Absence of tissue inhibitor of metalloproteinases 3 disrupts alveologenesis in the mouse
Contributions: S. E. Gill carried out or participated in all experiments and edited the manuscript.M. C. Pape participated in most of the experiments, maintained the animal colony and arranged most of the timed pregnancies.K. J. Leco carried out the rescue experiment presented in Figure 4, supervised the above personnel, acquired grant support and wrote the manuscript.
Abstract
Tissue inhibitors of metalloproteinases (TIMPs) regulate extracellular matrix (ECM) degradation by matrix metalloproteinases (MMPs) throughout lung development. We examined lungs from TIMP3 null mice and found significant air space enlargement compared with wild type (WT) animals during a time course spanning early alveologenesis (post-partum days 1, 5, 9 and 14). Trichrome staining revealed a similar pattern of collagen distribution in the walls of nascent alveoli; however, the alveolar walls of TIMP3 mutant mice appeared to be thinner than controls. Assessment of MMP2 and MMP9 activities by gelatin zymography demonstrated a significant elevation in the active form of MMP2 at post-partum days 1 and 5. Treatment of null pregnant dams with a broad spectrum synthetic metalloproteinase inhibitor, GM6001, on embryonic day 16.5 enhanced the formation of primitive alveoli during the saccular stage of lung development as evidenced by a partial, but significant, rescue of alveolar size in post-partum day 1 animals. We propose that increased MMP activity in the absence of TIMP3 enhances ECM proteolysis, upsetting proper formation of primitive alveolar septa during the saccular stage of alveologenesis. Therefore, TIMP3 indirectly regulates alveolar formation in the mouse. To our knowledge, ours is the first study to demonstrate that in utero manipulation of the TIMP/MMP proteolytic axis, to specifically inhibit proteolysis, significantly affects lung development.
Introduction
Alveolar development in the mouse begins with the genesis of sac-like structures (or primitive alveoli) at embryonic day (E) 16.5, which continue to develop until birth (the saccular stage). These structures are transformed into alveolar ducts and alveoli proper in a process known as alveolarization, which occurs after birth and continues until approximately 4 weeks of age in the mouse (alveolar stage) (Ten Have-Opbroek 1991). During the alveolar stage of development, the septa between the sacs and ducts, called the primary septa, fold to create secondary septa that subdivide the airspaces into smaller units and greatly increase the surface area within the lung (Burri 1984). This is followed by further remodeling of the extracellular matrix (ECM) and a thinning of the mesenchyme within the septa to form the mature septa, which have a single capillary network and a very thin interstitial layer (Burri 1984). Thus, the creation of these specialized structures in the lung requires a highly orchestrated interaction between cells of the developing organ and the surrounding ECM.
During embryogenesis, the ECM is in a dynamic flux, constantly being remodeled to accommodate developmental and physiological processes. The composition of ECM, its three dimensional structure and proteolytic remodeling all contribute to the tissue microenvironment that controls cell growth, differentiation and survival, which in turn dictates the progression of organogenesis (Vu & Werb 2000). The matrix metalloproteinases (MMPs) are the predominant effectors of ECM remodeling, whereas the tissue inhibitors of metalloproteinases (TIMPs) are naturally occurring protein inhibitors of MMPs, and therefore protect the ECM from degradation (Baker et al. 2002). It is believed that a shift in the balance between active MMPs versus TIMPs contributes to the design of tissue architecture during development (Vu & Werb 2000; Baker et al. 2002; Greenlee et al. 2007).
That TIMPs and MMPs play a role in sacculation and alveologenesis is implied by the fact that numerous MMPs and all four TIMPs are expressed throughout all stages of lung development (Nuttall et al. 2004). More convincing evidence for involvement of MMPs in genesis of lung airspaces is provided by two papers, which studied the effects of a null mutation for membrane-type 1 MMP (MT1-MMP) on lung development. Mice lacking MT1-MMP demonstrate immature alveolar development, which manifests in a 40% reduction in alveolar surface area at one month of age, with the walls of these alveoli having abnormal ultra-structure (Atkinson et al. 2005; Oblander et al. 2005). Conversely, mice expressing an MMP1 transgene in the lung developed severe air space enlargement with thin walled alveoli and dilated alveolar ducts detectable at one month post-partum (D’armiento et al. 1992).
