Study of the macrophyte assemblages and application of phytobenthic indices to assess the Ecological Status of the Marano-Grado Lagoon (Italy)
Abstract
Benthic macrophytes from 19 sites within the Marano-Grado Lagoon were sampled in July 2007 in order to update the available information on the flora and vegetation and to assess the Ecological Status (ES) within the European Water Framework Directive (WFD). Data on macrophytes were analysed using two phytobenthic indices (EEI and R-MaQI) recently set up to evaluate the ecological status of transitional environments in the Mediterranean eco-region. Notwithstanding the extension (c. 160 km2) that places this lagoon as the second largest Italian transitional environment, ecological studies are relatively few. The present research revealed a relatively poor flora, mainly characterized by the dominance of low-diversity settlements of Ulvaceae. Moreover, the absence of intensive aquaculture activities and commercial big harbours, which account for the new species introductions recorded in other lagoons, limits the presence of non-autochthonous species. The comparison with previous data (Curiel et al. 1998) highlighted the reduction of macrophyte coverage and biomass, especially Ulvaceae stands, and an increase in species richness. In particular, there was evidence of a worsening of the area north of the Grado bridge. This area, which in the past was colonized by widespread angiosperm coverage, at present is almost lacking in vegetation. According to these observations, by applying both the phytobenthic indices available in the literature for the transitional environments, the Marano-Grado Lagoon showed a ‘Good–High’ quality in the central part of the basin and near the lagoon inlets and a ‘Poor–Bad’ quality in the northern and southern areas. The evaluation of some physico-chemical parameters, coupled with the distribution of the angiosperms, also allowed a first delineation of the main lagoon water bodies.
Problem
The entering into force of the European Water Framework Directive (WFD, 2000/60/EC) stimulated many specific studies for the assessment of the Ecological Status (ES) of marine coastal waters and transitional environments. Macroalgae and angiosperms are two biological Quality Elements to be considered to assess the ES of coastal and transitional water bodies, because of their structural and functional key role in the marine ecosystems (EC 2000). Compared with the physico-chemical factors, the benthic macrophytes, characterized by sessile organisms with a relatively long life span, are expected to have different sensitivity to anthropogenic stressors, modifying their structure and function accordingly (Dennison et al. 1993; Orfanidis et al. 2001, 2003, 2008; Krause-Jensen et al. 2004; Panayotidis et al. 2004; SIBM 2004; Arévalo et al. 2007; Ballesteros et al. 2007; Mangialajo et al. 2007; Sfriso et al. 2007, 2009; Orlando-Bonaca et al. 2008).
As a general rule in coastal lagoons, eutrophication processes have been represented as a succession of primary producer communities, through the shift from pristine to altered states (Viaroli et al. 2008; and references therein). Pristine coastal lagoons are considered to be dominated by extensive angiosperm meadows that, under mesotrophic conditions, uptake nutrients from sediments. Under high nutrient loads (nitrogen rather than phosphorus) and turbid waters, species composition shifts from the dominance of angiosperms to blooms of opportunistic and nitrophilous macroalgae (Sfriso 1987; Sfriso et al. 1987; Orfanidis et al. 2008; Viaroli et al. 2008), due to their more efficient nutrient assimilation (Thompson & Valiela 1999) and lower light level demand (Congdon & McComb 1979; Lüning 1990). In their turn, macroalgal blooms induce further alterations in the ecosystem. In the latest states of species succession, in heavily degraded lagoons, macroalgae disappear and only phytoplankton or Cyanophyta may be present (Viaroli et al. 2008). The benthic vegetation succession along a nutrient gradient, explained in terms of life-cycle strategy and functional ecosystem complexity as a shift from k-selected/late successional species to r-selected/opportunistic ones, was utilized to evaluate the Ecological Status in transitional waters (Orfanidis et al. 2001, 2003, 2008). The transition from pristine to stressed conditions may be amplified by human activities and external stressors, inducing alterations in biogeochemical processes, although more direct and experimental evidence is needed to validate the conceptual model of alternative stable state theory (Schröder et al. 2005).
To meet the WFD (2000/60/EC) objectives, a sampling program to monitor macrophyte assemblages, aiming to assess the ES of this Marano and Grado Lagoon (North Adriatic Sea), was started in 2007. In spite of its importance, only few data are currently available on the macroalgal flora of this lagoon (Simonetti 1968, 1973; Corvi 1977–1978). The most extensive study was conducted during 1992–93 on soft-bottoms by Curiel et al. (1998). Other studies have been undertaken on intertidal sediment microphytobenthos at Marano basin (Sdrigotti & Welker 2002) and on hard substrata off-shore in the Grado basin (Curiel et al. 2000–2001).
This paper presents the results of a study aimed at (i) increasing and updating knowledge of the macroalgae and angiosperms which characterize the basins of Grado and Marano Lagoon and (ii) assessing the Ecological Status (ES) of the selected sampling sites using phytobenthic indices, recently proposed for the transitional environments of the Mediterranean eco-region.
Study area
The Marano and Grado Lagoon is part of the lagoon-delta system of the North Adriatic Sea, with an area of 160 km2 and a coast extension of c. 32 km. The mean distance between the internal edge and the barrier islands is about 5 km. Tides, which are the main driving forces of the lagoon hydrodynamics, are semidiurnal with an average tidal range of 65 cm (Gatto & Marocco 1993).
The Marano basin shows a wide shallow water body with few areas above the sea level and channels linking the numerous plain spring rivers flowing into its internal edge towards the sea. The transport of water in tidal cycles is essentially diffusive (Marocco 1995). In contrast, the Grado basin, characterized by morphological reliefs (islands) and tidal-marshes, is shallower than the Marano basin and has a more complex hydrographical network (Gatto & Marocco 1992, 1993). The transport of water during the tidal cycle is essentially advective (eastwards) and partially diffusive, towards Marano.
The average freshwater inflow is c. 78.0 and 20.5 m3·s−1 in the basins of Marano and Grado, respectively (Marocco 1995). Moreover, 22 water-scooping machines provide a significant freshwater supply to the lagoon of c. 200 million m3·year−1 (ARPA Friuli-Venezia Giulia 2006).
The mean salinity of the Grado basin is higher (28.5 psu) than the salinity of the Marano basin (22.2 psu). Water temperature, following the seasonal cycle, shows a gradient from the river mouths to the inlets, with mean values of 15.8 °C at Marano and 14.8 °C at Grado. The lagoon waters are well oxygenated, with a mean oxygen saturation near to equilibrium with the atmosphere (Mattassi et al. 2006). In spring–summer, during macrophyte or phytoplankton blooms, the water column may be oversaturated, whereas hypoxic and/or anoxic events have not been observed. The mean values of the total dissolved nitrogen (DIN) in the water column are c. 110 μm at Marano and c. 38 μm at Grado, whereas the mean reactive phosphorus (RP) ranges between c. 1.12 μm at Marano and c. 0.29 μm at Grado (Mattassi et al. 2006).
The present lagoon morphology is due to the last reclamation activities dating back to the first 20 years of the 20th century (Mattassi et al. 2006). After that period the lagoon was subject to many works (breakwaters, commercial port, many tourist ports) that have strongly affected water circulation (Mattassi et al. 2006). Moreover, at the river mouths, close to tourist ports and industrial areas, high concentrations of metals (especially Hg and As) are present (Marocco 1995; Brambati 1996; Adami et al. 1997). Owing to these conditions the major part of the lagoon and its hinterland has been included in the ‘polluted areas of national interest’.
