Expression of Progesterone Receptor in the Utero-tubal Junction After Intra-uterine and Deep Intra-uterine Insemination in Sows
Contents
The aim of this study was to investigate the expression of progesterone receptor (PR) in the utero-tubal junction (UTJ) of sows at 24 h after intra-uterine insemination (IUI) and deep intra-uterine insemination (DIUI) compared with conventional artificial insemination (AI) in pigs. Fifteen multiparous sows were used: AI (n = 5), IUI (n = 5) and DIUI (n = 5). The sows were inseminated with a single dose of diluted semen during the second oestrus after weaning at 6–8 h prior to ovulation (AI: 3000 × 106 spermatozoa, IUI: 1000 × 106 spermatozoa and DIUI: 150 × 106 spermatozoa). The UTJ was collected and subject to immunohistochemical staining using avidin-biotin immunoperoxidase technique with mouse monoclonal antibody to PR. In the oviductal part of the UTJ, the intensity of PR in the tunica muscularis and the proportion of PR-positive cells in the surface epithelium after DIUI were lower than AI (p < 0.05). The intensity and the proportion of PR-positive cells between AI and IUI in all compartments of the UTJ did not differ significantly (p > 0.05). When comparing between tissue compartments, prominent staining was observed in the muscular layer of the UTJ. It could be concluded that the expression of PR in the UTJ prior to fertilization after DIUI with a reduced number of spermatozoa was lower than that after AI. This might influence sperm transportation and the fertilization process.
Introduction
The mechanism of sperm transport from the insemination site to the fertilization area is complex and is regulated by many factors involving both the female genital tract and the spermatozoa (Rodriguez-Martinez et al. 2005). It is well established that the utero-tubal junction (UTJ) is the primary physical barrier to the oviduct and the caudal isthmus with the UTJ acting as a sperm reservoir to restrict sperm access to the fertilization site (Hunter 1981; Rodriguez-Martinez et al. 2005). Ovulation has been postulated to affect sperm transport by initiating the re-distribution of spermatozoa from the sperm reservoirs. This re-distribution is regulated by a change in the hormonal profile that occurs during ovulation (Hunter 1984). Mburu et al. (1996) recovered larger sperm numbers within the upper isthmus during the peri-ovulatory period than during the pre-ovulatory period.
Recently, two types of a specially-designed catheter for artificial insemination (AI) in pig have been established for non-surgical intra-uterine insemination (IUI) (Watson and Behan 2002; Sumransap et al. 2007) and deep intra-uterine insemination (DIUI) with reduction in the number of spermatozoa (Martinez et al. 2002; Vazquez et al. 2005). These procedures consist of a specially-designed catheter that can be passed through the cervix allowing the deposition of sperm in the uterine body (IUI) or uterine horn (DIUI). Using these techniques, the number of spermatozoa per dose can be reduced. It has been demonstrated that the IUI technique with a 3-times reduction in the sperm number per dose resulted in the same conception rate and litter size, under farm conditions, compared with AI (Watson and Behan 2002). However, Rozeboom et al. (2004) found that IUI with ≤1 × 109 spermatozoa per dose resulted in a smaller litter size compared with an AI with 4 × 109 spermatozoa per dose. For DIUI, earlier studies have shown that the catheter could be passed through the cervix completely in 90–95% of multiparous sows (parities 2–6; n = 147) (Martinez et al. 2001, 2002). The technique has also been used for advanced biotechnology procedures such as frozen-thawed semen, sex-sorted sperm and embryo transfer (Roca et al. 2003; Vazquez et al. 2003; Martinez et al. 2004). However, it was found that the number of spermatozoa in the sperm reservoir after DIUI with a reduced number of spermatozoa was significantly lower than that after AI (Tummaruk et al. 2007). Furthermore, poor litter size and low fertilization rate have also been observed for DIUI (Martinez et al. 2006).
It has been demonstrated that progesterone (P4) significantly increased soon after ovulation in pigs and influenced the transportation of spermatozoa and embryos (Mburu et al. 1996). The physiological mechanism of P4 on sperm transportation in the female reproductive tract is related to the expression of the progesterone receptor (PR) in the uterine horn of the pig (Sukjumlong et al. 2005) and it has been demonstrated that the PR in the pig oviduct (ampulla and isthmus) was more intense during the luteal phase compared with the follicular phase (Peralta et al. 2005). Furthermore, Sukjumlong et al. (2005) demonstrated that the PR was higher in inseminated sows compared with cyclic sows, and that the immunostaining of PR in the uterus was high during 5 or 6 to 70 h after insemination. Sperm distribution and fertilization after IUI and DIUI with a reduced number of spermatozoa have been demonstrated (Sumransap et al. 2007; Tummaruk et al. 2007). However, the expression of the PR after IUI and DIUI in pigs has never been investigated. This study was performed to investigate the expression of the PR in the UTJ of sows at 24 h after IUI and DIUI compared with that after AI in pigs.