TIMP3 can effectively inhibit all of the MMPs as well as a number of the disintegrin family of metalloproteinases (ADAMs), which are involved in shedding of ectodomains of many growth factors and growth factor receptors (Baker et al. 2002). As such, deletion of TIMP3 has potential repercussions on the maintenance of the ECM as well as growth factor homeostasis. We have previously published that the absence of TIMP3 impairs murine bronchiole branching morphogenesis (Gill et al. 2003), by a mechanism that involves excessive fibronectin degradation and a subsequent reduction in focal adhesion kinase signaling in the developing bronchiole tree (Gill et al. 2006). Further, TIMP3 null mice spontaneously develop air space enlargement as early as two weeks of age, which suggested a developmental defect in alveologenesis (Leco et al. 2001). Subsequently, we showed that dilation of alveolar ducts and enlarged air spaces could be detected as early as post-partum day 1 (PP1) (Gill et al. 2003).
Based on our previous experiments we set out to establish the extent of air space enlargement in the TIMP3 null lung over a developmental time course from PP1 through PP14. We also wished to confirm that the defect is due to excessive MMP activity. Therefore, in an attempt to rescue the phenotype in the developing pups in utero, we injected a broad spectrum synthetic metalloproteinase inhibitor, GM6001, into pregnant dams at E 16.5. Our working hypothesis was: in the absence of TIMP3, unregulated MMP activity in the developing lung results in inappropriate ECM degradation leading to aberrant air space morphogenesis.
Materials and methods
Mice
A full description of the establishment of TIMP3 null mice used in the current study is described elsewhere (Leco et al. 2001). Animals were cared for in accordance with guidelines from the Canadian Council on Animal Care, and animal protocols were approved by Animal Care and Veterinarian Services, University of Western Ontario. For these experiments, the mutant allele was back-crossed nine generations onto the C57/Black6 strain of mice for clone seven, and back-crossed eight times onto the C57/Black6 strain of mice for the independently targeted clone eight. Mice were maintained and bred as previously described (Gill et al. 2003) and both TIMP3 clonal null lines were used in all experiments.
Preparation and evaluation of lungs
Pups used in this study were removed from the nursing dam at PP1, PP5, PP9 and PP14. Pups were killed by lethal overdose of anesthetic. Lungs were either snap frozen on dry ice for protein analysis or fixed with freshly prepared phosphate-buffered saline (PBS) 4% paraformaldehyde as follows. After death, the body of the animal was removed below the diaphragm and the diaphragm was carefully punctured, avoiding puncture of the lungs. Under a dissecting microscope, the trachea was exposed and cannulated with fine gauge (0.012 inside diameter, 0.024 outside diameter) silicone tubing, attached to a 27 gauge needle and 0.5 mL syringe already filled with ice cold fixative to the end of the tubing. The cannulated trachea was then tied to the tubing with a suture. Fixative was carefully injected as follows; PP1: 50 µL; PP5: 100 µL; PP9: 150 µL; and PP14: 300 µL. A second suture around both the tubing and trachea was then tied securely and the tubing/trachea cut above the ligature. Lungs were removed from the animal, placed in 10 mL of ice-cold fixative and left overnight at 4°C. The following day, lungs were rinsed several times in PBS, dehydrated through an alcohol series and embedded in paraffin wax for sectioning. Sections were cut at 7 µm, mounted on Superfrost slides (VWR International, Mississauga, ON, Canada) and stored at room temperature until use. The H&E and Gomori's Trichrome staining were carried out according to standard histological procedures.
Mean alveolar intercept (a measure of distance between opposing alveolar walls) was determined essentially as described (Leco et al. 2001). Briefly, images of H&E stained lung sections were captured, the number of alveolar wall intercepts along a 500-µm line determined and the average size of alveoli along that line calculated. This was repeated on five random fields of view (always at the periphery of the lung section) and then averaged for each lung section to obtain a representative measurement. Statistical significance was determined using a two-tailed, independent Student's t-test between genotypes at each time point.