Material and methods
Macrophyte sampling
A qualitative pilot survey was carried out in April 2007 in three areas: near the salt-marshes of the Marano basin (area A, Fig. 1), in an angiosperm bed close to Porto Buso inlet (area B) and on a breakwater near the same inlet (area C). Sampling was carried out by scraping the bottom with a rake and/or collecting the macroalgae by hand in the shallowest areas. In July 2007, a more extensive study was carried out on 19 sampling sites spread out in both the basins (Fig. 1), selected to reflect the main hydro-geo-morphological and trophic gradients of the two basins.
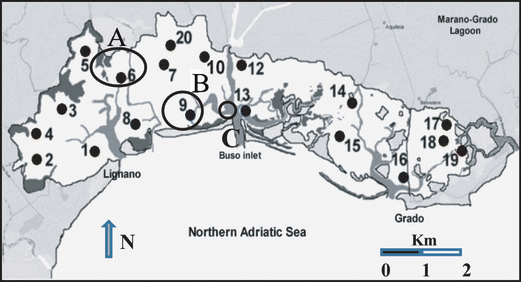
Marano and Grado sampling sites. The circles A, B and C areas concern the April 2007 sampling, the numbered sites the July 2007 one.
Sampling sites were numbered in the framework of the Friuli-Venezia Giulia Regional Environment Protection Agency (ARPA-FVG) monitoring program. Site 11 is missing because it was excluded during the field sampling. It was very similar to site 7 and the sampling was considered useless. The original numbering of the sampling sites was retained because in these stations other data (not inherent with this paper) were taken, for further comparisons.
For the floristic study in each sampling site, on a circular surface with a radius of c. 15–20 m, the highest number of macroalgae and angiosperm species was collected by SCUBA divers, at both soft and hard substrata. To apply the R-MaQI index (see Indices paragraph) the total coverage of benthic macrophytes was estimated by applying the Visual Census Technique (Cormaci et al. 2003) to the circle surface. This technique makes it possible to assess the coverage of the entire area by a visual estimation performed independently by two researchers. The result is the mean of the two coverage evaluations.
To measure the macrophyte biomass (as kg·m−2 fresh weight) and to apply the Ecological Evaluation Index (see Indices paragraph), all the macrophytes collected in three randomly selected replicates of 1 m2 were examined in the laboratory after fixation with 4% formaldehyde neutralized with hexamethylenetetramine.
When possible, all the macrophytes collected were identified to specific and infra-specific level on the basis of the most recent taxonomic literature. Cyanophyta, requiring specific sampling methodology and taxonomic skills for a serious investigation, were considered only in the presence of a significant coverage (turfs). Neither during samplings nor in laboratory analyses did they show a relevant presence.
To estimate the percent coverage of different species and functional groups, a transparent double-bottom square PVC container, filled with sea water and having at its bottom a square, was used. The surface covered by each sorted species in vertical projection floating in sea water was quantified as percentage of coverage (4 cm2 = 1% of the sampling surface). For species with an insignificant abundance, a nominal coverage value of 0.01% was allocated (Orfanidis et al. 2008; Orlando-Bonaca et al. 2008). The statistical analysis of macrophyte coverage was performed on data recorded in July. They refer to 62 species, because in April no quantitative data were collected.
Physico-chemical variables and nutrient concentrations
In July 2007 in each sampling site, temperature, dissolved oxygen, pH, and water transparency (Secchi disk) were measured and samples for chlorinity, chlorophyll a and nutrient concentrations in the water column were collected. Surface sediment samples were collected for the determination of sediment density, porosity, percentage of the fine fraction (<63 μm) and nutrient (phosphorus, nitrogen and carbon) concentrations.
The procedures used to determine environmental variables and nutrient concentrations in the water column and surface sediments, as well as the main sediment characteristics, are reported in Sfriso et al. (2003, 2005a,b).
Water bodies
An early delineation of the main water bodies of the Marano and Grado Lagoon was attempted by applying cluster analysis (Euclidean Distance – complete linkage) to the physico-chemical data and nutrient concentrations of the water column and surface sediments. These data have been integrated with the macrophyte distribution in the Marano and Grado Lagoon. In particular, these results were coupled with the presence/absence of Cymodocea nodosa and Zostera marina meadows and the complete absence of vegetation, except for Vaucheria submarina beds. In fact, macrophyte assemblages are related to the habitat conditions of the transitional environments and they can play an important cause–effect role in the quality of the environment (Sfriso et al. 2009).
Indices
The Ecological Status (ES) of the Marano and Grado Lagoon were assessed by applying two phytobenthic indices of easy and rapid application, proposed for the transitional environments of the Mediterranean eco-region: the Ecological Evaluation Index (EEI, Orfanidis et al. 2001, 2003) and the Rapid-Macrophytes Quality Index (R-MaQI, Sfriso et al. 2007, 2009). The EEI is based on macrophyte coverage and two morpho-functional groups are distinguished (ESG I = annual, opportunistic species, ESG II = perennial, late successional species). The R-MaQI is structured as a dichotomous key and is based on the recognition and the presence/absence of key species (angiosperms and/or macroalgae), together with the variability of some physico-chemical factors such as water transparency, salinity, oxygen concentration and sediment grain-size, estimated distinguishing between sand and silt + clay.
Results
Flora
In all, 70 species were identified (Table 1): of which 40 Rhodophyceae, 4 Phaeophyceae, 1 Xanthophyceae, 22 Ulvophyceae and 3 Angiosperms. Some of these included winter–spring species only recorded in April but the greater part of them were found in both April and July. Some species (i.e. Boergeseniella fruticulosa, Hydrolithon boreale) are new to the Lagoon, but are common on the marine coastal water of the Gulf of Trieste (Falace 2000; Falace et al. 2005), while Acrothamnion preissii was first reported in the North Adriatic Sea.
