Role of the PI3K-TOR-S6K pathway in the onset of cell cycle elongation during Xenopus early embryogenesis
Abstract
In the early embryogenesis of the frog, Xenopus laevis, cells proliferate by rapid and synchronous divisions, followed by cell cycle elongation and prolongation of the S phases, and then the appearance of the G2 and G1 phases after the midblastula transition (MBT). The beginning of cell cycle elongation was thought to depend on an increase in the nucleo-cytoplasmic (N/C) ratio in blastomeres and a decrease in cortical cytoplasmic factors necessary for cell cycle progression, although these factors are unknown. In the present study, we demonstrated that a regulatory subunit of PI3K (p85α) was localized in the cortical cytoplasm of the blastomere during the MBT. When the embryos were treated with a PI3K inhibitor, LY294002, or a TOR inhibitor, rapamycin, cell cycle elongation was initiated before the MBT. In addition, the inhibition of S6K expression by antisense morpholino oligo enhanced the initiation of cell cycle elongation. In contrast, the activation of PI3K-TOR by Rheb-S16H expression delayed the initiation of cell cycle elongation. These results indicate that a decrease in translational activity dependent on the PI3K-TOR-S6K pathway causes the initiation of cell cycle elongation at the onset of the MBT.
Introduction
During embryogenesis in all multicellular organisms, the number of cells increases rapidly to a level at which the embryo begins morphogenesis. In many animals, cells proliferate by rapid and synchronous divisions, called cleavages, during early embryogenesis. Following this stage, in Xenopus and zebrafish embryos, elongation of the cell cycle time and loss of synchrony begin at the midblastula transition (MBT), when the activation of transcription and cell motility occur (Graham & Morgan 1966; Newport & Kirschner 1982; Edgar et al. 1986; Kane & Kimmel 1993; Masui & Wang 1998; Iwao et al. 2005).
It has been demonstrated that the cell cycle times of blastomeres remain almost constant regardless of their cell size up to about 37.5 μm in radius, followed by the elongation of their cell cycle times in proportion to the inverse square of the cell radius after the MBT (Wang et al. 2000). The timing of the initiation of asynchronous divisions is determined by the nucleo-cytoplasmic (N/C) ratio of the blastomeres (Kobayakawa & Kubota 1981; Newport & Kirschner 1982; Edgar et al. 1986; Kane & Kimmel 1993; Clute & Masui 1995). We previously demonstrated that the asynchronous divisions are caused by variations in the lengths of the cell cycle phases, beginning with prolongation of the S phases, followed by the G2 and G1 phases, in that order (Iwao et al. 2005). The degradation of Cdc25A by Chk1 transiently activated during the MBT is involved in the initiation of G2 phase (Shimuta et al. 2002). The initiation of the G2 and G1 phases is probably accelerated by the localization of PTEN into the nuclei after the MBT (Ueno et al. 2006). The specific factor in the cytoplasm responsible for the extension of S phase in the initiation of cell cycle elongation is unknown. It is possible that the decrease in the rate of DNA synthesis is caused by the degradation of two proteins, Cyclin E1 and Xcdc6A, during the MBT (Tikhmyanova & Coleman 2003; Gotoh et al. 2007). Interestingly, the levels of both Cyclin E1 and Xcdc6A decrease drastically just after the MBT, and their mRNAs are degraded after the tail bud stage (Tikhmyanova & Coleman 2003; Gotoh et al. 2007). This suggests that the decrease of protein synthesis or the degradation of proteins including Cyclin E1 and Xcdc6A occurs at the MBT. However, the partial inhibition of Cyclin E1 translation before the MBT does not affect the cell cycle progression (Gotoh et al. 2007), so the function of Cyclin E1 for DNA synthesis seems to be restricted at the MBT. In general, cell growth is coordinated with cell cycle progression according to the cell size (Neufeld 2003). In vertebrates, the target of rapamycin (TOR) and the phosphatidylinositol 3-kinase (PI3K) are well-conserved factors that regulate cell growth and the cell cycle. In this respect, the PI3K-TOR pathway regulates translation through the ribosomal protein S6 kinase (S6K) and the eukaryotic translational initiation factor 4E-binding protein (4E-BP) (Neufeld 2003; Fingar & Blenis 2004). In the present study, we demonstrated that the PI3K-TOR-S6K pathway-dependent translational activity decreased with the progression of cleavage in the blastomeres during the MBT, which initiated cell cycle elongation.
