capsule. Some glomeruli showed nodular sclerosis, mesangial cell proliferation and reticulation of the mesangial area. IgG was positive, with a granular pattern along the capillary walls. This case is compatible with membranous glomerulonephritis accompanied by mesangial cell proliferation and mesangiolysis.
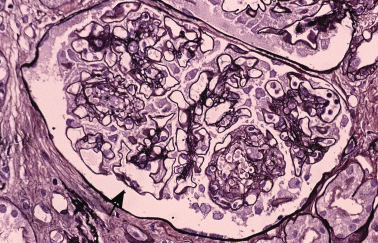
Forty-four-year-old man. The mesangial area is expanded with matricial increase or mesangiolysis (asterisk). There are focal spike formations of the glomerular basement membrane. HE stain; original magnification, ×40.
Another patient developed renal dysfunction accompanied by GVHD following allogeneic BMT. He had been treated with cyclosporine A (CsA). Renal biopsy revealed sparse spike formations and bubbles along the capillary walls without cellular proliferation or extracapillary lesions (Fig. 5a). IgG was positive along the capillary walls. Electron microscopy revealed subepithelial and mesangial deposits (Fig. 5b). Figure 6 also shows glomerular lesions accompanied by chronic GVHD, which developed into severe proteinuria. There were no significant findings except sparse double contour of GBM in glomeruli by PAM stain.
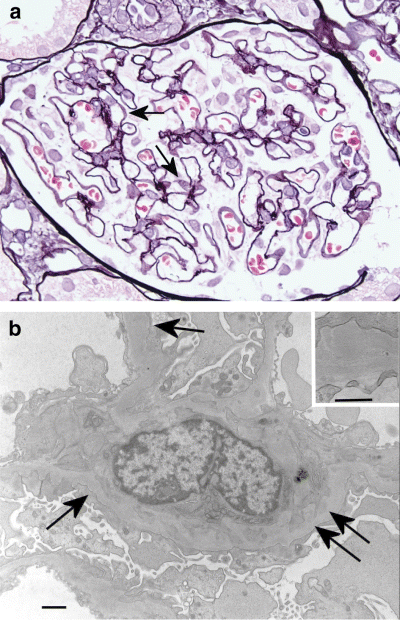
Twenty-four-year-old man. (a) There are bubbles (arrow) and spikes of the glomerular basement membrane. PAM stain; original magnification, ×40. (b) Deposits are noticed in the mesangial area (double arrows) and subepithelium (arrow). Bar, 1 µm. Inset: subepithelial deposits. Bar, 500 nm.
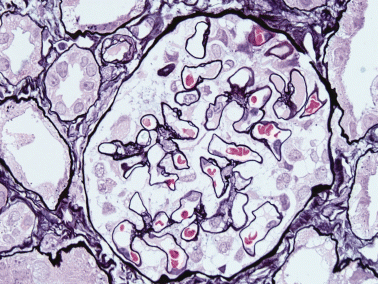
Sixty-nine-year-old man. There are a few double contours of the glomerular basement membrane. PAM stain; original magnification, ×40.
T-cell dysfunction is suggested to be responsible for the development of minimal change nephropathy after BMT. Traynor et al. reported a case of minimal change glomerulopathy associated with INF-α therapy for cutaneous T-cell lymphoma and its background of T-cell dysfunction is supported because the renal function recovered after discontinuation of interferon in that case.43 They concluded that lymphokine-mediated nephrotoxicity may play a role in the setting of lymphoproliferative disease and they therefore recommended caution and close observation of the renal function in T-cell disorders. A similar mechanism is suggested in human GVHD in relation to the development of abnormal cooperation of donor T cells and recipient B cells. IL-2 therapy enhances the ability of killer cells in MHC-restricted cytotoxicity against tumour cells. It is well known that CsA prevents GVHR in rodents44 and humans;45 however, it also breaks self-tolerance and induces killer cells with a specific reaction against class II MHC antigens.46 There are some reports that CsA enhances autoimmunity and also suggest that various kinds of cytokines relate to the glomerular lesion in GVHD.47 However, GVHD after BMT alone may cause glomerulonephritis like the murine model, although the influence of therapeutic agents must be taken into consideration.