| Ulvophyceae |
| Blidingia minima (Nägeli ex Kützing) Kylin |
| Blidingia ramifera (Bliding) Garbary et Barkhouse |
| Chaetomorpha linum (O.F. Müller) Kützing |
| Chaetomorpha ligustica (Kützing) Kützing |
| Cladophora albida (Nees) Kützing |
| Cladophora prolifera (Roth) Kützing |
| Cladophora sericea (Hudson) Kützing |
| Cladophora vadorum (Areschoug) Kützing |
| Cladophora vagabunda (Linnaeus) C. Hoek |
| Entocladia viridis Reinke |
| Gayralia oxysperma (Kützing) K. L. Vinogradova ex Scagel et al. |
| Phaeophila dendroides (P. et H. Crouan) Batters |
| Ulothrix flacca (Dilllwyn) Thuret |
| Ulothrix implexa (Kützing) Kützing |
| Ulva clathrata (Roth) C. Agardh |
| Ulva compressa Linnaeus |
| Ulva flexuosa Wulfen |
| Ulva intestinalis Linnaeus |
| Ulva laetevirens Areschoug |
| Ulva rigida C. Agardh |
| Ulva rotundata Bliding |
| Ulvella lens P. et H. Crouan |
| Rhodophyceae |
| Acrochaetium sp. |
| Acrothamnion preissii (Sonder) E.M. Wollaston |
| Anotrichium barbatum (C. Agardh) Nägeli |
| Antithamnion cruciatum (C. Agardh) Nägeli |
| Bangia fuscopurpurea (Dillwyn) Lyngbye |
| Boergeseniella fruticulosa (Wulfen) Kylin |
| Callithamnion corymbosum (J. E. Smith) Lyngbye |
| Ceramium cimbricum H. E. Petersen |
| Ceramium circinatum (Kützing) J. Agardh |
| Ceramium codii (H. Richards) Feldmann-Mazoyer |
| Ceramium siliquosum (Kützing) Maggs et Hommersand |
| Chondria capillaris (Hudson) M.J. Wynne |
| Dasya baillouviana (S.G. Gmelin) Montagne |
| Erythropeltis discigera (Berthold) F. Schmitz |
| Erythrotrichia carnea (Dillwyn) J. Agardh |
| Gastroclonium reflexum (Chauvin) Kützing |
| Gayliella flaccida (Harvey ex Kützing) T.O. Cho et L. McIvor |
| Gelidium pusillum (Stackhouse) Le Jolis |
| Gracilaria bursa-pastoris (S.G. Gmelin) P.C. Silva |
| Gracilaria gracilis (Stackhouse) Steentoft, L.M. Irvine et Farnham |
| Gracilaria sp. |
| Gracilariopsis longissima (S.G. Gmelin) Steentoft, L.M. Irvine et Farnham |
| Hydrolithon boreale (Foslie) Y. M. Chamberlain |
| Hydrolithon farinosum (J. V. Lamouroux) D. Penrose et Y. M. Chamberlain |
| Hypnea musciformis (Wulfen) J. V. Lamouroux |
| Laurencia obtusa (Hudson) J. V. Lamouroux |
| Lithophyllum pustulatum (J.V. Lamouroux) Foslie |
| Nitophyllum punctatum (Stackhouse) Greville |
| Palisada patentiramea (Montagne) Cassano, Sentíes, Gil-Rodríguez et M.T. Fujii |
| Pneophyllum fragile Kützing |
| Polysiphonia elongata (Hudson) Sprengel |
| Polysiphonia furcellata (C. Agardh) Harvey |
| Polysiphonia sanguinea (C. Agardh) Zanardini |
| Polysiphonia sertularioides (Grateloup) J. Agardh |
| Polysiphonia cf. subtilissima Montagne |
| Polysiphonia subulata (Ducluzeau) P. et H. Crouan |
| Porphyra leucosticta Thuret |
| Pterothamnion plumula (J. Ellis) Nägeli |
| Rhodophyllis divaricata (Stackhouse) Papenfuss |
| Spyridia filamentosa (Wulfen) Harvey |
| Phaeophyceae |
| Cystoseira barbata (Stackhouse) C. Agardh |
| Ectocarpus siliculosus (Dillwyn) Lyngbye var. hiemalis (P. et H. Crouan ex Kjellman) Gallardo |
| Fucus virsoides J. Agardh |
| Sphacelaria cirrhosa (Roth) C. Agardh |
| Xanthophyceae |
| Vaucheria submarina (Lyngbye) Berkeley |
| Angiosperms |
| Cymodocea nodosa (Ucria) Ascherson |
| Nanozostera noltii (Hornemann) Tomlinson et Posluzny |
| Zostera marina Linnaeus |
In the Marano and Grado Lagoon, macroalgae and angiosperms mainly colonize the central area of the whole basin (Table 2), whereas the southern and northern areas were poor both in biomass and species richness. In particular, the shallow waters between Lignano and Marano (stations 1, 2, 3, 4, 5), turbid and rich in nutrients because of the Stella and Aussa-Corno freshwater inputs, showed low species richness (5–13 species) and mean biomass of <0.1 kg·m−2 fresh weight (kg·m−2·fwt). Most of the area north of the Grado bridge, out of the first part of the main channel (st. 17–19), exhibited shallower bottoms and low species richness (2–3 species), low biodiversity and a negligible biomass (<0.1 kg·m−2·fwt) of Vaucheria submarina. In that area, angiosperms were missing and except for some Chlorophyta of the genus Blidingia, which colonized the pools along the channels and the banks of some islands, no macrophytes were found.
| stations | no. of species | mean coverage(%) | mean biomass (fwt·kg·m−2) | Hurlbert index ES (50) | Shannon index |
|---|---|---|---|---|---|
| 1 | 12 | 2 | <0.1 | 2.00 | 1.63 |
| 2 | 13 | 5 | <0.1 | 1.00 | 1.37 |
| 3 | 8 | 86 | <0.1 | 3.00 | 1.09 |
| 4 | 8 | 54 | <0.1 | 2.00 | 0.32 |
| 5 | 5 | 71 | <0.1 | 3.00 | 0.56 |
| 6 | 22 | 111 | 0.2 | 6.11 | 0.81 |
| 7 | 18 | 110 | 0.9a | 5.72 | 1.30 |
| 8 | 20 | 89 | 0.9 | 3.81 | 1.12 |
| 9 | 27 | 98 | 0.8a | 2.46 | 0.45 |
| 10 | 20 | 101 | 0.9a | 5.48 | 0.59 |
| 12 | 7 | 95 | 1.3 | 2.42 | 0.25 |
| 13 | 20 | 105 | 0.9a | 2.86 | 0.60 |
| 14 | 17 | 98 | 0.5 | 3.04 | 0.48 |
| 15 | 1 | 50 | 0.3a | 1.00 | 0.00 |
| 16 | 26 | 86 | 0.8a | 4.24 | 1.04 |
| 17 | 3 | 30 | <0.1 | 1.00 | 0.02 |
| 18 | 2 | 30 | <0.1 | 1.00 | 0.01 |
| 19 | 3 | 91 | <0.1 | 1.55 | 0.07 |
| 20 | 28 | 39 | 0.3 | 6.00 | 1.68 |
- aAngiosperm above-ground biomass.
The highest species richness (18–28 species) and coverage (39–110%) were recorded in the central area of the lagoon, chiefly in the shallow bottoms near Porto Buso (st. 6–10, 13, 20) and on the inlet of Grado (st. 16), where dense and well-structured angiosperm beds (Cymodocea nodosa and Zostera marina), with a biomass ranging between 0.8 and 0.9 kg·m−2·fwt, were present. Some sites close to the mainland (st. 12, 14) displayed a mean macroalgal biomass of 0.5–1.3 kg·m−2·fwt, essentially represented by Ulvaceae. In some cases, such as at station 15, located in a remote shallow area close to salt-marshes, the biomass was entirely constituted by Nanozostera noltii (above ground 0.3 kg·m−2·fwt). In this site the biodiversity was further reduced by the absence of epiphytes on Nanozostera leaves.
