Mesenchymal stem cells in immunoregulation
Abstract
Mesenchymal stem cells are present within the bone marrow cavity and serve as a reservoir for the continuous renewal of various mesenchymal tissues. Recent studies suggest that mesenchymal stem cells modulate immune reactions in vitro and escape from immune surveillance in vivo. We provide herein a discussion of issues including the current research progress on the in vitro interactions of mesenchymal stem cells with multiple subsets of immune cells (dendritic cells, T cells, B cells and NK cells), in vivo transplantation outcomes, the possible underlying mechanisms, future research directions as well as potential clinical implications.
Introduction
Since their discovery in the 1960s, mesenchymal stem cells (MSC) have gradually moved into the main stream focus by virtue of their plasticity and potential applications in various clinical situations, such as tissue regeneration and haematopoietic stem cell transplantation (HSCT).1, 2, 3 Both in vitro 4 and in vivo 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 studies have shown that MSC are not inherently immunogenic and can surpass the MHC barrier between genetically different individuals (Table 1). This withstanding of the immune rejection contradicts the well-established orthodoxy and has earned them the title of ‘universal suppressor of the immune system’.22 It has been suggested that MSC suppression involves several subsets of immune cells.19, 23 Nevertheless, an in-depth understanding of the underlying cellular and molecular basis of the observed phenomenon remains unknown. MSC may be one of the multilayer immune modulators and play specific roles in transplantation tolerance, autoimmunity, tumour evasion and metastases as well as maternal–fetal tolerance.
Under physiological conditions, MSC are quiescent in the bone marrow (BM) and constitute 0.001–0.01% of the total cell population. Once stimulated, for example, trauma, they are recruited into peripheral mesenchymal tissues and become functionally active. To date, the most common MSC isolation technique is still based on the plastic-adherence method described four decades ago.24 The attached cells are cultured in DMEM with 10% FBS and MSC are said to be isolated after obtaining appropriate results from further examinations, including (i) flow cytometry with mesenchymal markers (Stro-1, SH2-4, CD29, CD44, CD71, CD90 and CD109), haematopoietic markers (CD14, CD34 and CD45) and endothelial marker (CD31); and (ii) multilineage differentiation assays (usually osteogenesis, adipogenesis and chondrogenesis). MSC isolated by this method are still heterogeneous as evidenced by the different morphology and functional potentials observed, which may partially account for the discrepancies among current results. Most studies cited in the present review used this common method (adherent-expansion and DMEM medium culture with 10% FBS). However, a few other studies used different configurations (culture medium, negative selection), and such studies will be specified wherever applicable.
T cells: Well-defined targets for MSC suppression
T cells are a major executor of the adaptive immune response. In vitro and in vivo studies have confirmed the immunosuppressive effects of MSC on T cells.15, 25 MSC inhibit the proliferation of T cells stimulated by allogeneic T cells,26 cognate antigen stimuli27 as well as non-specific mitogenic stimuli.28, 29 The inhibition affects the expression of activation markers, antigen-specific proliferation (both for naive and memory T cells), CTL formation, IFN-γ production by Th1 cells and the IL-4 production by Th2 cells.23, 27 MSC constitutively express MHC-I and there is a certain inconsistency about the MHC-II expression with both no level and low level having been reported.14, 26, 28, 30 Two culture configurations were involved in these studies, including DMEM with 10% FBS and another α-MEM with 12.5% FBS and 12.5% horse serum. Although different culture media may produce a different mixture of heterogenous MSC, no match has been found between the culture configuration and the MHC-II expression level. Thus, the discrepancy observed has to be explained by other factors, such as the varying expression patterns of surface markers in culture as a function of time, different stages of differentiation or species diversity. Although MSC upregulate MHC-II expression after exposure to IFN-γ,31 it has been shown that MSC suppression is independent of MHC.26
One explanation for the observed suppression is that MSC have veto-like activity.30 Veto cells refer to a group of lymphoid cells that act as fraudulent APC and specifically inhibit T-cell precursor clones that interact with them.32 Veto activity in different species is mediated by different T-cell subsets. In humans and primates, CD8+ veto cells are termed graft-facilitating cells and trigger the accumulation of TGF-β1 mRNA and protein, causing the deletion of the responding T cells.33, 34 Studies suggest that TGF-β1 play a role in the MSC-induced suppression of CD8+ T-cell proliferation.28, 35 Therefore, MSC may possess veto-like activity per se or act indirectly through veto cells and trigger the suppressive downstream signals, leading to T-cell inhibition under both allogeneic and xenogeneic settings.26, 36