Materials and Methods
Animals, detection of oestrus and ovulation and insemination
Fifteen crossbred Landrace × Yorkshire multiparous sows were used in the experiment. On the day of weaning, they were brought from commercial farms to the Department of Obstetrics, Gynaecology and Reproduction, Faculty of Veterinary Science, Chulalongkorn University and were allocated to individual pens adjacent to adult boars. The sows were fed 3 kg/day (twice a day) with a commercial feed (Starfeed176®; BP Feed Co. Ltd, Saraburi, Thailand) containing 15% protein, 2% fat and 10% fibre. Water was provided ad libitum via water nipple. At arrival, the sows were randomly assigned to three groups according to ear tag, AI (n = 5), IUI (n = 5) and DIUI (n = 5) groups. The sows were subjected to boar contact and were observed for sign of pro-oestrus (e.g., swelling and reddening of vulva, boar interested) twice a day (am/pm). When the signs of pro-oestrus were observed, the sows were carefully examined for the onset of standing oestrus every 6 h by using a back pressure test in the presence of a mature boar. Transrectal ultrasonography (Echo camera SSD-550; Aloka Co. Ltd., Tokyo, Japan) was performed every 4 h, starting from approximately 10–12 h after the onset of oestrus, using a 5 MHz probe to examine the time when ovulation took place in all sows (Tummaruk et al. 2007). The sows were inseminated with a single dose of diluted semen during the second oestrus after weaning. The time of ovulation during the first oestrus was used to determine the timing of insemination, which was carried out at 6–8 h prior to the expected time of ovulation. The semen was collected from an adult Duroc boar. Semen with a motility of ≥70%, a concentration of ≥150 × 106 spermatozoa/ml and with normal sperm ≥85%, was extended with Beltsville thawing solution (Pursel and Johnson 1976). The sperm dose contained 3000 × 106 spermatozoa in 100 ml for AI, 1000 × 106 spermatozoa in 50 ml for IUI (Deep golden pig®; Minitube, Tiefenbach, Germany) and 150 × 106 spermatozoa in 5 ml for DIUI. The sows were inseminated by the AI, the IUI or the DIUI technique. Both the IUI and the DIUI techniques have been described previously by Sumransap et al. (2007) and Martinez et al. (2001), respectively.
Tissue collection and immunohistochemistry
The sows were generally anaesthetized at approximately 24 h after insemination. General anaesthesia was induced by azaperone (Stressnil®; Janssen Animal Health, Beerse, Belgium), 2 mg/kg, intramuscularly. Thirty minutes later, thio-pental sodium, 10 mg/kg, was given intravenously. The ovario-hysterectomy was performed by laparotomy. The reproductive organs were removed and immediately transferred to the laboratory. The oviducts and the proximal part of uterine horns (1 cm) on each side of the reproductive tracts were collected. The UTJ and all parts of the oviduct were fixed in 10% neutral buffer formalin. The samples were embedded in paraffin blocks, cut in 4 μm thick sections and placed on 3-aminopropyl-triethoxysilane coated slides (SIGMA-ALDRICH, Inc., Steinheim, Germany). The sections were deparaffinized in xylene and rehydrated in graded alcohol. The immunohistochemical protocol was modified after Sukjumlong et al. (2005). Briefly, the antigen retrieval technique was used to enhance the reaction between antigen and antibody by boiling in 0.01 m citrate buffer pH 6.0, 2 × 5 min in a microwave at 750 watt. A standard avidin-biotin immunoperoxidase technique (Vectastain® ABC kit; Vector Laboratories, Inc., Burlingame, CA, USA) was applied to detect the PR proteins. The primary antibodies used were mouse monoclonal antibody to PR (Immunotech, Hamburg, Germany; clone 10A9) at the dilution of 1 : 200 in a humidified chamber for 2 h at room temperature. A negative control was obtained by replacing the primary antibody with non-immune serum of the same concentration as the primary antibody. Normal sow oviducts known to express PR served as positive controls. The oviduct of the positive control sows was taken during day 1–2 of the oestrus cycle. In addition, UTJ from four non-inseminated sows (early dioestrous sows) were included as a non-inseminated control groups (NAI group). In the final step, the colour of the bound enzyme (brown colour) was obtained using 3,3′-diaminobenzidine (Vector Laboratories, Inc.). All sections were counterstained with Mayer’s haematoxylin and mounted with glycerine gelatin for investigation under a light microscope.