Gelatin zymography
Lung homogenation and gelatin zymography were carried out on a developmental series of murine wild type (WT) and null lungs (PP1, PP5, PP9 and PP14) as previously described (Gill et al. 2003). Quantification of the regions of gelatin degradation on inverse images of zymography gels was achieved using SimplePCI software (Nikon Canada, Inc., Mississauga, ON, Canada), and bands were normalized to a non-specific protein band within each lane on the gel to control for loading errors between samples. The values reported are averages of four replicates, expressed as a percentage of the WT control to accurately compare values from replicate gels. Averages were arc sine transformed before statistical analysis. Statistical significance was determined using a two-tailed, independent Student's t-test between genotypes at each time point.
GM6001 injections
Pregnant null dams were injected intra-peritoneally with carboxymethyl cellulose (CMC; Sigma-Aldrich, Oakville, ON, Canada) or 20 mg/kg GM6001 as a suspension in CMC (3.33 mg/mL GM6001 in CMC; Millipore Biosciences, Temecula, CA, USA) on E16.5, with the day of discovery of a vaginal plug considered as E0.5. Pups were removed from nursing mothers on either PP1 or PP14 and lungs were fixed and processed for H&E staining as described above. Mean alveolar intercept was determined from a minimum of ten random fields of view for PP1 lungs and twenty random fields of view for PP14, and average alveolar size was determined for each animal. Statistical significance was determined using a two-tailed, independent Student's t-test between treatments at each time point.
Results
TIMP3 null lungs demonstrate air space enlargement from birth
We previously demonstrated that the loss of TIMP3 results in enlarged air spaces in the lung at PP1 and PP14 (Leco et al. 2001; Gill et al. 2003). In the present study, we sought to quantify the extent of air space enlargement in the TIMP3 null lung compared with WT animals over a developmental time course spanning sacculation and early alveologenesis. Airspaces in mice lacking TIMP3 appear dilated at birth (PP1) when compared with WT counterparts and continue to diverge in size over the course of early alveolarization (PP5–PP14; Fig. 1A). Quantification of alveolar size at PP1 through PP14 is expressed as the average alveolar diameter in µm ± SEM (Fig. 1B). The average diameter of alveoli at each time point was significantly greater in null animals when compared with WT counterparts and this difference increased as animals aged. Compared with WT animals, the null alveoli were 26% larger at PP1, 27% larger at PP5, 32% larger at PP9, and 43% larger at PP14. The reduction in size of the alveoli in both genotypes over the time course reflects the process of septation, which subdivides the airspaces into smaller alveoli thereby increasing the area for gas exchange.
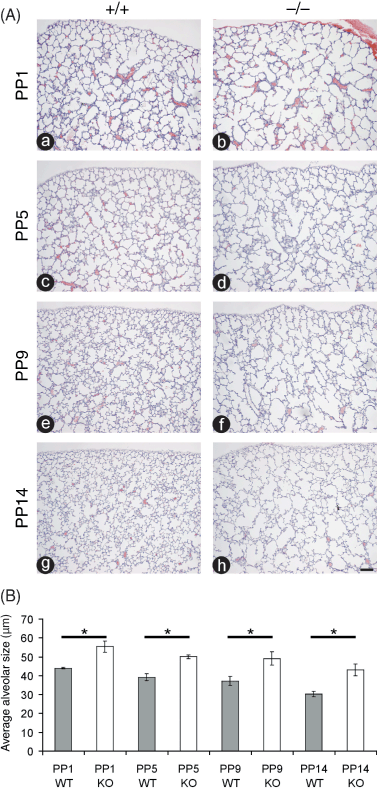
Airspaces in mice lacking tissue inhibitor of metalloproteinases 3 (TIMP3) already appear dilated at birth when compared with wild type (WT) counterparts. (A) Hematoxylin and eosin (H&E) staining of post-partum day 1 (PP1) (a and b), PP5 (c and d), PP9 (e and f), and PP14 (g and h) lung sections illustrate that both sacculation and alveolarization are impaired in TIMP3 null lungs (b, d, f and h) compared with lungs from WT mice (a, c, e and g). Lung pleura are visible at the top of each micrograph to demonstrate images were captured from similar regions of each lung (bar, 50 µm). (B) Quantification of alveolar size at PP1, PP5, PP9 and PP14 expressed as the average alveolar diameter (in µm). The average diameter of alveoli at each time point was significantly greater in null animals compared with WT counterparts and this difference increased as animals aged (*P < 0.05; n = 3 for each genotype and time point; error bars represent ± SEM).