The percent coverage of each species found in July, taking into consideration the two ecological state groups (ESGs) considered by Orfanidis et al. (2003) for the EEI application, is reported in Table 3. The ecological state classification (ESC) is plotted in Fig. 2, and the results obtained by applying the dichotomous key of the R-MaQI (Sfriso et al. 2007) are shown in Fig. 3. The two phytobenthic indices provide similar assessments of quality, ranging from ‘Bad’ to ‘High’ and encompassing all the five classes of quality provided by the WFD. Better environmental conditions, characterized by wide angiosperm meadows, were recorded in the central sector of the lagoon and in the surrounding lagoon inlets. ‘Bad’ and ‘Poor’ quality states were found in the western part of the Marano basin, in correspondence with the main fresh water outfalls, and in the eastern part of the Grado basin where, apart from a xantophyceae, no macrophytes were recorded.
| macrophyte TAXA | sampling sites | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | |
| ESG I | Average coverage % | ||||||||||||||||||
| Hydrolithon boreale | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.1 | 0.1 | 0.1 | 0 | 0.1 | 0 | 0 | 0.05 | 0 | 0 | 0 | 0.01 |
| Hydrolithon farinosum | 0 | 0 | 0 | 0 | 0 | 0.01 | 0.01 | 0.1 | 0.1 | 0.1 | 0 | 0.1 | 0 | 0 | 0.05 | 0 | 0 | 0 | 0.01 |
| Lithophyllum pustulatum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.1 | 0.1 | 0.1 | 0 | 0.1 | 0 | 0 | 0.05 | 0 | 0 | 0 | 0.01 |
| Pneophyllum fragile | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.05 | 0.05 | 0 | 0 | 0 | 0 | 0 | 0.05 | 0 | 0 | 0 | 0.01 |
| Cystoseira barbata | 0 | 0 | 0 | 0 | 0 | 0.25 | 3 | 0 | 0.5 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.5 |
| Fucus virsoides | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 |
| Nanozostera noltii | 0 | 0 | 0 | 0 | 0 | 10 | 10 | 50 | 0 | 0 | 0 | 10 | 0 | 50 | 40 | 0 | 0 | 0 | 20 |
| Zostera marina | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 90 | 0 | 0 | 40 | 0 | 0 | 0 | 0 |
| Cymodocea nodosa | 0 | 0 | 0 | 0 | 0 | 0 | 40 | 0 | 90 | 90 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ESG II | Average coverage % | ||||||||||||||||||
| Acrothamnion preissii | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.02 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Antithamnion cruciatum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.02 | 0 | 0 | 0.02 | 0 | 0 | 0 | 0.02 |
| Anotrichium barbatum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.01 |
| Bangia fuscopurpurea | 0.01 | 0 | 0 | 0 | 0 | 0.01 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.01 | 0 | 0 | 0 | 0.01 |
| Boergeseniella fruticulosa | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.04 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ceramium codii | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.03 |
| Callithamnion corymbosum | 0 | 0.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.03 |
| Ceramium cimbricum | 0.04 | 0.04 | 0.04 | 0 | 0 | 0.01 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.1 |
| Ceramium circinatum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.02 | 0 | 0 | 0 | 0 |
| Ceramium siliquosum | 0.04 | 0.04 | 0.04 | 0 | 0 | 0 | 0.04 | 0 | 0 | 0 | 0.04 | 0.04 | 0.4 | 0 | 0.02 | 0 | 0 | 0 | 0.1 |
| Chondria capillaris | 0.6 | 0.3 | 15 | 3 | 5 | 0.01 | 2 | 0.1 | 0.1 | 0.1 | 0.5 | 0.2 | 0.5 | 0 | 0.15 | 0 | 0 | 0 | 0.5 |
| Dasya baillouviana | 1 | 3 | 0 | 0.5 | 0.4 | 1 | 1.4 | 0.1 | 0 | 1.5 | 0 | 3 | 0 | 0 | 0.1 | 0 | 0 | 0 | 8 |
| Erythropeltis discigera | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.01 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Erythrotrichia carnea | 0.02 | 0 | 0 | 0 | 0 | 0 | 0 | 0.01 | 0 | 0.01 | 0 | 0 | 0 | 0 | 0.01 | 0 | 0 | 0 | 0.01 |
| Gastroclonium reflexum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.4 | 1 | 0 | 0.01 | 0 | 0 | 0 | 0 |
| Gayliella flaccida | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.02 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.1 |
| Gracilaria bursa-pastoris | 0 | 0 | 0 | 0 | 0 | 1.5 | 2.5 | 0 | 0.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Gracilaria gracilis | 0 | 0 | 0.5 | 0.1 | 5 | 1.5 | 0 | 2.5 | 0.3 | 0.8 | 3 | 0 | 0.5 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gracilaria sp. | 0 | 0 | 0 | 0.1 | 0 | 0 | 0 | 0 | 0.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Gracilariopsis longissima | 0 | 0 | 0 | 0 | 0 | 0 | 0.05 | 0 | 0 | 0.7 | 0 | 0.1 | 0.5 | 0 | 0.1 | 0 | 0 | 0 | 0.5 |
| Hypnea musciformis | 0 | 0 | 0 | 0 | 0 | 1.15 | 0.1 | 0.1 | 0 | 0.5 | 0.1 | 0 | 0.05 | 0 | 0 | 0 | 0 | 0 | 0 |
| Laurencia obtusa | 0 | 0 | 0 | 0 | 0 | 0.1 | 0.1 | 0 | 0 | 0.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.25 |
| Nitophyllum punctatum | 0.04 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Palisada patentiramea | 0 | 0 | 0 | 0 | 0 | 0.2 | 0.25 | 0.1 | 1.35 | 2.25 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Polysiphonia elongata | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0.5 |
| Polysiphonia furcellata | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Polysiphonia sanguinea | 0.04 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Polysiphonia sertularioides | 0 | 0.1 | 0 | 0.1 | 0 | 0.8 | 0.01 | 0 | 0.1 | 1.5 | 0 | 0.3 | 0.2 | 0 | 0.25 | 0 | 0 | 0 | 0.5 |
| Polysiphonia cf. subtilissima | 0 | 0.1 | 0.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Polysiphonia cf. subulata | 0 | 0 | 0 | 0 | 0 | 0 | 0.01 | 0 | 0.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pterothamnion plumula | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.01 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Rhodohyllis divaricata | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.1 | 0 | 0 | 0 | 0.1 |
| Spyridia filamentosa | 0 | 0 | 0 | 0 | 0.25 | 0.55 | 0 | 0.2 | 0.5 | 0 | 0 | 0 | 0.15 | 0 | 0.5 | 0 | 0 | 0 | 0.1 |