Classification of positively-stained cells
The UTJ was classified into two parts, the oviductal and the uterine parts. The oviductal part consisted of three compartments: surface epithelium, subepithelial layer of the stroma and muscular layer (tunica muscularis). The uterine part was classified into four compartments: surface epithelium, glandular epithelium, subepithelial layer of the stroma and myometrium. The glandular epithelium was also divided into superficial and deep glandular epithelium (Table 2). The results of the immunostaining were evaluated semi-quantitatively by a manual scoring method. The scoring of PR-positive cells was done by classification into three different levels of intensity: weak, 1; moderate, 2 and strong, 3. As not all cells stained positively in some compartments of the tissue, the proportion of positive to negative cells was also included for these tissues. The estimated proportions were classified into four different levels (marked 1–4): low proportion (<30% of positive cells, 1); moderate proportion (30–60% of positive cells, 2); high proportion (>60–90% of positive cells, 3) and almost all cells positive (more than 90%, 4) (Sukjumlong et al. 2005). In connective tissue stroma of the uterine and the oviductal part of the UTJ not all cells were positively stained. Therefore, the number of PR-positive cells per mm2 in the subepithelial layer was identified in each section. Five arbitrarily chosen microscopic fields were counted. The counting was performed at 400× magnification by using an ocular reticule (ocular micrometer, 0.13 × 0.13 mm, with 25 squares) placed in the eyepiece of the light microscope and by moving the ocular micrometer along the subepithelial layer of the stroma (Sukjumlong et al. 2003).
| Group of sows | Surface epithelium | Superficial gland | Deep gland | Stroma | Myometrium |
|---|---|---|---|---|---|
| AI | 1.6a/2.7A | 1.4a/2.4A | 1.4a/2.4A | 1.8a/1990A | 3.0a/4.0A |
| IUI | 1.4a/2.7A | 1.3a/2.4A | 1.4a/2.5A | 2.4a/2370A | 3.0a/4.0A |
| DIUI | 1.6a/2.7A | 1.2a/1.8A | 1.2a/1.9A | 1.8a/1933AB | 2.2b/3.8AB |
| NAI | 2.0a/3.3A | 2.3a/3.8A | 2.3a/3.8A | 1.5a/663B | 2.5ab/2.5B |
- The different superscript letters between rows are significantly different (p < 0.05).
- AI, artificial insemination; IUI, intra-uterine insemination; DIUI, deep intra-uterine insemination; NAI, non-inseminated control.
Statistical analysis
Data were analysed using SAS (Statistical Analysis System; SAS Institute Inc. 1996). The general mean of all parameters were calculated and were used to describe all data. The score of intensities and score of positive cells were compared between groups using Kruskal–Wallis’s test and Wilcoxon-rank sum test (NPAR1WAY procedure of SAS). A probability value of p < 0.05 was considered as statistically significant.
Results
Immunohistochemical staining of PR after AI, IUI, DIUI and NAI in the oviductal and the uterine parts of the UTJ are shown in Tables 1 and 2 and Fig. 1. In the oviductal part, the intensity of PR in the muscular layer and the proportion of PR-positive cells in the surface epithelium after DIUI were lower than that after AI and IUI (p < 0.05) (Table 1). In the tunica muscularis of the oviduct, the intensity of PR in the DIUI group were not significantly different compared with NAI groups (Table 1). In the uterine part, both the intensity and the proportion of PR-positive cells in all tissue compartments were not significantly different except for the myometrium in which a higher intensity was found for AI and IUI compared with DIUI (p < 0.05) (Table 2).
| Group of sows | Surface epithelium | Stroma | Tunica muscularis |
|---|---|---|---|
| AI | 1.8a/2.7A | 1.6a/1923A | 3.0a/4.0A |
| IUI | 1.4a/2.5A | 2.4a/2370A | 3.0a/4.0A |
| DIUI | 1.3a/1.8B | 1.7a/1475AB | 2.2b/3.7AB |
| NAI | 2.0a/3.8A | 1.5a/663B | 2.5ab/2.5B |
- The different superscript letters between rows are significantly different (p < 0.05).