Trichome staining reveals abnormalities in the structure of TIMP3 null alveolar walls
Previous work demonstrated a reduction in collagen content in null lungs compared with WT later in the animal's life (> 13 months of age) (Leco et al. 2001), or if the lung of mature adult animals was challenged in a model of sepsis (9–12 weeks of age) (Martin et al. 2003). Trichome staining detects type-I, type-III and type-IV collagen, which are the predominant isoforms of collagen in the lung (Madri & Furthmayr 1980; Kirk et al. 1984), and a green/blue precipitate represents total collagen. When examined by this technique, the lungs of null animals demonstrate little difference in abundance of collagen compared with controls; however, the total amount of staining represented in the micrographs is reduced in conjunction with the reduced number of alveolar walls in the null lungs (Fig. 2). Immunohistochemistry using antisera directed against collagens type-I and -IV also showed no difference in abundance between null and control lungs (data not shown). Qualitatively, it is apparent in the micrographs presented that the walls of the TIMP3 null alveoli are thinner than those of WT animals (enlarged inserts in Fig. 2).
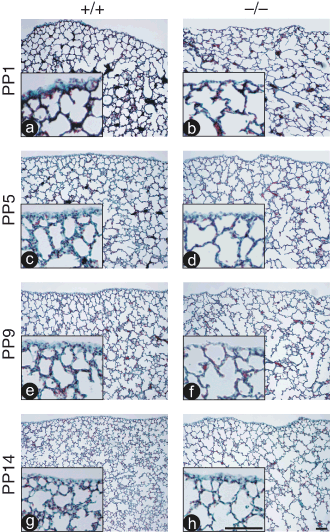
Collagen content does not appear to be altered in lungs of tissue inhibitor of metalloproteinases 3 (TIMP3) null mice during sacculation or alveologenesis. Gomori's Trichrome staining of post-partum day 1 (PP1) (a and b), PP5 (c and d), PP9 (e and f), and PP14 (g and h) lung sections reveal comparable collagen staining (blue/green stain in alveolar walls) in both wild type (WT) (a, c, e and g) and null lungs (b, d, f and h). Lung pleura are visible at the top of each micrograph to demonstrate images were captured from similar regions of each lung. Inset regions are higher magnification images of the lung periphery for each section demonstrating qualitatively that the walls of null lungs are thinner than those from control lungs (both bars in panel h, 50 µm).
Elevated MMP2 activation in newborn TIMP3 null lungs
One potential mechanism underlying the enlarged airspaces in the absence of TIMP3 may be an increase in MMP activation. We analyzed protein extracts from TIMP3 null and WT lungs by gelatin zymography, which detects latent, intermediate and active forms of MMP2 and MMP9. A significant increase was observed in abundance of active MMP2 in null lung extracts at PP1 and PP5 versus WT, but not at the later time points (Fig. 3). At PP1, active MMP2 was 180% elevated in null lungs compared with WT, and at PP5 this difference was 150%. Thus, we provide evidence that activation of at least one MMP is elevated in the newborn null lung.
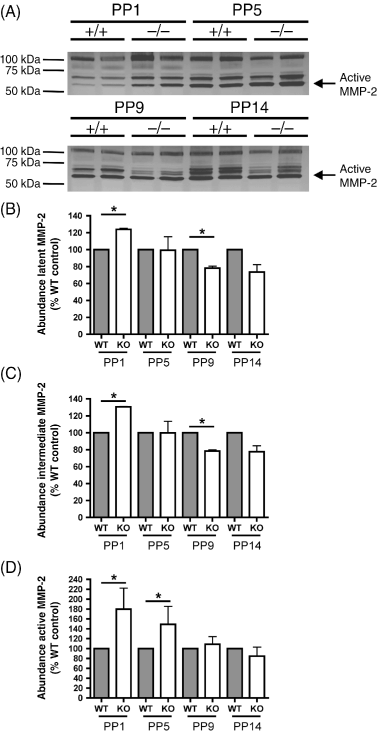
Lungs from tissue inhibitor of metalloproteinases 3 (TIMP3) null mice have increased matrix metalloproteinase 2 (MMP2) activation at birth (post-partum day 1 [PP1]) and at the beginning of alveologenesis (PP5). (A) Inverse images of zymography gels illustrate an increased abundance of active MMP2 gelatinolytic activity (62 kDa; arrow), in the lanes containing homogenate from null lungs compared with lanes containing homogenate from wild type (WT) lungs. (B,C) Quantification of the gelatin degrading activity (percentage of WT control) of latent MMP2 (B) and the intermediate form of MMP2 (C) demonstrates a significant increase in relative abundance in null lungs at PP1 but lesser amounts at PP9 and PP14. (D) Quantification of the gelatin degrading activity (percentage of WT control) by active MMP2 highlights a significant increase in gelatin degradation at PP1 and PP5. At PP9 and 14 the equivalent amount of active MMP2 in the null lungs versus WT would be expected to have enhanced activity in vivo, due to the absence of TIMP3. (*P < 0.05; n = 4 for each genotype and time point; error bars represent ± SEM).