HAEMOLYTIC URAEMIC SYNDROME
Since BMT has been introduced for the treatment of leukaemic malignancies, HUS has been reported as a delayed renal complication.48–50 The frequency of post-BMT HUS has been estimated at about 6% to 25%.51–53 It occurs gradually after 1–6 months54,55 or 4–12 months post-BMT. Arends et al. reported that arteriolo-arteriolar and glomerular microangiopathy and apoptotic cells in glomerular endothlium were detected frequently in post-BMT patients who had plasma exchange therapy.49 Histologically, the initial phase is characterized by endothelial damage in glomeruli and vessels followed by thrombosis. Glomerular endothelial cells are swollen, and capillary lumina become narrowed and disappear. Subendothelial and mesangial areas show oedema in addition to subendothelial oedema, including those of small interstitial vessels. Crescent formation is rare; however, mesangial cell proliferation and intimal fibrosis with smooth muscle cell proliferation in arterioles can be found. Prognosis is worse when massive tubular necrosis develops with cortical necrosis. Figure 7 shows a characteristic renal lesion of HUS that developed in a 4-year-old male with neuroblastoma after autologous peripheral stem cell injection combined with irradiation and chemotherapy. Cytomeglovirus infection, GVHD, immunosuppressive reagents such as CsA and FK506, chemotherapy and irradiation are the possible candidates for HUS post-BMT. CsA has been recognized as being effective for GVHD since the report that among 20 patients receiving CsA for longer than 44 days, only one died of GVHD.46 Scanning electron microscopic examination revealed some vascular endothelial damage, which is thought to play an important role in the pathogenic process in HUS that followed allogeneic BMT.56 Although CsA is seen to be the main cause of this condition,57 HUS has occurred in three patients not treated with CsA where immune deposits are supposed to have been responsible for their pathogenesis.58 In humans, serum concentration of chemotherapeutic agents is usually well controlled and we rarely see patients who have received high doses; however, it is still suggested that chemotherapy could induce HUS. There is another case report of HUS developing after autologous BMT without irradiation or chemotherapy.59 The aetiopathogenic cause of HUS is still not clear, but therapeutic agents might be one of the leading factors affecting renal tissue so as to induce it.
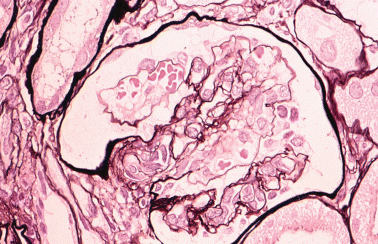
Four-year-old boy. Apparent mesangiolysis and double contour of the glomerular basement membrane are compatible with haemolytic uraemic syndrome. PAM stain; original magnification, ×40.
RADIATION NEPHROPATHY
Irradiation is used to avoid GVHD or host-versus-graft disease after allogeneic BMT. Nephropathy, interstitial pneumopathy, and renal and pulmonary fibrosis are major resulting complications after the combination of irradiation and BMT. Radiation nephropathy is a slowly progressive and non-inflammatory disease and manifests in quite severe tissue damage. Luxton has classified it into acute and chronic phases, with or without proteinuria, benign hypertension and malignant hypertension.60 Acute radiation nephropathy usually occurs 6–12 months after irradiation.
Primary glomerular lesions of radiation nephropathy include mesangial cell proliferation, circumferential mesangial interposition, necrotizing lesions and sclerotic changes. Arterial changes tend to lead to ischaemic or sclerotic glomeruli as secondary changes. Immunofluorescent study shows the same findings as those of microangiopathy; however, deposition of IgM is conspicuous and is also distributed in arterial walls. Aviolo et al. have reported that renal blood flow is decreased when the irradiation increases above 400 rad.61 Radiation nephropathy can be avoided if the shielded area covers one-third of the renal volume.62,63 Renal shielding in post-BMT irradiation is common to date; however, its protective effect is not fully recognized. Lawton et al. have reported that the rate of BMT-associated HUS is reduced from 26% to 6% with a decreased radiation dose.64 Irradiation may be an important factor in inducing HUS after BMT; however, other agents could be responsible for an increased severity of the condition.
In the murine model, late complications following total body irradiation showed glomerular lesions with clinical symptoms of elevated blood urea nitrogen, a fall in haematocrit, and haemolysis consistent with HUS.65
CONCLUSION
BMT is an effective therapy for various kinds of cancers. However, its side effects vary and are hard to avoid. Many factors, such as donor T cells, therapeutic agents, and irradiation followed by released cytokines, can cause prolonged renal lesions after BMT. The nature of side effects and their severity may depend on the patient's individual immune response. The number of cases with glomerulonephritis accompanied with GVHD after BMT is increasing. It is important to take note when autoantibodies are detected. Their mechanisms and crucial interactions are still obscure and further research needs to be done in this area.




