Occurrence and distribution pattern of Palaemon spp. shrimps in a shallow submarine cave environment: a study case in South-eastern Italy
Abstract
The common shrimps of the genus Palaemon are often reported as living in Mediterranean submarine caves. The present study quantifies the distribution pattern of a Palaemon spp. populations throughout 1 year of observations in a shallow submarine cave in South-eastern Italy, Ionian Sea. The cave was subdivided into six sectors from the entrance towards the inner portion of the ‘blind cave’ and 16 dives (four per season, during both day and night) were performed with SCUBA equipment. The population was estimated in situ using visual census. Four different species of Palaemon were identified. This study has shown that Palaemon spp. assemblages live throughout the year in submarine caves. Moreover, the results suggest submarine caves play an important role in the biology of Palaemon spp. populations, by providing refuge, juvenile recruitment and food resources.
Problem
Submarine caves are peculiar habitats of rocky coasts and represent an important shelter for many sessile (Balduzzi et al. 1989; Bussotti et al. 2006; Riedl 1966; Denitto et al. 2007) and vagile (Gili & Macpherson 1987; Bianchi et al. 1988; Zander 1990; Bussotti et al. 2002) organisms, which find favourable conditions to survive and spawn.
Although crustaceans are typical inhabitants of Mediterranean submarine caves, the only available information is mostly qualitative and limited to decapod species (Gili & Macpherson 1987; Bianchi et al. 1988; Pessani & Manconi 1994; Pastore & Denitto 2002; Manconi & Pessani 2003).
Within decapods, Palaemon serratus Pennant 1777 represents the only Palaemonidae found in these coastal environments (Gili & Macpherson 1987; Bianchi et al. 1988; Pessani & Manconi 1994; Arko-Pijevac et al. 2001; Manconi & Pessani 2003; Oertel & Patzner 2007). By contrast, Palaemon species are frequently observed in different habitats in the Mediterranean Sea and along the European Atlantic coasts (Zariquiey Álvarez 1968), such as Posidonia oceanica meadows (Fresi et al. 1984; Zupi & Fresi 1985), shallow bays (Guerao 1995; Guerao & Ribera 1996; Łapiñska & Szaniawska 2006) and estuaries (Attrill & Thomas 1996; González-Ortegón et al. 2006). For a long time, palaemonids have been described by many authors as omnivorous, feeding on a wide variety of benthic organisms including large quantities of organic detritus and sand (Berglund 1980; Marchand 1981; Sorbe 1983; Figueras 1986). During the last three decades, several papers dealing with trophic relationships among marine organisms have been published (Zupo & Mazzocchi 1998 and references therein). In particular, thanks to improvements in research on the food webs in several coastal ecosystems, most of the Palaemon species have been studied in detail, revealing specific and very different feeding habits (Guerao & Ribera 1996). For example, Palaemon xiphias Risso, 1816 is one of the best represented species in the food web of the eelgrass Cymodocea nodosa (Guerao 1995) and the seagrass P. oceanica (Fresi et al. 1984; Zupi & Fresi 1985). It is important not only from an ecological standpoint, but also from an economic one, as it is intensively fished in several Mediterranean regions (Fresi et al. 1984). Fresi et al. (1984), Zupi & Fresi (1985) and Guerao (1995), through examination of the gut contents of P. xiphias, demonstrated its nocturnal predation of both slow-moving and highly mobile benthic invertebrates such as small decapods, copepods and amphipods.
Among the most cited and well-known palaemonid species, P. serratus is a very common and widespread Atlanto-Mediterranenan shrimp (González-Ortegón & Cuesta 2006). It lives in a large variety of environments and its diet consists mainly of benthic invertebrates such as molluscs and crustaceans (Guerao & Ribera 1996). As with many other Palaemon species (see Berglund 1980; Fincham & Furlong 1984; Zupi & Fresi 1985), P. serratus shows circadian predatory activity with a peak at night (Guerao & Ribera 1996). By contrast, Palaemon elegans, a common shrimp living in tidal rockpools and in seagrasses (González-Ortegón & Cuesta 2006; Bilgin et al. 2008), is a generalist, feeding on algae, small vagile prey, detritus and sand grains (Köhn & Gosselck 1989).