| Blidingia minima | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.1 | 0 |
| Chaetomorpha linum | 0.04 | 0.04 | 0.04 | 0 | 0 | 0 | 0 | 0 | 0.05 | 0.05 | 0 | 0.05 | 0.05 | 0 | 1 | 0.05 | 0.05 | 0 | 0 |
| Chaetomorpha ligustica | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.01 | 0 | 0 | 0.01 | 0 | 0 | 0 | 0 |
| Cladophora albida | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.05 | 0 | 0 | 0 | 0 | 0.1 | 0 | 0.05 | 0 | 0 | 0 | 0.5 |
| Cladophora sericea | 0 | 0.02 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.05 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cladophora prolifera | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.01 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cladophora vagabunda | 0 | 0 | 0 | 0 | 0 | 0.01 | 0.01 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.15 | 0 | 0 | 0.05 | 0 | 0 | 0 |
| Cladophora vadorum | 0 | 0 | 0 | 0 | 0 | 0 | 0.01 | 0.05 | 0.05 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Entocladia viridis | 0 | 0 | 0 | 0.01 | 0 | 0.01 | 0 | 0.01 | 0.01 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Phaeophila dendroides | 0 | 0 | 0 | 0 | 0 | 0.01 | 0.01 | 0.01 | 0.01 | 0 | 0 | 0.01 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ulothrix flacca | 0 | 0 | 0 | 0 | 0 | 0.01 | 0 | 0 | 0 | 0.01 | 0 | 0 | 0 | 0 | 0.01 | 0 | 0 | 0 | 0 |
| Ulothrix implexa | 0 | 0.01 | 0 | 0 | 0 | 0 | 0 | 0 | 0.01 | 0 | 0 | 0 | 0.01 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ulva compressa | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.05 | 0 | 0 | 0 | 0 |
| Ulva clathrata | 0 | 0 | 0 | 0 | 0 | 0.01 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.05 | 0 | 0 | 0 | 0 |
| Ulva intestinalis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.5 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ulva laetevirens | 0.4 | 0.4 | 35 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 90 | 0.3 | 2 | 0 | 1.5 | 0 | 0 | 0 | 2.5 |
| Ulva rigida | 0 | 0.4 | 35 | 50 | 60 | 4 | 50 | 25 | 4 | 1.5 | 0 | 0.3 | 2 | 0 | 0 | 0 | 0 | 0 | 2.5 |
| Ulvella lens | 0 | 0 | 0 | 0 | 0 | 0.01 | 0 | 0 | 0.01 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sphacelaria cirrhosa | 0.04 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.04 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Vaucheria submarina | 0.12 | 0.1 | 0 | 0.1 | 0 | 90 | 0 | 0 | 0 | 0 | 0 | 0 | 90 | 0 | 0 | 30 | 30 | 90 | 0 |
| Total | 2 | 5 | 86 | 54 | 71 | 101 | 56 | 28 | 7 | 10 | 94 | 5 | 98 | 0 | 4 | 30 | 30 | 90 | 18 |
| Average coverage % | |||||||||||||||||||
| ESG I | 0 | 0 | 0 | 0 | 0 | 10 | 53 | 60 | 91 | 91 | 1 | 100 | 0 | 50 | 82 | 0 | 0 | 1 | 21 |
| ESG II | 2 | 5 | 86 | 54 | 71 | 101 | 56 | 28 | 7 | 10 | 94 | 5 | 98 | 0 | 4 | 30 | 30 | 90 | 18 |
| Ecological state classification | |||||||||||||||||||
| EEI | M | M | B | P | B | B | M | G | H | H | B | H | B | G | H | M | M | B | M |
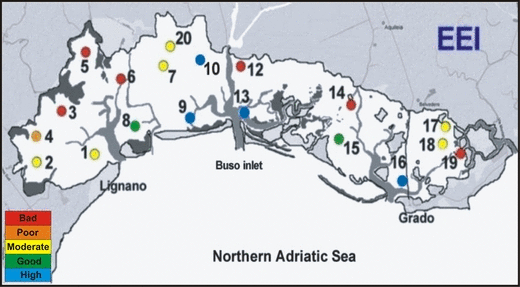
ES assessment with the Ecological Evaluation Index.
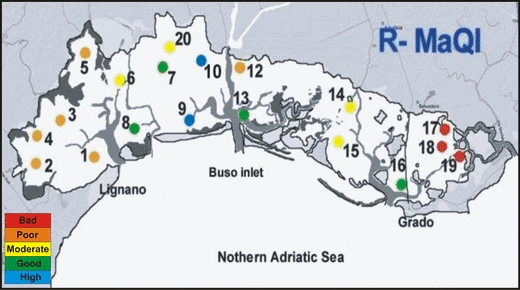
ES assessment with the Rapid-Macrophyte Quality Index.
The cluster analysis (Fig. 4) applied to the macroalgae and angiosperm coverage data (Bray–Curtis Similarity, transformed square root) separated the 19 sampling sites into five groups, plotted in Fig. 5. Group A consists of the sampling sites (st. 1–5) characterized by Ulva rigida and Chondria capillaris, while station 12 (cluster B) is distinguished by the presence of Ulva laetevirens (90% similarity). The sites of group C (st. 7, 9 and 10) are characterized by Cymodocea nodosa, which represents more than 58% of the similarity of the group, together with U. rigida and Cystoseira barbata. The clusters D and F show the greatest uniformity. Group D (st. 6, 14, 17–19) is characterized by V. submarina (95% similarity), while cluster F groups the sampling sites (st. 8, 13, 15, 16 and 20) that present a 76% similarity due to the presence of Z. marina and N. noltii. The subdivision of the sampling sites has been verified by the ANOSIM test (Global R: 0.867, significance level of sample statistic 0.1% and number of permuted statistics greater than or equal to Global R: 0).
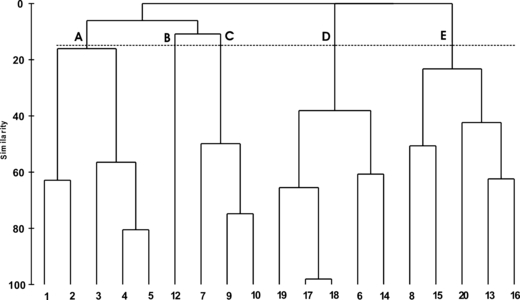
Hierarchical cluster dendrogram based on Bray–Curtis Index showing the relationship of the 19 sites and macrophyte communities.
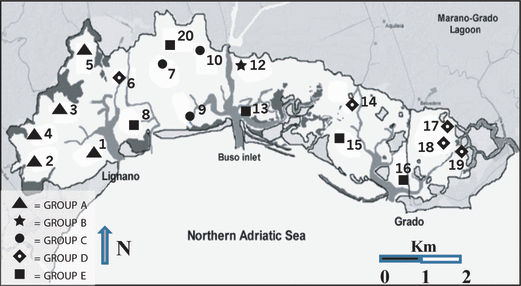
Distribution in the Marano and Grado Lagoon of the five clusters originating from the application of Bray–Curtis Index on community quantitative data.
Physico-chemical variables and nutrient concentrations
The environmental data are reported in Table 4. Some data concerning the trophic state of the lagoon, such as the nitrogen and phosphorus concentrations in the water column and surface sediments and the concentration of chlorophyll-a, were plotted in maps reported in Facca et al. (2008). Even if restricted to one sampling, it is possible to highlight a range of trophic conditions in the Marano and Grado basins. The highest nutrient concentrations, in both water column and surface sediments, showed a gradient from Marano to Grado basin and from the outflows of Stella, Aussa-Corno and other minor rivers and canals to the sea. This trend was more evident for DIN, mainly represented by nitrates, which showed the highest concentrations at stations 4 (63.8 μm) and 12 (60.3 μm), near the outflows of the Stella and Aussa-Corno rivers, decreasing toward the sea and the Grado basin. There was a more homogeneous distribution of orthophosphates, with the highest concentration (0.87 μm) at station 1, near Lignano. In surface sediment, predominately fine sediment (fraction <63 μm ranging from 60.0% to 97.8%), both phosphorus and nitrogen were the highest near the river outflows (peak values: 536 μg·g−1 dry weight and 1.9 mg·g−1·dwt, respectively, at station 5). However, a high nitrogen concentration (1.6 mg·g−1·dwt) was also recorded at station 9 in seagrass meadows.