Materials and methods
Construction of cDNAs and recombinant plasmids – morpholino oligonucleotides
cDNA fragments of Xenopus p85α, p55γ and Rheb (GenBank accession nos. AY062922, BC077814, BC043818) were isolated by polymerase chain reaction (PCR) from a Xenopus oocyte cDNA library using specific primers for each. To construct wild-type Rheb (Rheb-WT) or an enhanced green fluorescent protein (EGFP)-conjugated p85α, p55γ (EGFP-p85α, EGFP-p55γ), the XbaI (and BamHI)-cut PCR fragments were subcloned into the pT7G (UKII+) or the pT7G (UKII+)-N-L-EGFP plasmids (Iwao et al. 2005). A constitutively active Rheb-S16H mutant (Ser16→His) was constructed by site-directed mutagenesis using appropriate primers from Rheb-WT as a template. All constructs were cut singly at the NotI site, located downstream of the 3′-poly(A) tail, and then transcribed in vitro using the MEGA Script T7 Kit (Ambion) with a 5′-cap analog (NEB). A morpholino oligonucleotide (MO) against S6K (S6K-MO; 5′-TCAATGTCAAACA- CCCCAGCCATTG-3′) was designed and supplied by Gene Tools, LLC.
Microinjection and embryology
Fertilized Xenopus eggs were obtained by insemination in vitro, de-jellied in a cysteine solution (3% L-cysteine hydrocholoride monohydrate, 1% NaOH, pH 8.5) and maintained in 30% modified Barth’s solution (MBS; 88 mmol/L NaCl, 1 mmol/L KCl, 2.4 mmol/L NaHCO3, 0.82 mmol/L MgSO4, 0.33 mmol/L Ca(NO3)2, 0.41 mmol/L CaCl2, 10 mmol/L HEPES-NaOH, pH 7.6). For the overexpression of the Rheb-S16H protein or the adequate level of expression of the EGFP-p85α and EGFP-p55γ proteins, 4.6 or 1.9 ng mRNA was microinjected into one-cell stage embryos in 30% modified Barth’s solution with 4% Ficoll, respectively. For the inhibition of S6K translation, S6K-MO (66 ng) was microinjected into each one-cell stage embryo. The injected embryos were maintained in 30% MBS until the 128-cell stage and then transferred to 10% MBS. The stage of embryos was determined as previously described by Nieuwkoop & Faber (1967).
Antibodies and western blot analysis
Routinely, the amount of proteins equivalent to one embryo was loaded onto 10% or 15% polyacrylamide gels for western blot analysis with an anti-p85 antibody (1:100 dilution; sc-423, Santa Cruz) or anti-4E-BP antibody (1:1000 dilution; #9452, Cell Signaling Technology), or onto 12.5% Anderson gels for immunoblotting using an anti-S6K antibody (1:2000 dilution; AF8962, R&D Systems). The signals were detected with an horse radish peroxidase (HRP)-conjugated anti-rabbit immunoglobulin G (IgG) goat antibody (1:2000 dilution; A-6667, Sigma) and detected using an enhanced chemiluminescence system (ECL+; GE Healthcare).
Culture of dissociated blastomeres
The culture and statistical analyses of the dissociated blastomeres were performed using methods previously described by Wang et al. (2000). To dissociate blastomeres, the embryos were transferred into modified low Ca Stearns’ solution (74.6 mmol/L NaCl, 2.4 mmol/L KCl, 0.63 mmol/L Na2HPO4, 0.14 mmol/L KH2PO4, 1.9 mmol/L Na2SO4, 0.5 mmol/L CaCl2 0.5 mmol/L MgCl2, 1 mmol/L ethylenediaminetetraacetic acid [EDTA], 0.1% bovine serum albumin [BSA], 0.1% dimethyl sulfoxide, pH 8.3) and maintained at 20°C. Fertilization membranes were manually removed using fine forceps and blastomeres of the animal hemispheres were isolated using a fine glass rod. To culture the blastomeres, each was transferred using a small glass pipette into a droplet (5 μL) of the low Ca Stearns’ solution as a culture medium, which had been placed on the bottom of plastic-based culture dishes coated beforehand with a dry layer of laminin and fibronectin. The droplets were covered with mineral oil and each dish was placed on the stage of an inverted microscope, where the behavior of the blastomeres was recorded by time-lapse imaging (2 min intervals) using a digital camera. Cell sizes were determined by measuring the diameters of the blastomeres when they appeared morphologically rounded, which typically occurred a few minutes before the appearance of the cleavage furrow. The timings of the cell divisions were determined by observing the appearance of cleavage furrows.