The other explanation is that MSC function through suppressive T cells. Apart from two well-defined T-cell subsets, namely, Th cells and CTL, there is a third type of T cells termed regulatory T cells (Treg), which negatively regulate immune responses.37 To date, three subsets of Treg have been identified. First, naturally occurring Treg are CD4+CD25highfoxP3+ and act in an antigen-non-specific manner, whereas the induced Treg are CD4+CD25+/− and act in an antigen-specific manner, including IL-10-secreting Treg1 and TGF-β-secreting Th3. Finally, a distinct CD8+CD28− subset termed T suppressor has been identified in humans recently.38 The findings of the present study with CD4+CD25highfoxP3+ Treg are conflicting. When using MSC cultured in Mesencult medium (Stem Cell Technologies, WA, USA) with MSC supplement, the Treg were seen to be highly activated.22 However, two other studies using MSC cultured in DMEM medium with 10% FBS resulted in both positive and negative results.23, 27 This lack of standardized culture configuration together with the heterogeneous populations produced makes it difficult to compare the results from different studies. Once this issue is solved, many downstream signals could be the potential research targets, including IL-2-related suppressive signals, Smad-mediated IL-10 secretion and costimulatory molecule-induced antigen-specific unresponsiveness, such as CD28 and CTLA4.39, 40, 41
Other explanations include T-cell anergy, suppressive soluble factors, physical cell–cell contact and apoptosis. T-cell recognition of an antigenic peptide–MHC complex sometimes results in a state of unresponsiveness called clonal anergy with the inability of cells to proliferate. Whether clonal expansion or clonal anergy ensues is determined by the presence or absence of a costimulatory signal, for example, the interaction of CD28 with B7 on APC. As MSC do not express some of the important costimulatory molecules, such as B7-1, B7-2, CD40 or CD40L, it is assumed that MSC carry out the suppressive effect through the induction of T-cell anergy. However, the findings from different groups were not consistent. In murine models, it has been suggested that T-cell tolerance was induced because lymphocytes did not proliferate after removal of MSC.29, 42 However, studies on other murines or humans found that the unresponsiveness was transient and relieved once MSC were removed from the culture.27, 28 Apart from the various culture conditions, the diverse stimuli used in different study designs may partially account for the variation observed. Soluble factors have been proposed to be responsible for the inhibition of CTL formation, including TGF-β1 and hepatocyte growth factor, prostaglandin E2 (PGE2), IFN-γ and indoleamine 2,3-dioxygenase (IDO).23, 28, 43, 44 Nevertheless, IDO-mediated tryptophan degradation is in disagreement with the reports that MSC do not lead to T-cell apoptosis,28, 45 which normally resulted from the tryptophan depletion (with one exception of observed apoptosis46). Other studies bring forward the possibility of physical inhibition as cell contact is essential in some experiments.19, 27 Alternatively, MSC may have their own system of immunoregulation through a specific pattern of inhibitory cytokines, such as the proposed ‘autocrine loop’ evidenced by simultaneous upregulation of both TGF-β1 and TGF-β1R on MSC.47
The remarkable correlation between T cells and MSC dates back to the earlier ontogenic stage both in the BM and thymus.48, 49 During the developmental process, mesothelium-derived MSC localize to the BM by the invasion of the vasculature and subsequently provide physical and functional support for HSC. This process occurs at the same time when primitive haematopoietic progenitors appear. From the ontogenic point of view, the spatiotemporal proximity between HSC and MSC implies the intimacy of two stem cell subpopulations. It may also account for the cross-reaction of MSC surface markers, such as Stro-1, SH-2 with vasculature endothelial and epithelial cells.50 After originating from HSC, primitive T cells migrate to the cortex zone of the thymus and undergo postmarrow maturation by positive and negative selections. In the murine model, both allogeneic donor-derived cells and self stromal cells (another term for MSC) migrate into the thymus and participate in the positive selection of thymocytes.51 Furthermore, it has been reported that stromal cells can provide sufficient signals to support T-cell development in the absence of the thymus52 and the early thymocytes (T precursors) preferentially adhere to BM stromal cells, suggesting the BM stroma as an extrathymic site for T lymphopoiesis.53 Phenotypically, MSC share some surface molecules with the epithelial component, thymic nurse cells, of the thymic microenvironment, including vascular cell adhesion molecule (VCAM)-1, intercellular adhesion molecule (ICAM)-1 and lymphocyte function associated antigen type 3 (LFA-3) (Figure 1).54 We assume that MSC not only provide stromal support for HSC in the BM, but also play a role in the earlier ontogenic event, such as the maturation process of T cells in the central immune organ – thymus and, therefore, contribute to the renewal of the T-cell repertoire. MSC may therefore play a more essential role in the process of haematopoiesis than has been suggested before.