| St. 1 | St. 2 | St. 3 | St. 4 | St. 5 | St. 6 | St. 7 | St. 8 | St. 9 | St. 10 | St. 12 | St. 13 | St. 14 | St. 15 | St. 16 | St. 17 | St. 18 | St. 19 | St. 20 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| water column | |||||||||||||||||||
| temp (°C) | 27.5 | 28 | 27.7 | 28.5 | 29 | 30.1 | 29.8 | 29.4 | 29.2 | 29 | 28.8 | 28.5 | 29.0 | 29 | 27.7 | 28.3 | 28.5 | 28.6 | 29.8 |
| salinity (psu) | 27.8 | 27.1 | 19.6 | 21.7 | 22.9 | 29.6 | 29.5 | 31.9 | 34.3 | 29.9 | 30.2 | 35.0 | 34.9 | 35.8 | 35.0 | 31.7 | 30.0 | 31.7 | 29.2 |
| OD (%) | 116 | 115 | 140 | 129 | 121 | 135 | 137 | 125 | 203 | 165 | 127 | 130 | 105 | 119 | 130 | 85.0 | 78 | 76 | 164 |
| pH | 8.20 | 8.46 | 8.48 | 8.29 | 8.2 | 8.41 | 8.29 | 8.3 | 8.37 | 8.47 | 8.34 | 8.4 | 8.21 | 8.43 | 8.4 | 8.34 | 8.33 | 8.31 | 8.42 |
| Chl a (μg·dm−3) | 0.80 | 0.80 | 1.80 | 1.20 | 1.80 | 0.90 | 0.24 | 0.80 | 0.80 | 0.24 | 0.70 | 0.60 | 0.30 | 0.5 | 0.20 | 0.48 | 0.67 | 0.56 | 0.20 |
| Phaeo a (μg·dm−3) | 1.30 | 1.30 | 2.40 | 0.48 | 2.26 | 0.64 | 0.26 | 0.67 | 0.47 | 0.40 | 0.63 | 0.52 | 0.33 | 0.2 | 0.29 | 0.45 | 0.85 | 0.56 | 0.29 |
| Chl a tot (μg·dm−3) | 2.10 | 2.10 | 4.21 | 1.68 | 4.07 | 1.54 | 0.50 | 1.47 | 1.27 | 0.64 | 1.33 | 1.12 | 0.63 | 0.7 | 0.49 | 0.93 | 1.52 | 1.12 | 0.49 |
| silicates (μm) | 13.75 | 14.00 | 13.90 | 14.80 | 13.55 | 5.00 | 6.35 | 5.95 | 7.40 | 3.85 | 14.25 | 7.10 | 6.30 | 4.65 | 3.70 | 9.00 | 8.50 | 6.70 | 4.20 |
| RP (μm) | 0.87 | 0.27 | 0.21 | 0.13 | 0.16 | 0.14 | 0.14 | 0.12 | 0.12 | 0.14 | 0.27 | 0.04 | 0.13 | 0.1 | 0.14 | 0.10 | 0.13 | 0.12 | 0.17 |
| ammonium (μM) | 12.62 | 7.76 | 5.22 | 11.89 | 12.25 | 6.89 | 18.56 | 6.57 | 16.97 | 7.11 | 13.78 | 3.77 | 6.24 | 3.63 | 5.80 | 9.43 | 6.48 | 8.34 | 3.34 |
| nitrites (μM) | 1.01 | 0.93 | 0.58 | 0.80 | 0.65 | 0.33 | 0.40 | 0.30 | 0.38 | 0.40 | 0.68 | 0.25 | 0.30 | 0.18 | 0.25 | 0.43 | 0.35 | 0.25 | 0.33 |
| nitrates (μM) | 18.2 | 17.5 | 23.2 | 51.1 | 23.4 | 7.1 | 7.1 | 10.5 | 11.3 | 11.6 | 45.9 | 6.43 | 4.03 | 3.28 | 3.09 | 5.74 | 4.82 | 3.95 | 4.59 |
| DIN (μM) | 31.8 | 26.2 | 29.0 | 63.8 | 36.3 | 14.3 | 26.0 | 17.3 | 28.7 | 19.1 | 60.3 | 10.5 | 10.6 | 7.09 | 9.14 | 15.6 | 11.65 | 12.54 | 8.26 |
| surface sediment (5 cm) | |||||||||||||||||||
| density (g·dwt·cm−3) | 1.02 | 0.84 | 0.78 | 0.72 | 0.58 | 0.71 | 0.80 | 0.66 | 0.95 | 0.85 | 0.68 | 1.10 | 0.88 | 0.72 | 0.90 | 0.66 | 0.59 | 0.78 | 0.93 |
| fraction <63 μm (%) | 73.1 | 60.0 | 94.5 | 85.3 | 97.8 | 80.4 | 77.7 | 94.0 | 79.8 | 65.6 | 93.4 | 65.1 | 86.2 | 93.6 | 64.4 | 98.3 | 94.0 | 94.0 | 70.3 |
| porosity (%) | 0.63 | 0.69 | 0.70 | 0.73 | 0.74 | 0.73 | 0.72 | 0.76 | 0.64 | 0.70 | 0.75 | 0.59 | 0.67 | 0.72 | 0.66 | 0.76 | 0.78 | 0.70 | 0.67 |
| Ctot (mg·g−1·dwt) | 73.0 | 83.4 | 69.1 | 74.8 | 71.0 | 69.2 | 67.5 | 66.3 | 74.6 | 72.8 | 61.8 | 76.9 | 67.3 | 62.7 | 77.2 | 67.0 | 66.5 | 73.8 | 67.8 |
| Cinorg (mg·g−1·dwt) | 68.0 | 71.4 | 55.1 | 58.8 | 52.0 | 55.2 | 56.5 | 50.3 | 61.6 | 62.8 | 47.8 | 71.9 | 57.3 | 48.7 | 67.2 | 51.0 | 53.5 | 60.8 | 58.8 |
| Corg t (mg·g−1·dwt) | 5.0 | 12.0 | 14.0 | 16.0 | 19.0 | 14.0 | 11.0 | 16.0 | 13.0 | 10.0 | 14.0 | 5.0 | 10.0 | 14 | 10.0 | 16.0 | 13.0 | 13.0 | 9.0 |
| Ntot (mg·g−1·dwt) | 0.5 | 1.2 | 1.4 | 1.6 | 1.9 | 1.4 | 1.1 | 1.6 | 1.3 | 1.0 | 1.4 | 0.5 | 1.0 | 1.4 | 1.0 | 1.6 | 1.3 | 1.3 | 0.9 |
| Ptot (μg·g−1·dwt) | 272 | 249 | 430 | 402 | 536 | 275 | 276 | 331 | 246 | 257 | 362 | 209 | 372 | 334 | 265 | 388 | 414 | 439 | 282 |
| Pinorg (μg·g−1·dwt) | 198 | 200 | 304 | 283 | 344 | 184 | 191 | 218 | 181 | 190 | 227 | 160 | 208 | 206 | 187 | 242 | 258 | 292 | 200 |
| Porg (μg·g−1·dwt) | 74 | 49 | 126 | 119 | 192 | 91 | 85 | 113 | 64 | 67 | 135 | 49 | 164 | 128 | 79 | 145 | 156 | 147 | 83 |
All stations, except for the area north of the bridge which connects Grado with the mainland (st. 17–19), showed oversaturation. However, no relevant peaks of chlorophyll-a were found (maximum values 1.8 μg·dm−3 at st. 3 and 5).