- AI, artificial insemination; IUI, intra-uterine insemination; DIUI, deep intra-uterine insemination; NAI, non-inseminated control.
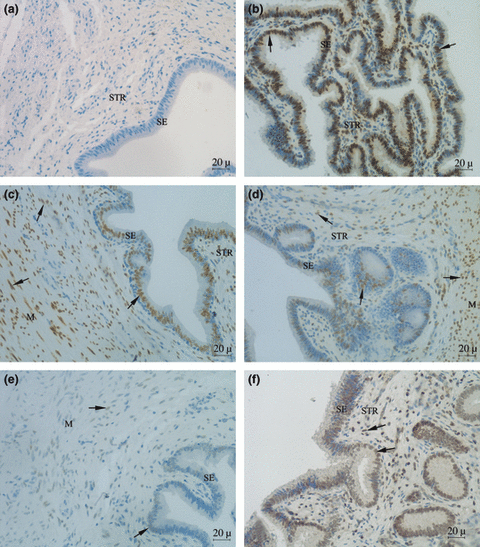
Expression of progesterone receptor (PR) in the utero-tubal junction (UTJ) of sows: (a) negative control, (b) positive control, (c) artificial insemination (AI), (d) intra-uterine insemination (IUI), (e) deep intra-uterine insemination (DIUI) and (f) non-inseminated sows (NAI). SE, surface epithelium; STR, stroma layer; M, myometrium; black arrow indicate positive staining cell
The proportion of PR-positive cells in the stroma and the myometrium in the NAI group was significantly lower than the AI and IUI groups (p < 0.05) in both the oviductal (Table 1) and the uterine parts of the UTJ (Table 2). The intensity and the proportion of PR-positive cells did not differ significantly between IUI and AI groups in all compartments of the UTJ (p > 0.05). For AI and IUI, high intensities and high proportion of PR-positive cells were observed in myometrium compartments of the UTJ, while low intensities of PR-positive cells were always observed in the tunica muscularis of DIUI group (Fig. 1). When comparing between tissue compartments, the prominent stating was observed in the muscular layer of the UTJ for all groups (Table 3).
| Tissue | Surface epithelium | Connective tissue stroma | Tunica muscularis or myometrium |
|---|---|---|---|
| Oviduct | 1.6a/2.3A | 1.7a/1699A | 2.6a/3.9A |
| Uterus | 1.6a/2.7A | 1.8a/1962A | 2.6a/3.9A |
- The different superscript letters between rows are significantly different (p < 0.05).
The intensities of the PR in each side of the reproductive tract were compared in each individual sows in all groups. In the AI group, the intensities of the PR between left and right UTJ in all tissue layers in both the oviduct and the uterine compartments were similar in all sows. In the IUI group, the intensities of PR between left and right UTJ in the surface epithelium and myometrium of the oviduct and the uterine compartments were similar in all sows and the intensities of PR in the stroma layer of the oviduct and the uterine compartments and in the glandular layer of the uterine compartment were similar in four out of five sows. For the DIUI group, the intensities of PR between left and right UTJ in the surface epithelium of the uterine compartment, myometrium of the oviduct and the uterine compartments, glandular epithelium of the uterine compartment were similar in all sows, and the intensities of PR in the stroma of the oviduct were similar in four out of five sows and in the stroma of the uterine compartments were similar in three out of five sows (data not shown).