Imaging of cellular localization of EGFP-p85α or EGFP-p55γ in translucent blastomeres and neurula embryos
The one-cell embryos were microinjected with EGFP-p85α or EGFP-p55γ mRNA. The culturing and statistical analyses of translucent blastomeres were performed based on the methods of Iwao et al. (2005). For the observation of epithelial cells during the neurulation stage, embryos were cultured until stage 18, the fertilization membranes were manually removed, and then the embryos were placed on a glass base-dish (3910-035, IWAKI). The images of EGFP-p85α and EGFP-p55γ were captured using a confocal laser-scanning microscope (LSM510Meta, Carl Zeiss). The cells were scanned with 6 μm thick optical sections of the translucent blastomeres or with 4 μm thick sections during neurulation.
Results
Expression and localization of the regulatory subunits of PI3K, p85α and p55γ during Xenopus embryogenesis
In X. laevis, p85α and p55γ are known as regulatory subunits of PI3K, but their roles in early embryogenesis have not been fully investigated. We examined changes in their expression during embryogenesis by determining the amount of p85α and p55γ proteins in each embryo by western blotting using an antibody against the N-terminal SH2 domain of human p85α, which reportedly recognizes both subunits with similar N-SH2 domains in mammals. The antibody can also recognize both p85α and p55γ in Xenopus, since they have similar N-terminal SH2 domains (Fig. 1A). Not only p85α, but also p55γ, was consistently present in Xenopus embryos from the morula (stage 6) to the neurula (stage 22) (Fig. 1B). During these stages, the cell number in one embryo increased rapidly, while the amount of p85α and p55γ protein was nearly constant. Next, we investigated changes in the localization of p85α and p55γ in the cytoplasm of blastomeres during embryogenesis, especially around the MBT. Immunocytochemical localization of the PI3K regulatory subunits (p85α and p55γ) showed localization not only in the peripheral cytoplasm near the plasma membrane, but also around the nucleus at the morula (stage 6) (Fig. 2A). From the MBT (stage 8.5) to the gastrula (stage 12), the distribution of the PI3K regulatory subunits in the peripheral cytoplasm and around the nucleus was maintained (Fig. 2B,C), but marked localization near the plasma membrane was observed at the gastrula (Fig. 2C). Since the antibody recognized both p85α and p55γ, it is difficult to distinguish the localization of these subunits in cells by this method. Thus, we then analyzed differences in localization between these subunits by the expression of EGFP-p85α or EGFP-p55γ in developing embryos with translucent blastomeres (Fig. 3). We estimated the timing of the MBT by measuring the blastomere size (about 37.5 μm in radius) and the length of the cell cycle (at the beginning of cell cycle elongation). EGFP-p85α was distributed in the cytoplasm near the plasma membrane during the MBT, and a similar localization was observed after the MBT (Fig. 3A). In contrast, another subunit of EGFP-p55γ was observed not only in the cytoplasm near the plasma membrane, but also around the nucleus during the MBT (Fig. 3B). After the MBT, most of the EGFP-p55γ was localized in the nucleus (Fig. 3B). This differential localization between EGFP-p85α and EGFP-p55γ was clear during neurulation (stage 18) (Fig. 3C). These results indicate that most of the p85α localizes in the cortical cytoplasm, but not in the nucleus, whereas p55γ localizes preferentially in the nucleus during embryogenesis. However, both EGFP-p85α and EGFP-p55γ localized throughout the whole cytoplasm in M phase, when the nuclear envelope had broken down. In the case of cleavage without cell growth, the area of the plasma membrane increased in one embryo, otherwise the amount of PI3K subunits remained nearly constant and, in particular, p85α in the cortical cytoplasm appeared to decrease. It has been proposed that the initiation of cell cycle elongation at the onset of the MBT is caused by a decrease in the nucleo-cytoplasmic (N/C) ratio in blastomeres, and a decrease in the cell surface around which the factors necessary for cell cycle progression are localized (Masui & Wang 1998). Thus, we hypothesized that the decrease in PI3K activity in the cortical cytoplasm with a reduction in the size of the blastomere leads to decreased protein synthesis and the promotion of cell cycle elongation. Thus, the p85α subunit may be a cortical cytoplasmic factor involved in the cell cycle progression during the MBT.