Palaemon spp. also represent the majority of decapods living in estuaries (Cuesta et al. 2006). In these transitional waters, they become the prey of many fish species (Dauvin & Desroy 2005; Sa′et al. 2006) and are one of the most important components of the trophic structures in these ecosystems (Attrill & Thomas 1996; Dauvin & Desroy 2005).
To date, there are no detailed ecological studies of Palaemon spp. in coastal submarine caves. Salento Peninsula (SE Italy) is one of the most important marine ‘cave hot-spots’ in the Mediterranean basin. More than 77 submarine caves have been explored to date along the Salento coast (see Denitto & Belmonte 2008 and references therein).
The aim of the present study is to quantify, for the first time, the occurrence and distribution pattern of Palaemon spp. over an annual cycle of observations in a shallow submarine cave in SE Italy, Ionian Sea. A list of crustacean decapods found in the cave during the year of investigation is also provided.
Material and Methods
Study area
The study site (Fig. 1) was the shallow submarine cave ‘Grotta di Ciolo’, located in the Salento Peninsula (SE Italy, Ionian Sea, Mediterranean Sea) (39°50′38′′ N; 18°23′11′′ E). The ‘blind cave’ (sensuHarmelin 2000) is a simple horizontal tunnel approximately 120 m long and 5 m wide, with a single semi-submerged entrance and air chambers along the tunnel. The bottom is characterised by pebbles (from the entrance to 40 m) and thin sand (from 40 m to the innermost part). The biology of the cave has been recently widely investigated by several authors (Bussotti et al. 2002, 2006; Pastore & Denitto 2002; Todaro & Shirley 2003; Denitto & Licciano 2006; Todaro et al. 2006; Denitto et al. 2007; Moscatello & Belmonte 2007).
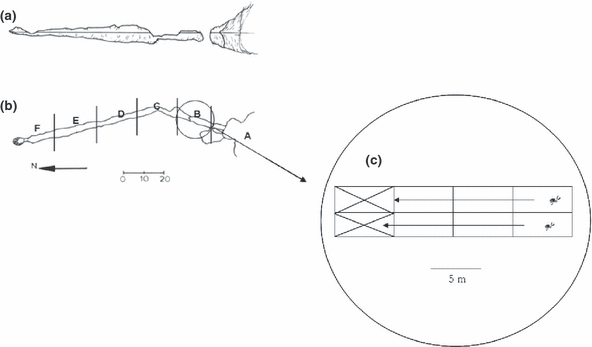
(a) Longitudinal section of the cave (39°50′38′′ N; 18°23′11′′ E); (b) plant of the cave divided into six sectors (A–F); and (c) schematic representation of one sector subdivided into eight areas (= replicates) 5 m × 2 m each. For statistical reason, the last two areas in each sector were ignored during the samplings.
Sampling design and analysis of samples
A modified ‘strip-transect’ visual census method (Bussotti et al. 2002, 2003) was adopted to investigate the distribution pattern of Palaemon spp. shrimps inside the cave ‘Grotta di Ciolo’. The cave has been subdivided into six ‘Sectors’ (A–F) approximately 20 m long, from the entrance towards the inner portion of the cave.
As only through laboratory observation has it been possible to detect the different species, the statistical analysis was performed to investigate the complete Palaemon spp. population dynamics and distribution. In each cave sector, censuses of palaemonid shrimps were taken along each side of the rocky wall (with eight replicates per sector, 5 m long × 2 m wide each). To avoid spatial autocorrelation among replicate units in different sectors, the last two replicates within each sector were ignored and only six replicates per sector were considered (n = 6 in the statistical analysis; see Fig. 1C for a better description of the design). Sampling was performed during the four ‘seasons’ (S1 = Autumn 2002; S2 = Winter 2002/2003; S3 = Spring 2003; S4 = Summer 2003). Twelve SCUBA dives (‘Times’ as a factor in the expected design) – one per month, at midday – were performed with 12 SCUBA divers.
A second experimental design was used to evaluate the spatio-temporal distribution of the palaemonid shrimps at night. Four dives (one per season, at midnight) were performed by SCUBA divers using the same procedure of the first experimental design.
For the species identification in the laboratory, some specimens of different size were caught at each dive using a hand-towed net, stocked in plastic bottles and fixed in 70% ethanol immediately after collection. The same method was used for other crustacean specimens collected during the dives.