Discussion
Flora and biomass changes
The small amount of existing data on macrophytes did not permit accurate appraisal of the temporal changes occurring in the Marano and Grado Lagoon over a long period, such as for the lagoon of Venice and other coastal areas of the North Adriatic Sea (Falace 2000; Falace & Bressan 2003; Curiel et al. 2004; Falace et al. 2005; Ceschia et al. 2007; Sfriso & Curiel 2007; Sfriso & Facca 2007). Some citations concerning macroalgae date back to the early 1970s (Giaccone 1974), but no checklist or quantitative data were supplied. The first floristic studies were produced in the late 1970s by Corvi (1977–1978) (Table 5). During that period the areas north of the Grado bridge were colonized by widespread meadows of C. nodosa, N. noltii and Ruppia cirrhosa (Petagna) Grande, together with stands of C. barbata and Fucus virsoides. Between the late 1980s and the early 1990s, as described also for the Venice Lagoon (Curiel et al. 2004; Sfriso & Facca 2007), abnormal growth of nuisance macroalgae and macrophyte assemblage shifts occurred, most likely due to nutrient enrichment. In particular, in the Grado basin, Curiel et al. (1998) highlighted a significant reduction of angiosperms, which were replaced by large amounts of Ulvales (mean biomass 2–4 kg·m−2·fwt, with spring–summer peaks of 5–6 kg·m−2·fwt). Concurrently, the Marano basin was characterized by blooms of Ulvaceae, Gracilariaceae (Gracilaria gracilis and Gracilariopsis longissima) and Cladophoraceae (mostly Chaetomorpha spp.), with mean biomasses of 4–5 kg·m−2·fwt. During that period the meadows of Z. marina, C. nodosa and N. noltii were restricted to areas close to the sea inlets. However, in spite of these marked changes, during the samplings performed in 1992–93 in different seasons, Curiel et al. (1998) recorded a higher number of species than Corvi (1977–1978), and no Xantophyceae (Table 5).
| Ulvophyceae | Rhodophyceae | Phaeophyceae | Xanthophyceae | Liliopsida | total | |
|---|---|---|---|---|---|---|
| 1977–78 (Corvi 1977–1978) | 10 | 11 | 2 | 1 | 3 | 27 |
| 1992–93 (Curiel et al. 1998) | 16 | 18 | 6 | 0 | 4 | 44 |
| 2007 (present study) | 22 | 40 | 4 | 1 | 3 | 70 |
In the present study, the macrophyte qualitative and quantitative data in both the lagoon basins highlight the presence of low-diversity settlements. Furthermore, in the Marano basin on shallow muddy bottom, previously covered by thick biomasses of Ulvaceae, a great increase of V. submarina was detected, similar to what was already observed in the Venice Lagoon. The biomasses recorded in all 19 sampling sites were lower than in the early 1990s (Curiel et al. 1998). The dominant species are still Ulvaceae, but with a maximum standing crop of 3 kg·m−2·fwt and a medium of 0.5–1.3 kg·m−2·fwt. Moreover, north of the Grado bridge, angiosperms completely disappeared, except for the first part of the main channel characterized by high water turnover, where some narrow beds of C. nodosa and Z. marina survive. At present, canopy-forming species such as Cystoseira spp. and F. virsoides and the extended Ulvaceae beds, recorded by Corvi (1977–1978) and Curiel et al. (1998), respectively, are lacking and only V. submarina colonizes the muddy sediments of the channel boundaries of this area, but with a mean biomass lower than 0.1 kg·m−2·fwt. The reason of these significant changes observed north of the Grado bridge is unknown and is the object of studies in progress.
The species recorded in this study, even if restricted to the spring–summer period, noticeably increase the floristic check-list of these lagoon basins (Table 1). However, the total number of macrophytes in the Marano and Grado Lagoon is very low when compared with the Venice Lagoon flora (c. 280 species, Sfriso & Curiel 2007), in spite of their similar surface area and features, probably because of the almost total absence of suitable hard substrata. Moreover, the reduced presence of non-autochthonous species, compared with their spread in other Italian transitional waters such as Venice Lagoon or Mar Piccolo at Taranto (Cormaci et al. 2004; Curiel et al. 2006a) may be related to the absence of big commercial and tourist harbours and the limited aquaculture activities, which are likely to be the main causes of their introduction.
The general decrease of macroalgal biomasses and angiosperm stands noticed in the Marano and Grado basins is in accordance with what is occurring in the Venice Lagoon, where the coverage and biomass production are approximately one order of magnitude lower than in 1980 (Miotti et al. 2007; Sfriso & Facca 2007). Furthermore, in Venice and Marano and Grado Lagoons the biomass reduction observed is coupled with an increase in the total number of species, although it is important to underline that variations of the number of recorded species may often be difficult to interpret. The differences noticed may depend on ‘natural factors’ (e.g. diversity of habitats or environmental gradients) but also on sampling methods, specific systematic knowledge, and nomenclatural discrepancies. Moreover, as there are strong connections between the sampling scale and the processes which influence macroalgal biodiversity, there are further difficulties connected with the comparison of species lists concerning the definition of sampling areas, which makes reproducibility of monitoring at different times problematic (Falace 2000; Falace et al. 2005; Ceschia et al. 2007).
Similar patterns of macrophyte assemblage changes have been reported for several shallow lagoons in the Mediterranean region (i.e. Sacca di Goro, Orbetello, Valli di Comacchio), which until the mid-1970s were characterized by widespread Ruppia spp. and Zostera spp. meadows (Viaroli et al. 2008). More recently, human pressures and nutrient loads have caused the loss of angiosperms and their replacement by bloom-forming macroalgae (mainly Ulva spp. and Gracilariopsis/Gracilaria spp.) or mixed communities of nanoplancktonic green algae and cyanobacteria, with a general impoverishment in ecosystem components and loss of complexity (Bombelli & Lenzi 1996; Andreoli et al. 1998; Piccoli 1998; Viaroli et al. 2006).
Water body identification
A first screening of the main water bodies of the Marano and Grado basins was obtained by elaboration of the environmental variables. The cluster analysis separates the sampling areas into two main groups (Fig. 6): one that associates stations 3–5, 8, 12, 14, 15, 17–19, characterized by a higher trophic level and high fine sediment content (on average 93.1%) and another that grouped stations 1, 2, 6, 7, 9, 10, 13, 16 and 20, which exhibited lower nutrient concentrations and lower fine sediment (on average 71.4%). However, because the physico-chemical data refer to one summer sampling, that classification was further improved taking into consideration the distribution of angiosperms and, except for V. submarina, the absence of macrophytes. The final result is plotted in Fig. 7, which subdivides the Marano and Grado Lagoon into four main water bodies: (i) marine areas, (ii) mesotrophic areas, (iii) eutrophic areas, and (iv) confined and polluted areas. This preliminary description is similar to that obtained in the Venice Lagoon by Solidoro et al. (2004), who analysed the temporal and spatial changes of the most important water quality parameters. Those data confirm and provide evidence of the key role played by the lagoon tributaries and the sea-water exchanges in the determination of the ecological status of the Marano and Grado Lagoon.
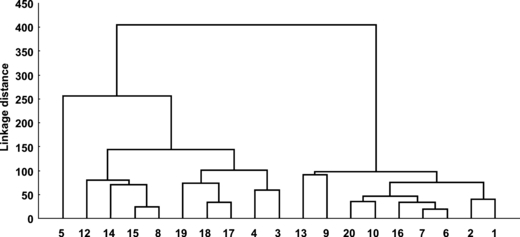
Hierarchical cluster dendrogram (Euclidean Distance) of the chemico-physical parameters of the water column and surface sediments.
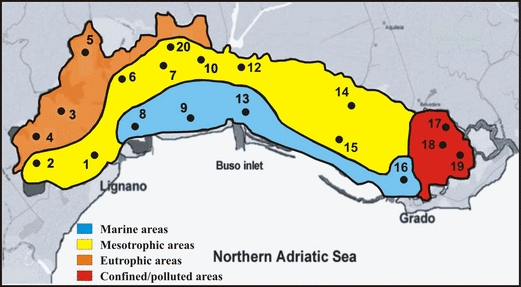
Identification of the main water bodies, by the analysis of the environmental parameters integrated with the macrophyte distribution.