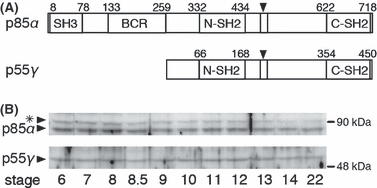
Expression patterns of the regulatory subunits of PI3K during embryogenesis. (A) A schematic illustration of the regulatory subunits of PI3K, p85α and p55γ. Arrowheads indicate a binding site of a catalytic subunit of PI3K (p110). N-SH2 domains in both p85α and p55γ could be recognized by an antibody against the human p85α SH2 domain (Santa Cruz, sc-423). (B) The constant expression of the p85α and p55γ proteins during embryogenesis. The protein equivalent to one embryo in each lane was analyzed by western blot with anti-human p85α SH2 domain antibodies. The asterisk indicates non-specific bands. Nieuwkoop-Faber (N/F) stages are shown at the bottom.
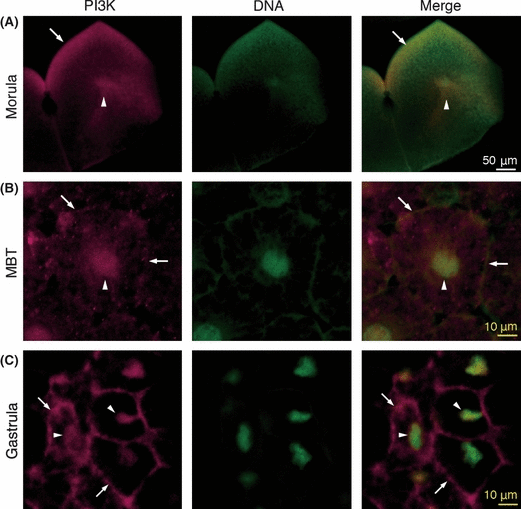
Intracellular localization of endogenous regulatory subunits of PI3K during embryogenesis. PI3K localization was determined by confocal fluorescent microscopy with anti-human p85α SH2 domain antibodies. Optical cross-sections of embryos at morula (A, stage 6), the midblastula transition (MBT) (B, stage 8.5) and late gastrula (C, stage 12). Left panels, anti-human p85α SH2 domain antibody-staining; middle panels, DNA stained by Sytox green; right panels, a merge of these images. PI3K localization in the peripheral cytoplasm near the plasma membrane (arrows) was observed from the morula to gastrula. The PI3K in the nucleus became apparent after the MBT (arrowheads). Scale bars, 50 μm (white in A); 10 μm (yellow in B and C). The whole-mount immunochemistry was performed by the methods according to Ueno et al. (2006).
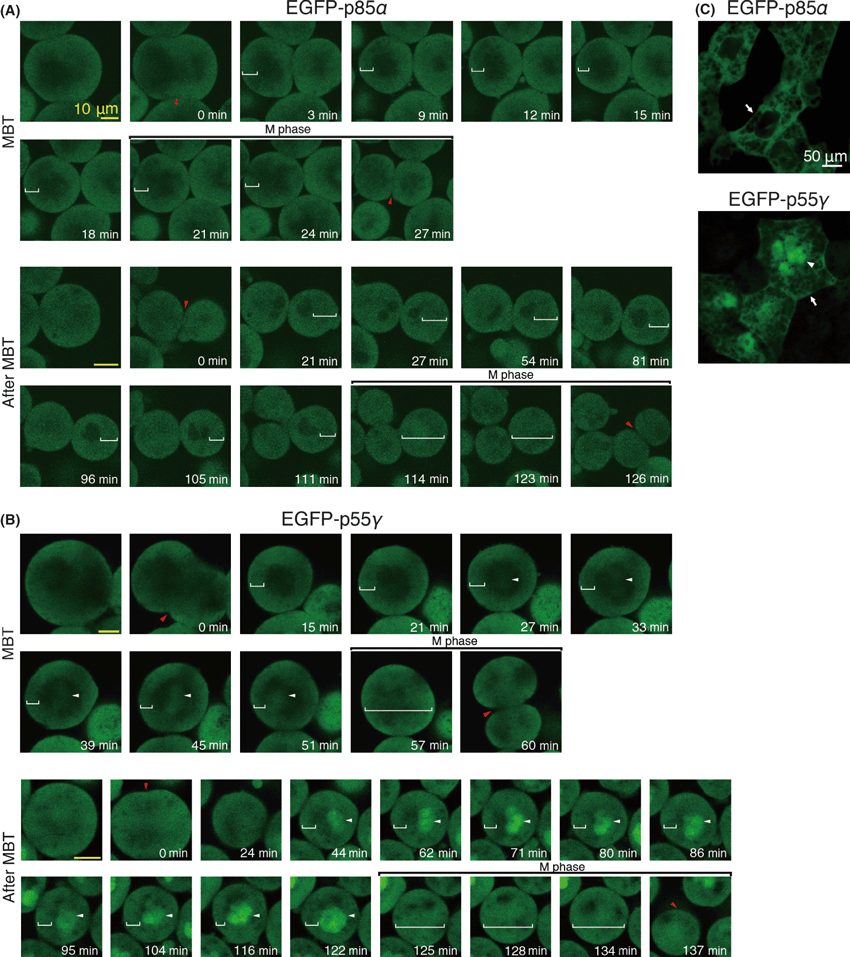
Differential localization of p85α and p55γ during the midblastula transition (MBT). (A) Enhanced green fluorescent protein (EGFP)-p85α in the translucent blastomeres during the MBT showing localization in the cytoplasm near the plasma membrane (white brackets), but throughout the whole cytoplasm at the M phase. (B) EGFP-p55γ localized in the cytoplasm near the plasma membranes (white brackets) and in the nuclei (white arrowheads) during the MBT. The amount of EGFP-p55γ in the nuclei increased after the MBT, but did so throughout the entire cytoplasm at the M phase. Red arrowheads, the sites of cleavage furrow; scale bar, 20 μm. The time represents the lapsed time after the cleavage of each cell cycle. (C) Localization of EGFP-p85α, -p55γ in the cells of the embryo surface at stage 18. EGFP-p85α and -p55γ was localized to the cytoplasm (arrows), whereas EGFP-p55γ was distributed in the nuclei (arrowhead). Scale bar, 50 μm (white in C); 10 μm (yellow in A and B).
Decrease of S6K activity after MBT
In the downstream portion of the PI3K-TOR signaling cascade, the activities of two independent factors, S6K and 4E-BP, are regulated by phosphorylation. When a direct target of TOR, S6K, is activated, the translation in the cytoplasm should be promoted. When a negative regulator of translation, 4E-BP, is phosphorylated, the suspension of translation should cease. Therefore, we investigated the changes in the phosphorylation of these factors during the MBT by western blotting to determine whether the activity of S6K or 4E-BP in each cell was reduced according to the decrease in the cortical