The interactions between T cells and MSC have significant clinical implications in HSCT. MSC have been shown to lessen complications, such as graft-versus-host disease (GVHD) after HSCT.7 Traditionally, one strategy for treating GVHD is to carry out alloreactive T-cell depletion of the donor graft. However, after receiving this procedure, patients tend to have a much higher relapse rate than the non-depletion counterparts, mainly because the mature donor T cells play a very important role in graft-versus-leukaemia effect.55 The procedure was optimized later by the selective removal of host-reactive T cells, but the outcomes were still not very reliable.56 Among all the efforts, cotransplantation of MSC has emerged with a unique rejection–suppressive effect on the recipients.2 After high-dose chemotherapy/radiotherapy, cotransplanted MSC may exert a trophic effect on the microenvironment by producing local growth factors, cytokines and chemokines, help to recover damaged stroma and participate in the early developmental process of T lymphopoiesis.4 All these effects will accelerate the haematopoiesis reconstitution, which is crucial for the successful HSCT.
Dendritic cells: First line to encounter MSC
Dendritic cells (DC) are termed ‘professional APC’ because of their exclusive role in naive T-cell stimulation during the primary immune response. They are also involved in the sensing and activation of B cells either indirectly through Th cells or directly through soluble factors (including IL-12). Therefore, DC are critical to both cell-mediated immunity and humoral immunity.57 Recent evidence showed that MSC inhibited the differentiation, maturation and activation of co-cultured DC. Specifically, MSC inhibited the initial differentiation of monocytes to DC by downregulating the expression of CD1a, CD86 and HLA-DR and later DC maturation by suppressing the expression of CD83.58 For mature DC, MSC skew their phenotype towards immature status by reducing CD83 expression59 and alter cytokine secretion, such as decreased TNF-α of DC1 and increased IL-10 secretion of DC2.23 DC have also been involved in an indirectly suppressive mechanism wherein MSC are suggested to inhibit T cells through contact-dependent induction of regulatory APC with suppressive functions.19
There are several possible ways in which the suppressive signals can be produced (Figure 1). First, MSC decrease the capacity of DC to secrete IL-12, which is critical to the promotion of effective cell-mediated immunity by activating and differentiating T cells towards the Th1 pathway.58 Second, MSC secrete TGF-β1, a factor that inhibits the in vitro activation and maturation of DC.28, 60 In addition, the preferential activation of CD4+CD25+ Treg may contribute to the delayed maturation of DC.61 Finally, MSC caused mature DC1 to decrease TNF-α secretion and mature DC2 to increase IL-10 secretion, leading to a state of immunotolerance. IL-10 tends to have a significant inhibitory effect on several aspects of APC function, such as downregulating the expression of surface markers (CD40, CD80, CD83 and CD86) and IL-12 secretion. IL-10-secreting DC show minimal or no stimulatory effect in primary mixed lymphocyte culture and are markedly inhibitory to T-cell proliferation.62, 63, 64 Therefore, IL-10-producing DC are functionally and phenotypically inhibitory cells and are putatively tolerogenic.19, 65 However, as it has been suggested that MSC tend to synthesize IL-10 in a continuous manner in vitro, whether the current finding is applicable in vivo needs further confirmation.66 DC precursors, following the original arising from HSC, differentiate through either the myeloid pathway (and will thus become epidermal Langerhans cells, interstitial and/or circulating DC) or the lymphoid pathway (and will become plasmacytoid DC). Lymphoid-derived DC are a special type of DC, which are believed to primarily mediate the regulatory effector function rather than the stimulatory effector functions by preferentially activating Th2 lymphocytes. As a result of the limitations of current isolation techniques, most studies involve only monocyte/CD14+-derived DC1, with one exception of plasmacytoid DC2.23