A synthetic metalloproteinase inhibitor rescues the alveolarization defect in the TIMP3 null lung
In order to confirm that the defect in alveolarization seen in the TIMP3 null lung is due to excessive MMP activity, we injected null pregnant dams with the broad spectrum synthetic metalloproteinase inhibitor GM6001, a technique we have used previously with success (Gill et al. 2006). Pregnant null females were injected with GM6001 or vehicle at E16.5 (beginning of sacculation), and pups were collected at either PP1 or PP14, the lungs inflated with fixative, and processed for histological examination. Injection of GM6001 caused a partial rescue of the air space enlargement at PP1 compared with vehicle treated lungs, which was not sustained at PP14 (Fig. 4A). Quantification confirmed that the GM6001 treated lungs at PP1 showed a modest, but significant, reduction in mean alveolar size (Fig. 4B).
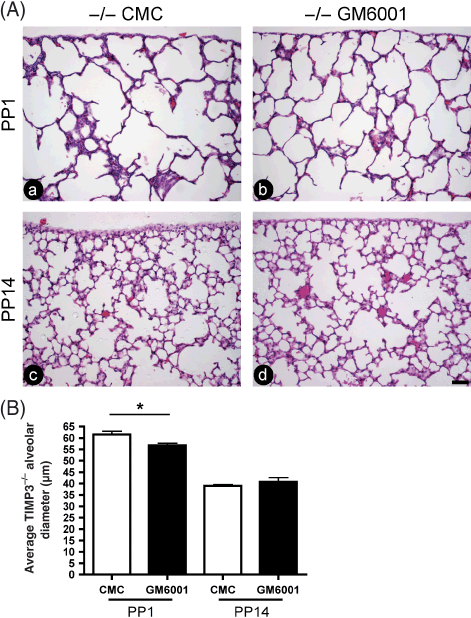
Synthetic metalloproteinase inhibitor rescues defect in tissue inhibitor of metalloproteinases 3 (TIMP3) mutant lungs at post-partum day 1 (PP1). (A) Hematoxylin and eosin (H&E) staining of PP1 (a and b) and PP14 (c and d) lung sections illustrates that administration of broad spectrum synthetic metalloproteinase inhibitor (GM6001; b and d) to TIMP3 null mice at embryonic day 16.5 (E16.5) rescues the airspace defect at PP1, but not at PP14, compared with vehicle treated counterparts (carboxymethyl cellulose [CMC]; a and c). Lung pleura are visible at the top of each micrograph to demonstrate that images were captured from similar regions of each lung (bar, 50 µm). (B) Quantification of alveolar size at PP1 and PP14 expressed as the average alveolar diameter (in µm). The average diameter of alveoli in GM6001 treated null lungs was significantly smaller compared with control treated counterparts at PP1 (*P < 0.01; null CMC, n = 6; null GM6001, n = 9), but this rescue was not maintained at PP14 (null CMC, n = 3; null GM6001, n = 6; error bars represent ± SEM).