p85α prior to the MBT. The upward size shifts of protein mobility of S6K showed their phosphorylation, which indicated increased phosphorylation at the P3 and P4 positions (Fig. 4A). Prior to the MBT, the amount of highly phosphorylated S6K (mobility size shift at the P3 level) per embryo was nearly constant. Just after the MBT, the phosphorylation of the S6K proteins was reduced to that of the P2 level and the rate of phosphorylation was constant until gastrulation. These results indicated that the activity of S6K in each cell decreased until the MBT according to the increase in the cell number, and that the activity of S6K in each cell was markedly reduced by the total activity of S6K per embryo after the MBT. We then determined whether the activity of S6K was reduced by the treatment of embryos with the PI3K or TOR inhibitors. The PI3K inhibitor, LY294002, rapidly decreased the rate of phosphorylation of S6K even before the MBT (mobility size shift to below the P2 level, and mainly to the P1 level) (Fig. 4A). Treatment of the embryos with the TOR inhibitor, rapamycin, decreased the rate of phosphorylation of S6K before the MBT (mobility size shift to below the P2 level, and mainly to the P1 level). We investigated whether S6K was activated by the upregulation of the PI3K-TOR pathway. It was reported that a GTP-binding protein, Rheb, causes the TOR pathway to activate based on the activity of PI3K-Akt. Alternatively, the overexpression of a constitutively active form of Rheb (Rheb-S16H) can activate the TOR regardless of the endogenous PI3K-Akt activity (Yan et al. 2006). When the embryos were injected with Rheb-S16H mRNA, the rate of phosphorylation of S6K remained high even after the MBT (mobility size shift mainly to the P3 level) (Fig. 4A). Furthermore, the decrease in the rate of S6K phosphorylation in the LY294002-treated embryos before the MBT was blocked by the injection of Rheb-S16H mRNA. The rate of phosphorylation of S6K in the injected embryos was more similar to that of the non-treated embryos than to that of the LY294002-treated embryos (mobility size shift mainly to the P2 level, and also remained at the P3 level until the MBT) (Fig. 4A). In addition, the rate of phosphorylation of 4E-BP was determined by the mobility shifts of the protein. The 4E-BP protein was slightly phosphorylated at 2.5 h before the MBT (smeared size shift from the P1 to the P2 level). The rate of 4E-BP phosphorylation was gradually reduced mainly to the P1 level at 1 h before the MBT (Fig. 4B). When the embryos were treated with LY294002 or rapamycin, the rate of phosphorylation of 4E-BP during the MBT was similar to that of the non-treated embryos (Fig. 4B). Furthermore, Rheb-S16H injection did not affect the rate of phosphorylation of 4E-BP during the MBT. These results indicated that the change of 4E-BP activity was independent of the PI3K-TOR activity during the MBT. Taken together, the protein synthesis dependent on the TOR pathway in each cell appeared to decrease in relation to the amount of phosphorylated S6K until the onset of MBT, which suggested that a reduction in the efficiency of protein synthesis is a necessary condition for cell cycle elongation.