Unfortunately, the MSC application may compromise DC-based cancer therapy during clinical practice. The new strategy to either treat or prevent cancer by using cancer antigen-immunized DC vaccines has hitherto been regarded as having great potential.67 When combined with MSC, however, the performance of DC may be crippled to a certain extent in that the immune system will reduce its response to the tumour antigen-loaded DC vaccine in the presence of immunosuppressive MSC (e.g. MSC supplementation in HSCT for the DC vaccine-treated leukaemia patients). Under physiological circumstances, it has been suggested that the immune system has the potential to eliminate neoplastic cells as evidenced by rare, but well-documented instances of spontaneous remissions in renal cell carcinoma and melanoma. The reason why only a few cancer cell populations finally develop into clinical cancer is not yet clear. Similarly, it has been observed that immunocompromised individuals (transplantation recipients, congenital immune deficiency and AIDS patients) show an increased incidence of rare neoplasms, which correlates to the degree of immunosuppression. MSC infusion could damage the natural protection of the body and compromise self-defence if cancer cells are developing at this stage or negatively interfere with DC-based cancer vaccines when cancer cells have not yet developed. MSC infusion has been shown to favour tumour growth during the tumour model induction process.68, 69 In future, concurrent administration of MSC should be carried out with caution during DC-based cancer therapy.
NK cells: A peripheral figure to interact with MSC
NK cells can lyse targets without the aid of prior immunization and are further activated by IL-2, IL-12, IL-15, IL-18, IL-21, IFN-α and IFN-β.70 MSC are not lysed by freshly isolated allogeneic NK,71 but are susceptible to lysis by IL-15 activated NK.72 It has been suggested that MSC downregulate IFN-γ production of IL-2-stimulated NK (Figure 1),23 and suppress the proliferation, cytokine secretion and cytotoxicity of those stimulated by IL-15.71 NK target cells that have aberrant MHC expression, notably a reduction in the display of MHC-I molecules by some tumour cells and virus-infected cells. As MSC express normal levels of MHC-I, they should not be targeted by NK. However, in any individual's NK repertoire there are cells that only express single or certain (as the quantity may vary) killer cell immunoglobulin-like receptors (KIR). If mismatched allogeneic MSC do not have this specific class I allele group, then they trigger NK alloreactivity and are thereby eliminated under the instruction of unblocked KIR.70, 73 Several subtypes of NK have been identified, including CD56+dimCD16+ and CD56brightCD16−. Current isolation methods are limited by their lack of specific surface markers, and NK acquired through negative selection are therefore heterogeneous, including several subtypes in different ratios. The results of current studies may not represent the response from a single pure NK fraction. MSC have been shown to inhibit the expression of IL-2 receptor (CD25) on PHA-activated lymphocytes.74 Whether a similar effect will be executed on NK is worth further investigation as CD56+brightCD16− NK constitutively express high-affinity CD25.75 Different NK stimulators should also be brought into consideration in future studies, because only IL-2 and IL-15 are currently used to stimulate NK.
MSC infusion may be beneficial in correcting the abnormal activation of uterine NK cells (uNK) during recurrent pregnancy loss (RPL). Pregnancy loss, more commonly referred to as ‘miscarriage’, is the most common complication of pregnancy. During early gestation, uNK appear in close proximity to the embryo site.76 The maternal immune response to the fetal allograft is somehow regulated by uNK as evidenced by the abnormal upregulated cytotoxicity of uNK in RPL.77, 78 Two therapies currently used are leucocyte immunization and i.v. immunoglobulin infusion, intending to down-regulate the maternal immune response towards the embryo. However, no benefit has been confirmed so far.79 Although there are functional differences between uNK and peripheral NK, namely decidual uNK show weaker cytotoxic activity and express a broader cytokine profile, uNK function can be readily increased after IL-2 stimulation and kill trophoblast cells afterwards. Therefore, uNK may actually derive from peripheral NK under the influence of local hormone signals, such as progesterone. MSC may help to suppress overactivated uNK in RPL and reduce the lysis of trophoblast cells. Mechanically, in utero MSC transplantation has been successfully carried out in the first-trimester fetal sheep model.80 Whether a similar procedure is feasible in human trials is still open.