Discussion
When the TIMP3 knock-out animal model was first developed, the animals were shown to develop spontaneous air space enlargement, beginning at PP14 and proceeding to a fatal loss of lung function with advancing age (Leco et al. 2001). Thus, we knew there was a developmental defect in lung organogenesis, and we set out to establish the extent of air space enlargement in the TIMP3 null lung over a developmental time course. This study has demonstrated that the TIMP3 null lungs show a significant and substantial defect in alveolarization, such that at PP14 there is a 43% enlargement in alveolar diameter compared with WT lungs. The difference in size of the alveoli between null and control at PP14 is considerably greater in the current study than what we reported previously (Leco et al. 2001). This discrepancy can be attributed to refinement in our inflation technique. That is, the lungs in the current study were inflated with fix, then tied off and fixed overnight, whereas the lungs in the previous study were inflation fixed for only 1 min, allowed to collapse and then fixed overnight.
Trichrome staining and immunohistochemistry for type-I and type-IV collagen did not reveal any gross differences in the abundance of collagen in the alveolar walls of TIMP3 null animals. Previously, we showed that collagen type-I abundance in the lungs declines as TIMP3 null animals age. We also suggested, based on ultrastructure analysis, that there is turnover of fibrillar collagen within the interstitium of alveoli, with consequent synthesis and assembly of disorganized fibers, but only in animals of advanced age (Leco et al. 2001). The point to be made here is that with no difference in collagen abundance, it is unlikely that a potential mechanism of enhanced collagen degradation in the absence of TIMP3 is responsible for the observed enlarged alveoli in our model of early lung development. This is in contrast with the results of D’Armiento et al. (1992), who showed that inappropriate expression of MMP1 causes severe, early onset air space enlargement due to enhanced collagen type-I degradation. This is not to say that reduction in collagen content during lung development does not cause air space enlargement; it clearly does in the above-mentioned work. Rather, loss of structural collagens is not likely responsible for the phenotype of enlarged alveoli observed during lung development in the TIMP3 knock-out mice.
We previously reported that the loss of TIMP3 has significant repercussions on the branching of the bronchiole tree during lung development (Gill et al. 2003; Gill et al. 2006). The main conclusions of those studies were that in the absence of TIMP3, unregulated MMP activity caused degradation of the ECM protein fibronectin, which led to a reduction in focal adhesion kinase activity, and reduced epithelial cell proliferation during branching morphogenesis. In the current study, we examined the abundance of a number of different ECM molecules, most notably fibronectin, by several techniques including histology, immunohistochemistry and Western blot analysis (data not shown). While we did detect a slight reduction in fibronectin abundance, the reduction was not deemed statistically significant until PP14 (not shown).
While fibronectin abundance is not diminished in PP1 TIMP3 null lungs, perhaps degradation of a different ECM molecule not yet tested could result in decreased epithelial cell proliferation during sacculation and septation yielding the enlarged alveoli observed. For example, laminin is comprised of one α, one β and one γ chain, many of which are expressed in the developing mouse lung. There are 15 different heterotrimeric laminin isoforms assembled from combinations of five α, three β and three γ chains, with different isoforms having distinctive functions in lung development (Nguyen & Senior 2006). Most notably, laminin α5 null animals demonstrate defects in alveologenesis and epithelial cell proliferation, differentiation and maturation (Nguyen et al. 2005). Future studies in our lab will address the possibility that laminin α5 is specifically degraded in the absence of TIMP3.
We provide evidence of an increase in the amount of MMP2 activity in TIMP3 null lung protein extracts at PP1 and PP5, supporting our hypothesis that the balance between active MMPs and TIMPs is disrupted during alveolar development. At first glance, the analysis of the latent, intermediate and active forms of MMP2 draws a somewhat confusing picture; however, several points can be concluded. At PP1 there is a significant increase in all forms of MMP2 in the TIMP3 null lungs versus WT. One potential explanation is that in the absence of TIMP3 there is modification of some (as yet unknown) factor's bioavailability, which leads to an increase in the synthesis of MMP2. Future experiments will follow up this observation to determine if this apparent upregulation of MMP2 is at the transcriptional or translational level and determine the identity of the hypothetical factor. The increase in the amount of active MMP2 at PP1 and PP5 in null lungs clearly demonstrates that in the absence of TIMP3 one potential substrate of MMP2, that being the intermediate form MMP2, shows heightened auto-activation. Further, the decrease in the intermediate form of MMP2 at PP9 and PP14 can be interpreted to mean that in the absence of TIMP3, membrane type-MMP activity is elevated, therefore enhancing the conversion of pro-MMP2 to the intermediate form. Finally, even though there is not a significant increase in active MMP2 at PP9 or PP14 in TIMP3 null extracts compared with WT, it should be noted that an equivalent amount of active MMP2 in null lungs would be expected to have enhanced activity in vivo, due to the absence of TIMP3. Others have shown that MMP2 activation is enhanced in cultured TIMP3 null fibroblasts (English et al. 2006), and our results support their conclusions under in vivo conditions.