The phosphorylation rates of S6K and 4E-BP during the midblastula transition (MBT). (A) Proteins equivalent to one embryo in each lane were analyzed by western blotting using an anti-S6K antibody. The rates of phosphorylation were determined by the mobility-shift corresponding to the phosphorylation (P1–P4: P4 indicates higher phosphorylation state, P1 indicates lower phosphorylation state). In the embryos treated with 100 μmol/L LY294002 or 5 μmol/L rapamycin at the 128-cell stage, the phosphorylation of S6K decreased before the MBT. In the embryos expressed with Rheb-S16H, the phosphorylation of S6K was constant after the MBT. (B) The protein equivalent to one embryo in each lane was analyzed by western blotting using an anti-4E-BP antibody. The rates of phosphorylation were determined by the mobility-shift corresponding to the phosphorylation (P2 indicates higher phosphorylation state, P1 indicates lower phosphorylation state). In all embryos, the phosphorylation of 4E-BP decreased slightly at the onset of the MBT with nearly the same pattern. None, non-treated embryos.
Elongation of cell cycle time caused by inhibition of PI3K and TOR activities
We demonstrated that the decrease in S6K activity during the MBT is dependent upon the PI3K-TOR pathway. Meanwhile, the cell cycle time began to lengthen at the onset of the MBT. To examine the involvement of the PI3K-TOR-S6K pathway in the initiation of cell cycle elongation, we treated blastomeres with LY294002 or rapamycin to inhibit PI3K or TOR, respectively. The size of the blastomeres and their cell cycle time during the next cell division were measured to investigate the relationship between the initiation of cell cycle elongation and the cell sizes (Fig. 5). The dissociated, non-treated blastomeres continued to undergo cell divisions with cell cycle times of about 30–40 min until the radius of the average blastomere fell below about 38 μm at the onset of the MBT. Thereafter, the cell cycle times increased in an inverse relation to the cell radius after the MBT (Fig. 5A). The inhibition of PI3K activity by LY294002 treatment caused elongation of the cell cycle time in the larger blastomeres (about 45 μm in radius) relative to the non-treated blastomeres (Fig. 5A). In addition, the inhibition of TOR activity by rapamycin treatment also caused elongation of the cell cycle time in the larger blastomeres (about 47 μm in radius) (Fig. 5B). Furthermore, to examine the involvement of S6K activity in the initiation of cell cycle elongation, we injected the morpholino oligo of S6K into a one-cell embryo to reduce the expression of S6K (Fig. 5F). The inhibition of S6K expression caused elongation of the cell cycle time in the large blastomeres (about 42 μm in radius) in the same manner as the inhibition of PI3K or TOR (Fig. 5C). These results indicated that the PI3K-TOR-S6K pathway-dependent reduction of protein synthesis initiated cell cycle elongation at the onset of the MBT. We found that the overexpression of Rheb-S16H activated the TOR-S6K pathway despite a decrease in PI3K-Akt activity after the MBT. To examine whether the decrease in TOR-S6K activity is necessary for the initiation of cell cycle elongation at the onset of the MBT, we measured the relationship between the cell size and the cell cycle time of Rheb-S16H-expressed blastomeres. When the TOR-S6K activity was increased by the expression of Rheb-S16H, the initiation of cell cycle elongation was delayed (about 35 μm in radius) (Fig. 5D). Furthermore, when the Rheb-S16H-expressed blastomeres were treated with LY294002, the cell cycle time was not changed compared with that of non-treated blastomeres (about 38 μm in radius) (Fig. 5E). These results support the notion that the TOR-S6K pathway-dependent decrease in protein synthesis initiates cell cycle elongation at the onset of the MBT.