B cells: Potential targets for MSC?
B cells are responsible for humoral immunity. Compared with other immune cells, B cells remain the least studied. Glennie et al. reported recently that, in mice, the proliferation of B cells stimulated by anti-CD40 antibody and IL-4 was inhibited in the presence of MSC (MSC : B cells was equal to 1:10).42 Another murine study suggested the involvement of the programmed death-1 pathway in B-cell inhibition by MSC.47 The latest human studies proposed a multilevel intervention model where MSC affect the proliferation, antibody production and chemotaxis of B cells.81 The suppressive effect of MSC on B-cell proliferation in this study was significant at 1:1 ratio of MSC cells : B cells, different from the earlier murine published work at a ratio of 1:10. The reasons may be the heterogeneous MSC mixture produced by the different culture regimens used in the two studies, the intrinsic diversities among species or the different stimulation cocktail used. The stimulator in the human study was more powerful in triggering B-cell proliferation; thus more MSC were needed to suppress B cells. Another phenomenon observed was that the supernatants from co-cultured, but not confluenced MSC inhibited B-cell proliferation, suggesting that paracrine signals from B cells are required for the release of inhibitory soluble factors from MSC.81 However, no in vivo evidence of the suppressive effect of MSC on B cells is available presently.
B cells originate from the BM and keep close contact with stromal cells throughout the developmental process. It is estimated that in mice 90% of the B cells die of negative selection and clonal deletion without ever leaving the BM and reaching the circulating pool. Such a high elimination rate has been attributed entirely to the classic immune cell-mediated negative selection against B cells, which results from the cross-linking of self-antibodies on immature B cells with self-antigens present on stromal cells. However, the stromal cells (including MSC) involved may function not merely as venue-providers, but also as effector-carriers. MSC have been implicated in the negative regulation of B lymphopoiesis in the BM as the engagement of LY-6A/E protein found in the BM stromal cell line leads to increased production of GM-CSF, which inhibits B-lymphocyte development.82, 83 Hormones can alter B lymphopoiesis as well. Mesenchyme-derived stromal cells in the BM assist both oestrogen-exerted suppressive effects and androgen-exerted suppressive effects on B lymphopoiesis.84 The effect of androgen is stromal dependent and is mediated by androgen receptor expressed on the stromal cell surface. One potential product of MSC, TGF-β, has been shown to participate in B-cell inhibition,47 as it induces downregulation or blockade of stromal cell-derived IL-7.85, 86 Although the mesenchyme-derived stromal cells used in the study were cultured in RPMI-1640, a different medium from DMEM for MSC, they do have some similarities with MSC, such as adherent-dependence, extensive proliferation and the secretion of TGF-β and IL-7 (Table 2).
The interactions between B cells and MSC have significant implications in autoimmune disease. Castration of normal male mice induces the expansion of B cells in BM. Androgen is responsible for this effect in the presence of marrow stromal cells. Androgen replacement therapy has been shown to reduce autoantibody levels in hypogonadal patients with systemic lupus erythematosus (SLE).88 Non-specific polyclonal B-cell activation can be induced by a variety of viruses and bacteria in the absence of Th cells. The proliferation of B cells results in the release of autoantibodies in the forms of IgM and/or IgG, such as rheumatoid factors in rheumatoid arthritis and antinuclear antibodies in SLE. Ideal treatment should be aimed at reducing only the autoimmune response, while leaving the rest of the immune system intact. However, current immunosuppressive drugs (e.g. corticosteroids and cyclophosphamide) do not distinguish between a pathological autoimmune response and a protective immune response. Such drugs reduce the severity of autoimmune symptoms at the cost of depressing the whole immune response. This places the patients at a greater risk of infection or to the development of cancer. Intriguingly, allogeneic MSC have been shown to produce suppressive TGF-β,47 reduce both IgM and IgG production in humans81 and inhibit the proliferation, activation as well as IgG secretion of B cells from the BXSB mouse, which is an experimental model for human SLE.89 Using the regulatory signals produced, the infusion of MSC could help to protect the autoimmune patients from their own oversensitive immune system while leaving the rest of the normal immune functions intact.