Ecological status evaluation
The EEI and R-MaQI indices used to assess the ESC provided similar results in the ‘High–Good’ classes but differed in part in the lower quality classes. In particular, areas with few species and low coverage are classified by the EEI as ‘Moderate’, while the R-MaQI considers them ‘Bad’ or ‘Poor’ (e.g. st. 17 and 18). The divergences observed are basically due to the different structure of the two indices. The EEI is a quantitative index based on the percentage of macrophyte coverage, with two morpho-functional groups distinguished: the foliose, filamentous and coarsely branched upright genera (most of them are r-selection species) and the perennial well-structured genera (mainly k-selection species). As already observed for the Venice Lagoon (Curiel et al. 2006b; Sfriso et al. 2007), the EEI presents numerous ESG II species but few species belonging to ESG I group. The latter is composed of marine angiosperms, with a coverage up to 100%, some Corallinaceae, which cover the angiosperm leaves, and a reduced number of other species. However, some species which are inserted into ESG I group, such as Sargassum muticum (Yendo) Fensholt, Undaria pinnatifida (Harvey) Suringar and C. barbata, in the Venice Lagoon are common in hypertrophic and polluted waters. In contrast, some species characteristic of marine areas in the lagoon inlets where the environmental conditions are good, such as those belonging to Rhodophyceae: Bonnemaisonia asparagoides (Woodward) C. Agardh, Ceramium cimbricum, Palisada patentiramea, Hypnea musciformis and the Phaeophyceae: Dictyopteris polypodioides (A.P. De Candolle) J.V. Lamouroux and Cladosiphon zosterae (J. Agardh) Kylin, are inserted in the group ESG I. These results raise doubts of their accuracy when assessing the environment quality simply based on the environmental assessment on macrophyte morphology.
The R-MaQI is a qualitative index based on the presence/absence of indicator species on the grounds of expert judgment and the sensitivity of each species/community to pollution and/or to different environmental stressors. The index was calibrated together with the E-MaQI (Expert Macrophyte Quality Index) in many Italian transitional environments and validated in 20 stations of the Venice Lagoon, where many environmental parameters and nutrient concentrations in the water column and surface sediment were recorded monthly for 1 year. The index was further validated in five stations, corresponding to the five classes of ecological status established from the WFD (2000/60/CE), where metal and organic pollutant concentrations in surface sediments were also available (Sfriso et al. 2009).
In the presence of a low number of species or in the absence of macrophytes, the determination of some environmental parameters such as oxygen availability and sediment grain-size is also taken into consideration. For example, for stations 17 and 18, classified by the EEI index as ‘Moderate’, the assignment by the R-MaQI index to the class ‘Bad’ is in accordance with the physico-chemical parameters recorded in the same stations.
However, despite the different appraisal of the areas of low quality obtained with the two indices, the general classification of the lagoon is in agreement with the main existing human pressures that exist in these basins.
As data series monitoring for benthic macrophytes are not available for the Marano and Grado Lagoon, the results of this study have to be confirmed by long-term investigations on different scales. One of the main constraints of using benthic macrophytes as a quality element is the high temporal and spatial variability of communities between and within habitats (Orfanidis et al. 2008; Viaroli et al. 2008). The ESC assessment of the Marano and Grado Lagoon using EEI and R-MaQI refers to the spring–summer flora, but it is worth mentioning that the macrophyte assemblages present a seasonal variability that is particularly evident in the unstable environment of the North Adriatic Sea (Falace 2000; Falace & Bressan 2003; Falace et al. 2005). Additional studies, carried out in different seasonal periods, are necessary to obtain a more comprehensive characterization of the flora. Furthermore, the seasonal variations may be more evident in the critical zones classified as ‘Poor’ or ‘Bad’, as for example on shallow bottoms close to the fresh-water outfalls or in confined areas where, in the absence of the most stable late-successional species (i.e. angiosperms), the opportunistic ones (i.e. Ulvaceae or Gracilariaceae) may cyclically dominate. These episodic blooms may be the result of a complex interplay of several ecological factors, such as long-term changes in abiotic parameters (i.e. nutrients, light, currents, sedimentation rates and sand movements), which, in their turn, are related to general climatic changes (i.e. winds of Bora or Scirocco, rainfalls, etc.). On this basis, an accurate assessment of the ES requires continuous monitoring, over several years, to better appreciate the dynamics and the biological characteristics of this lagoon.
This is the first study aiming at evaluating the ecological state of the Marano and Grado Lagoon. To date, no water bodies or surveillance sampling sites have been selected by the Friuli-Venezia Giulia Regional Environment Protection Agency (ARPA-FVG). Considering the similar results obtained by the analyses of the macrophytes and the proposed subdivision in main water bodies, we suggest selecting our sampling sites for the future institutional (ARPA-FVG) macrophyte monitoring. Obviously, they can be integrated with additional sampling sites if necessary, especially in the critical areas where the environmental changes are the greatest.
Conclusions
The data presented in this paper indicate that a great part of the transitional environments of the Marano and Grado Lagoon hosts a quite poor marine flora. Furthermore, the absence of strong economic and tourist development almost completely prevents the introduction of non-autochthonous species.
Although the literature is scarce, the previous floristic data confirm that the Marano and Grado Lagoon was subject to long-term changes in species richness, biomass and distribution, as observed in the Venice Lagoon (Sfriso & Facca 2007) and in the coastal area of the Gulf of Trieste (Falace 2000; Falace & Bressan 2003; Falace et al. 2005; Ceschia et al. 2007). After an increase of eutrophic species, mainly belonging to Ulvaceae and Gracilariaceae, that occurred in the 1990s (Curiel et al. 1998), the lagoon was affected by a reduction of these stands and an increase in the number of species. However, this environmental improvement was not observed in the northern part of the Grado basin, where macrophytes are almost completely absent.
By the analysis of some water column and surface sediments parameters, coupled with the macrophyte distribution, four main water bodies have been identified: (i) marine areas characterized by coarse sediments prevalently colonized by C. nodosa and Z. marina; (ii) eutrophic areas characterized by relatively high nutrient concentrations and fine sediments; (iii) mesotrophic areas which present intermediate conditions and (iv) confined and polluted areas lacking of vegetation (with the exception of Vaucheria).
The first results, obtained by applying the available indices for the ecological state of transitional environment assessment according to WFD, show that the Marano and Grado Lagoon has a high environmental differentiation, with areas ranging from ‘Poor–Bad’ conditions near the river outflows and north of the Grado bridge to ‘Good–High’ conditions in the areas colonized by angiosperms.
At present, further sampling campaigns are in progress to verify the main results of this work, to better define the water bodies and also to apply the Rhodophyta/Chlorophyta ratio (Sfriso et al. 2002; Curiel et al. 2004) and the Expert-MaQI (Sfriso et al. 2007, 2009). These indices set up and validated in the Venice Lagoon have been calibrated in many Italian lagoons (Sfriso et al. 2009). Nevertheless, an official index for the assessment of the transitional environment ES according to the WFD, which is acknowledged by the National Agencies (ARPA) and/or by the European Community, is not yet available.
Acknowledgements
The authors thank Dr Giorgio Mattassi of the ARPA Friuli-Venezia Giulia and all his staff for the logistic facilities. Thanks are also addressed to Dr Chiara Facca and Sonia Ceoldo for their technical assistance and facilities.