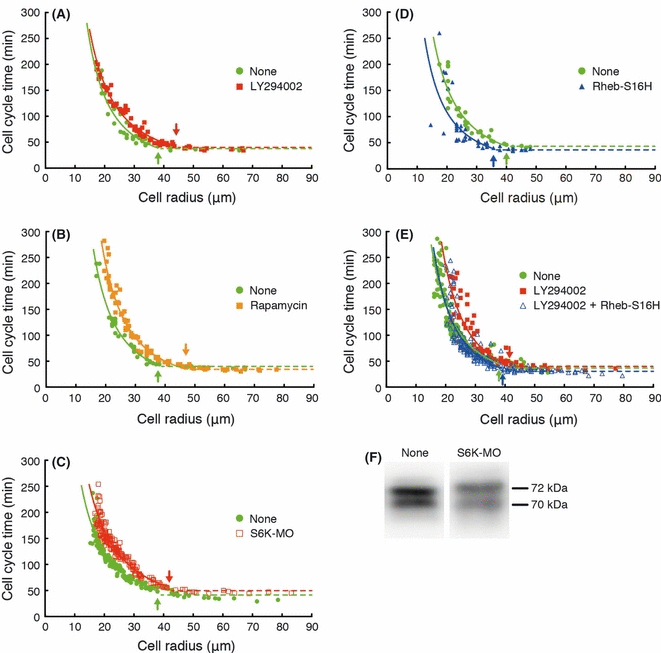
Correlation between cell size (cell radius) and cell cycle time in dissociated blastomeres. All embryos for each experiment were obtained from the same batch of eggs. Blastomeres of about the same size were selected at the 128 cell stage. The cell radius was measured just before each cell division and the cell cycle time was measured between the points at which the cleavage furrows appeared. Both the treated and non-treated blastomeres in each experiment were prepared from the eggs of the same batch to precisely determine the changes in cell cycle time. (A) Inhibition of PI3K activity by treatment with 100 μmol/L LY294002 (red squares) caused cell cycle elongation in the large cells in comparison with non-treated blastomeres (green circles). (B) Inhibition of target of rapamycin (TOR) activity by treatment with 5 μmol/L rapamycin (orange squares) caused cell cycle elongation in the large cells in comparison with non-treated blastomeres (green circles). (C) The decrease in the level of S6K protein by anti-S6K morpholino oligo (open red squares) caused cell cycle elongation in the larger cells in comparison with the non-treated blastomeres (green circles). (D) Activation of TOR by Rheb-S16H injection (blue triangles) caused a delay in the initiation of cell cycle elongation after the midblastula transition (MBT). (E) Activation of TOR blocked the effect of the inhibition of PI3K activity (open blue triangles). Arrows in the graphs indicate the point of the initiation of cell cycle elongation, at which time the blastomeres began to elongate the cell cycle constantly in an inverse relation to the cell radius. (F) The S6K protein levels at the onset of the MBT were analyzed by western blotting using an anti-S6K antibody. The S6K protein level was decreased by the anti-S6K morpholino oligo (S6K-MO) in comparison with the non-treated embryos (None).
Discussion
To clarify the role of the PI3K-TOR-S6K pathway for the initiation of cell cycle elongation in early embryonic development of the frog, Xenopus, we investigated the function of this pathway in translational regulation by analyzing cell cycle times in dissociated, translucent blastomeres, their immunohistochemistry, and the expression of several types of mRNA. We demonstrated that the decrease in translational activity dependent on the reduction of cortical cytoplasmic PI3K initiated cell cycle elongation at the onset of the MBT.
Relationship between the cell size and cell cycle progression
It takes many hours for somatic cells to grow large enough to divide into two daughter cells in the next cell cycle. Cell cycle progression is coordinated with the cell growth rate to maintain a constant cell size. The PI3K-TOR-S6K pathway plays an important role by monitoring the cell size and regulating cell growth and the cell cycle. In large cells, the PI3K-TOR-S6K pathway is activated and enhances the protein synthesis necessary for cell cycle progression (Neufeld 2003; Fingar & Blenis 2004). In early Xenopus embryogenesis, the size of the blastomere remains large until the MBT, at which point the cells divide about every 30 min. In the present study, we showed that the PI3K-TOR-S6K pathway is necessary to coordinate the blastomere size with the initiation of cell cycle elongation at the onset of the MBT. After the MBT, the cell cycle time was elongated in an inverse correlation with the cell size when the radius of the blastomeres became less than about 37.5 μm (Wang et al. 2000). This response could be changed to delay the initiation of cell cycle elongation in a small cell or to start it earlier in a large cell by the activation or inhibition of the PI3K-TOR-S6K pathway, respectively (Fig. 5). The PI3K-TOR-S6K pathway represents a universal system for coordinating the progression of the cell cycle with cell size.