Conclusion
The immunomodulatory potential of MSC remains controversial yet hopeful. Many immune cells participate in this process (Figure 1).
-
Definite inhibitory effect of MSC on T cells, which holds great promise in HSCT
-
MSC play a role in regulating lymphopoiesis
-
MSC interact with DC and this may compromise the DC-based cancer therapy
-
MSC may inhibit NK cells and/or B cells and thus benefit the treatment of RPL, autoimmune disease etc.
However, more issues still need to be addressed, including how many the immune cell subsets participate in MSC immunoregulation? Which pathway(s) is/are involved? What is the diverse systemic response towards MSC in different disease settings? Are there any safety issues during further clinical trials? The MSC can be safely and routinely applied in the future only after these unexplored territories have been clarified.
Acknowledgement
Xi Chen is supported by overseas research student award from Universities UK, 2003–2006.
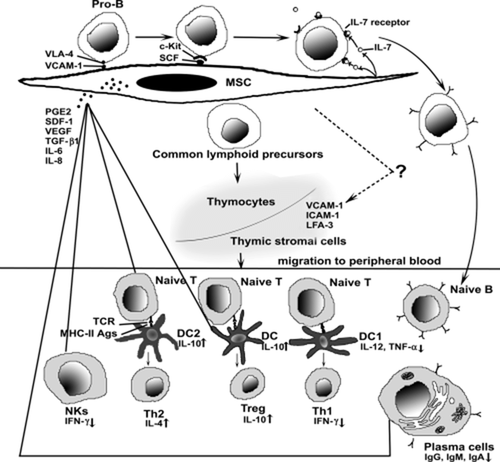
Mesenchymal stem cells (MSC) regulate lymphopoiesis and suppress immune response. Bone marrow MSC participate in the developmental process of both T lymphocytes and B lymphocytes through growth factors, cytokines, adhesion molecules etc. Some crucial surface molecules, such as vascular cell adhesion molecule (VCAM)-1, intercellular adhesion molecule (ICAM)-1 and lymphocyte function associated antigen type 3 (LFA-3), are expressed on both MSC and thymic stromal cells, indicating similarities between two different microenvironments of bone marrow compartments and thymus. Moreover, MSC mediate immunoregulatory effects on both innate and adaptive immunity through either indirect soluble factors or direct physical contact. The general effects are to skew the immune response towards anti-inflammatory/tolerant phenotypes, including the shift from Th1 towards Th2, downregulation of IFN-γ production from NK and reduction in the antibody productions of B cells. PGE2, prostaglandin E2; SCF, stem cell factor; SDF-1, stem cell-derived factor 1; TCR, T cell receptor; TGF-β1, transforming growth factor β1; VEGF, vascular endothelial growth factor; VLA-4, very late antigen 4.
| Reference | Recipient condition | Donor cell source | Surgical procedure | Publication year |
|---|---|---|---|---|
| Dai et al. 5 | Myocardial infarction model of Fischer rat | Allogeneic | 2 × 106 MSC injected into the scar of a 1-week-old myocardial infarction | 2005 |
| Price et al. 6 | Myocardial infarction model of swine | Allogeneic | 3.2 ± 0.4 × 108 MSC i.v. infusion | 2005 |
| Zhang et al. 7 | Intervertebral disc degeneration model of rabbit | Allogeneic | 1 × 105 MSC direct injection | 2005 |
| Le Blanc et al. 8 | 9-year-old patient with severe GVHD | Allogeneic | 1 × 106 to 2 × 106 MSC/kg i.v. infusion | 2004 |
| Deng et al. 9 | Lethally irradiated mice | Allogeneic | 5 × 105 MSC i.v. infusion | 2004 |
| Natsu et al. 10 | Half-stratum laceration model of Sprague–Dawley rat | Allogeneic | Fibrin block with ∼3.5 × 105 MSC transplanted into muscle defect | 2004 |
| Mahmud et al. 11 | Baboon with TBI + HBI | Allogeneic | 10 × 106 MSC/kg by either i.v. infusion or IBM | 2004 |