This is not necessarily the only example of enhanced activation of MMPs in the TIMP3 null lung, but rather the only one detected by gelatin zymography. Evidence that the defect observed in the present study is due to enhanced MMP activity is provided by the fact that GM6001, a broad spectrum synthetic metalloproteinase inhibitor, partially rescues the defect (GM6001 treated null animals are born with smaller alveoli). Although GM6001 treatment increased septation during sacculation in utero, this rescue was not sustained at PP14, suggesting that the continued presence of TIMP3 is required to maintain proper septation throughout the post-partum process of alveologenesis.
Virtually all MMPs are expressed at some point during lung development (Nuttall et al. 2004); therefore future experiments will aim to establish if MMPs other than MMP2 are similarly activated with the loss of TIMP3 in the developing lung. The balance between active forms of MMPs and TIMPs would seem to be the key to proper lung morphogenesis, since tipping the balance by selective loss of MT1-MMP also results in improper alveologenesis, again yielding enlarged alveoli (Atkinson et al. 2005; Oblander et al. 2005). It becomes apparent that the orchestration of MMP and TIMP expression and/or activation is such that any perturbation has dramatic effects on lung development.
Further, it is now well established that MMPs function not only in remodeling of the ECM. These multifunctional enzymes can cleave a wide range of bioactive substrates, and this may indeed be the predominant function of MMPs in vivo (Lopez-Otin & Overall 2002). Further, TIMP3 is also a potent inhibitor of a distinct class of metalloproteinases, the ADAMs (a disintegrin and metalloproteinase). The primary purpose of the ADAMs is the cleavage of the extracellular domains of many membrane proteins from the cell surface in a process termed ‘ectodomain shedding’ (Kheradmand & Werb 2002). Proteolysis by both MMPs and ADAMs modulates bioactivity of growth factors by the release of stored growth factors from the ECM, activation of cell surface growth factors by shedding, by the cleavage of growth factor binding proteins, and in some cases, via degradation of the growth factors themselves (Blobel 2005; Murphy et al. 2008). One such example is the enzyme TACE (tumor necrosis factor α converting enzyme), which is responsible for cleavage of cell surface bound TNFα to yield the soluble form. Work from the Khokha lab has shown that in the absence of TIMP3, TACE activity is increased in the liver, resulting in constitutive release of TNFα, activation of TNFα signaling in the liver and eventual liver failure. This phenotype was reversed by breeding the TIMP3 knockout into a TNF receptor I deficient background (Mohammed et al. 2004). With this precedent in mind, we examined the abundance of the membrane bound (26 kDa) and soluble forms (17 kDa) of TNFα in a developmental series of WT versus TIMP3 null lungs. Western blot analysis demonstrated no differences in the relative abundance of either form of TNFα at E16.5 through PP3, and thus, it is unlikely that dysregulated TACE activity is responsible for the enlarged alveoli in the TIMP3 null lung (data not shown).
Finally, there are many other examples where deletion or overexpression of growth factors, cytokines or their receptors have serious repercussions on lung development, too numerous to review here. Many of these models phenocopy the TIMP3 knockout lung, including deletion of molecules involved in TGFβ and epidermal growth factor (EGF) signaling (Kheradmand et al. 2002; Sterner-Kock et al. 2002; Bonniaud et al. 2004; Chen et al. 2005). The challenge we now will address is to go beyond the somewhat simplistic model of ECM degradation, and systematically approach growth factor processing and its effect on the dysfunctional morphogenesis that occurs in the TIMP3 null lung.
Acknowledgments
This work was supported by grants from the Canadian Institutes of Health Research (K.J.L.), and the Canada Foundation for Innovation (K.J.L.). Salary support for S.E.G. was provided by the Ontario Graduate Scholarship Program. K.J.L. is a recipient of the Ontario Premier's Research Excellence Award.