Discussion
This study demonstrated the expression of PR after IUI and DIUI in pigs compared with that after AI. Intra-uterine insemination and DIUI are new techniques for insemination in pigs, and the expression of PR after IUI and DIUI have never been reported before. It is known that P4 mediates changes in pig reproductive tissue during the oestrous cycle and is important for the fertilization process. During recent years, the expression of PR in pigs as well as in other species has been investigated (Hartt et al. 2005; Peralta et al. 2005; Sukjumlong et al. 2005). An earlier study has demonstrated that the immunostaining of PR in the uterus was high during 5 or 6 to 70 h after insemination (Sukjumlong et al. 2005). The sows in all groups of this study were slaughtered at 24 h after insemination and the expression of PR in some compartments of the UTJ in the DIUI groups was significantly lower than that in the AI and IUI groups. The number of spermatozoa and the volume of semen used for DIUI are 20 times less than AI. It has been demonstrated that E2 up-regulates PR in the pig uterus (Sukjumlong et al. 2005). As the boar semen contain a certain amount of E2, a low volume of semen used for DIUI might also influence the expression of PR due to the lower amount of E2. Wu et al. (2006) demonstrated that PR influences the capacitation process of spermatozoa prior to fertilization. The role of PR expression in the oviduct on the fertilization rate after DIUI with a low number of spermatozoa is still unclear. In our previous study, the mean number of spermatozoa in the crypt of the sperm reservoir (both UTJ and caudal isthmus) after DIUI was significantly lower than that after AI and IUI (Tummaruk and Tienthai 2008). In this study, expression of PR in the tunica muscularis/myometrium of the oviductal and the uterine part of the UTJ after DIUI was lower than that after AI and IUI. These findings indicate that DIUI with 150 × 106 spermatozoa significantly reduced the number of spermatozoa in the sperm reservoir and reduced the expression of PR in the UTJ. This might influence the re-distribution of the spermatozoa and the fertilization process. In clinical research, a low fertilization rate and poor quality of embryos after DIUI with a small number of spermatozoa have also been observed (Martinez et al. 2006). Although DIUI with a 20-fold reduction in number of spermatozoa resulted in a similar pregnancy rate compared with AI, a higher number of partial fertilizations, unilateral fertilizations and lower litter size were also observed (Martinez et al. 2006).
In general, the amount of spermatozoa recommended to be used for IUI was three times less than AI. This study indicates that the reduction in the number of spermatozoa per insemination by IUI technique dose not alter the expression of PR in the UTJ. P4 influences the transportation of spermatozoa both before and after fertilization (Mburu et al. 1996). This study suggests that IUI could be used without any effect on the expression of PR in the sperm reservoir prior to fertilization. The sperm acrosome reaction is required for mammalian fertilization. It has been suggested that P4 is a physiological inducer for sperm acrosome reaction (Wu et al. 2006). In our previous study, the mean number of spermatozoa in the sperm reservoir after IUI was not significantly different from that after AI (Tummaruk and Tienthai 2008). In this study, expression of PR in both the oviductal part and the uterine part of the UTJ after IUI was not significantly different from that after conventional AI. These findings indicate that IUI with 1000 × 106 spermatozoa is sufficient to obtain a certain number of spermatozoa in the sperm reservoir and does not alter the expression of PR in the UTJ.
In the uterus of the sow, there are several communications between the uterine epithelial cells and the spermatozoa. These mechanisms depend on many factors, e.g., viability of the spermatozoa, concentration of the semen, presence of seminal plasma, receptors and some mediators (Rath et al. 2008). For instance, it was found that spermatozoa have a regulating influence on epithelial cytokine expression, and that three of five tested cytokines were down-regulated to baseline levels in the presence of spermatozoa (Rath et al. 2008). In our previous study, a number of spermatozoa were observed in the crypt of the sperm reservoir after AI, IUI and DIUI (Tummaruk and Tienthai 2008). The up-regulation of PR in the epithelial cells of the UTJ observed in this study might be, at least in part, due to the presence of spermatozoa in the epithelial crypt. It was found that direct contact between spermatozoa and the epithelium of the UTJ is required to allow the sperm go through the UTJ to form the sperm reservoir (Rath et al. 2008). Furthermore, the maintenance of the sperm reservoir involved several factors, e.g., mucous secretion, oviductal fluid, temperature gradient and receptor–ligand interaction between spermatozoa and oviductal epithelial cells (Tienthai et al. 2003; Rodriguez-Martinez et al. 2005; Rath et al. 2008). In this study, changes in PR expression in the oviductal epithelium reveal that the modification of the AI technique and the reduction in number of spermatozoa and semen volume might influence the mechanism involving sperm reservoir formation and perhaps also the re-distribution of spermatozoa heading to the fertilization site.
It could be concluded that the expression of PR in the tunica muscularis and the myometrium of the UTJ prior to fertilization after DIUI with a 20 times reduced number of spermatozoa was significantly lower than that after AI and IUI. This might influence sperm transportation and the fertilization process by the mechanisms which involve the expression of PR.
Acknowledgements
This study was funded by the Thailand research fund (IUG5080002) and Ratchadaphiseksomphot Endowment Fund, Chulalongkorn University. We would also like to thank Chula Unisearch, Chulalongkorn University for the contribution to language editing.
Author contributions
P. Tummaruk designed study, analysed data and drafted paper, P. Tienthai, S. Manee-In and S. Srisuwatanasagul read paper and technical support.