| Arinzeh et al. 12 | Critical-sized segmental defect model of dog | Allogeneic | 4.25 × 104 MSC/HA/TCP ceramic implantation | 2003 |
| Tsuchida et al. 13 | Femoral segmental defect model of Fischer rat | Allogeneic | 8 × 106 MSC implantation in 6-mm transverse segment of the central diaphysis | 2003 |
| Fouillard et al. 14 | Patient with severe idiopathic aplastic anaemia | Allogeneic | 2 × 106 to 6 × 106 MSC/kg i.v. infusion | 2003 |
| Devine et al. 15 | Normal baboon | Autologous and allogeneic | 18.5 × 106 to 30.3 × 106/kg MSC i.v. infusion | 2003 |
| Horwitz et al. 16 | Children with osteogenesis imperfecta | Allogeneic | 1 × 106 to 5 × 106 MSC/kg i.v. infusion | 2002 |
| Bartholomew et al. 17 | Baboon skin graft transplantation | Allogeneic | 20 × 106 MSC/kg i.v. infusion | 2002 |
| Devine et al. 18 | Baboon with TBI and haematopoietic support | Allogeneic | 3 × 106 to 30 × 106/kg MSC i.v. infusion | 2001 |
| Almeida-Porada et al. 19 | Day 55 to day 60 fetal sheep | Xenogeneic (human) | 5 × 104 to 7.5 × 105 MSC together with 0.7 × 104 to 6.5 × 104 hae matopoietic stem cells i.p. injection | 2000 |
| Archambault et al. 20 | Femoral gap model of ACI rat | Allogeneic | Osteoconductive matrix loaded with MSC | 2000 |
| Lazarus et al. 21 | HSCT supplementation | Allogeneic | 1 or 2.5 × 106 MSC/kg i.v. infusion | 2000 |
- ACI, a cross strain between the August and Copenhagen–Irish strains; GVHD, graft-versus-host disease; HA/TCP, hydroxyapatite-tricalcium phosphate; HBI, hemibody irradiation; HSCT, haematopoietic stem cell transplantation; IBM, intrabone marrow; MSC, mesenchymal stem cells; TBI, total body irradiation.
| Adhesion molecules | VCAM |
| ICAM-1, ICAM-2, ICAM-3 | |
| HCAM | |
| ALCAM | |
| NCAM | |
| L-selectin | |
| LFA-1, LFA-3 | |
| Integrins: VLA-α1, VLA-α2+/−, VLA-α3+/−, VLA-α5, VLA-α6+/− | |
| VLA-β1, VLA-β2+/-, VLA-β3, VLA-β4+/− | |
| Vitronectin R β-chain | |
| Cytokine and growth factor receptors | IL-1, IL-3, IL-4, IL-6, IL-7R |
| IFN-γ R | |
| TNF-α-I/IIR | |
| TGF-β-I/IIR | |
| FGFR | |
| EGFR | |
| PDGFR | |
| Transferrin receptor | |
| Growth factors | |
| IL-1, IL-6, IL-7, IL-8, IL-11, IL-12, IL-14, IL-15‡ | |
| LIF | |
| SDF-1 | |
| OSM | |
| BMP-4 | |
| Flt-3 ligand | |
| SCF | |
| G-CSF | |
| M-CSF | |
| GM–CSF | |
| Extracellular matrix proteins | Collagen type I, III, IV, V, VI |
| Fibronectin | |
| Hyaluronan | |
| Laminin | |
| Vimentin | |
| Proteoglycans |
- † Based on Deans and Moseley,3 Minguell et al. 50 and Roberts.87
- ‡ Under stimulation of IL-1. ALCAM, activated leucocyte cell adhesion molecule; BMP-4, bone morphogenetic protein 4; EGFR, epidermal growth factor receptor; FGFR, fibroblast growth factor receptor; FL, Flt-3 ligand; G-CSF, granulocyte-colony stimulating factor; HCAM, the homing-associated cell adhesion molecule; ICAM, intercellular adhesion molecule; IL-R, interleukin receptor; LFA, lymphocyte function associated antigen; LIF, leukemia inhibitory factor; M-CSF, macrophage-colony stimulating factor; NCAM, the neural cell adhesion molecule; OSM, oncostatin M; PDGFR, platelet-derived growth factor receptor; SCF, stem cell factor; SDF-1, stem cell-derived factor 1; VCAM, cell adhesion molecule; VLA, very late antigen.