Cortical cytoplasmic factors necessary for translational activity
We previously mentioned that cell surface-related factors play important roles in the transition from synchronous to asynchronous divisions at the onset of the MBT (Iwao et al. 2005). In the present study, we showed that a PI3K-subunit (p85α) in the cortical cytoplasm was one of the best candidates for a cell surface-related factor of the sort needed for cell cycle progression. In the cell cortex, the binding of insulin-like growth factor (IGF) with an IGF receptor activates the insulin receptor substrate (IRS), and the p85α phosphorylation by the IRS leads to PI3K activation (Dominici et al. 2005). However, while the maternal expression of the IGF receptor and the IRS has been confirmed (Shuldiner et al. 1991; Perfetti et al. 1994; Groigno et al. 1996, 1999), the expression of insulin and IGFs during cleavage has not been confirmed. The binding of fibronectin with α5β1 integrin (fibronectin receptor) also activates focal adhesion kinase (FAK), and then the phosphorylation of p85α by FAK causes the activation of PI3K (Zeng et al. 2006). Fibronectin, α5β1 integrin, and FAK are known to be expressed maternally in Xenopus embryos (Ransom et al. 1993; Joos et al. 1995; Zhang et al. 1995; Winklbauer 1998). In the present study, we cultured blastomeres on glass-based dishes coated with fibronectin so that the FAK-dependent pathway could function as an activator of PI3K in both the intact embryogenesis and in our culture of blastomeres.
The role of PI3K-TOR-S6K pathway during early embryogenesis
The PI3K-TOR-S6K pathway-dependent inhibition of protein synthesis initiates cell cycle elongation even in the large cell condition before the MBT, but did not affect further cell cycle elongation after the MBT (Fig. 5). In the initiation of cell cycle elongation during the MBT, the elongation of the S phase occurred for the first time without the appearance of the G2 or G1 phases (Iwao et al. 2005). This finding suggested that the factors necessary for promoting DNA synthesis are reduced by the PI3K-TOR-S6K pathway-dependent inhibition of protein synthesis. After the MBT, the level of the Cyclin E1 and Xcdc6A proteins decreased markedly, but their mRNAs were degraded after the tail bud stage (Tikhmyanova & Coleman 2003; Gotoh et al. 2007). Therefore, the reduction of translation for these proteins may trigger the initiation of cell cycle elongation at the onset of the MBT. In Xenopus and zebrafish embryos, the inhibition of the PI3K-Akt pathway or TOR caused several defects during gastrulation and neurulation (Peng et al. 2004; Finkielsztein & Kelly 2009; Moriyama et al. 2011). These previous findings showed that the PI3K-Akt pathway is linked to the GSK-3β/β-catenin pathway, which is regulated by Wnt signaling in Xenopus embryogenesis (Peng et al. 2004). These findings suggest that while cell cycle elongation after the MBT is dependent on the translational regulation downstream of TOR, several pathways downstream of PI3K-Akt are still necessary for further development after gastrulation.
The progression of cell cycle elongation after MBT
The duration of interphase is markedly prolonged by the appearance of the G2 and G1 phases following the elongation of the S phase after the MBT. During the prolonged interphase, several karyomere structures are integrated into one nucleus as previously observed in somatic cells (Montag et al. 1988; Lemaitre et al. 1998). In the present study, we showed that a short-type PI3K subunit (p55γ) is localized to the nucleus after the MBT (Fig. 3B). Since the overexpressed N-terminal p55γ localizes to the nucleus and blocks cell cycle progression at G1 phase in MCF-7 cells (Xia et al. 2003), it is possible that the structural changes in the nucleus after the MBT permit the nuclear localization of p55γ which plays a role in cell cycle regulation after the appearance of the G1 phase.
In summary, we demonstrated that a decrease in PI3K-TOR-S6K pathway activity initiated cell cycle elongation just after the MBT. However, the molecular mechanisms responsible for coordinating the initiation of cell cycle elongation with the appearance of the G2 and G1 phases following the MBT have not yet been determined. Future studies should be aimed at identifying these molecular mechanisms in order to elucidate the coordination between cell proliferation and morphogenesis.
Acknowledgments
This work was partially supported by a Grant-in-Aid for Young Scientists (B) (No. 21770236) to S. Ueno and a Grant-in-Aid for Scientific Research (No. 19570207) to Y. Iwao from the Japanese Society for the Promotion of Science.