Abiotic variables of the water layer near the bottom such as temperature, light, salinity, and conductivity were seasonally measured using a multiparametric portable probe at each of the three cave sectors (A, C, E, as referred to in Fig. 1C). Finally, hydrodynamics along the cave axis was evaluated in May 2003 on the basis of the plaster balls technique (Muus 1968). These plaster balls were positioned in three sectors (A, C, and E, as mentioned above) which represented the entrance, the central and the inner sectors of the cave, respectively. In the selected sectors three replicates each consisting of three plaster balls were distributed at a distance of about 5 m from each other along a longitudinal transect.
Data analysis
The experimental design for the diurnal investigations consisted of three factors: ‘Season’ = S, four levels, fixed; ‘Time’ = T, three levels, random and nested within ‘Season’; and ‘Sector’ = Se, six levels, fixed and crossed to ‘Season’ and ‘Time’, with n = 6 per combination of factors, for a total of 432 observation units. A three-way ANOVA was used to assess differences in the mean abundance of the Palaemon spp. populations among ‘Times’ and ‘Sectors’ within the cave during the day.
A two-way ANOVA was used to assess differences in the mean abundance of Palaemon spp. among ‘Times’ and ‘Sectors’ within the cave during the night. This second experimental design consisted of two factors. In this case, the analysis considered the factor ‘Season’ (four levels) as random, and ‘Sector’ (six levels) as fixed and crossed to ‘Season’, with n = 6 per combination of factors for a total of 144 observation units.
Two-way ANOVA was performed to assess differences in hydrodynamics (consumption of plaster balls) among sectors (fixed, orthogonal factor), and among areas (random, nested in sectors). Prior to analyses, the homogeneity of variance was tested using Cochran’s test and data were appropriately transformed, if necessary. The Student–Newman–Keuls’ (SNK) test (P < 0.05) was used for post hoc comparisons of the means (Underwood 1997). All ANOVAs were performed using the GMAV5 package from the University of Sydney.
Results
Table 1 shows the hydrological features of the sampling sectors. The temperature of the investigated water layer ranged from a minimum of 14.71 °C in S2 at sector A to a maximum of 23.91 °C in S4 at sector C. Depending on seasonal differences in runoff, salinity ranged from 35.6 ppt in S2 at sector E to 39.32 ppt in S4 at sector A. The highest value of pH (8.28) was recorded in S1 at sector A, and the maximum photosynthetically active radiation (PAR) (2755 μW·cm−2) occurred in S4 at the same sector.
| Abiotic factors | S1 | S2 | S3 | S4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | C | E | A | C | E | A | C | E | A | C | E | |
| Temperature (°C) | 18.62 | 18.82 | 18.81 | 14.71 | 14.78 | 14.97 | 15.82 | 15.86 | 15.97 | 23.87 | 23.91 | 23.53 |
| Conductivity (mS·cm−1) | 49.73 | 49.65 | 49.28 | 45.29 | 44.93 | 43.51 | 47.47 | 47.45 | 45.91 | 57.54 | 57.47 | 56.78 |
| pH | 8.28 | 8.24 | 8.14 | 8.26 | 8. 23 | 8.00 | 8.16 | 8.16 | 8.15 | 8.19 | 8.19 | 8.15 |
| Salinity (ppt) | 37.72 | 37.47 | 37.17 | 37.45 | 37.06 | 35.60 | 38.38 | 38.33 | 36.86 | 39.32 | 37.06 | 37.45 |
| P.A.R. (μW·cm−2) | 2322 | 658 | 0 | 2441 | 1072 | 0 | 2060 | 595 | 0 | 2755 | 1116 | 0 |
Overall, 13 species of decapod crustaceans were collected (Table 2) during the study period. Some, such as Gnathophyllum elegans, Lysmata seticaudata, Ilia nucleus, Achaeus cranchii and Macropodia rostrata, were represented by only one or a few individuals, and some of them were apparently related to particular seasons: G. elegans and L. seticaudata were collected during the cold period (S1 and S2), whereas single specimens of I. nucleus and M. rostrata were found in spring (S3).
| Decapod species | Sector | Season | Notes |
|---|---|---|---|
| Stenopus spinosus Risso 1827 | C, D | S3, S4 | On the rocky walls, ♀ ov. |
| Gnathophyllum elegans (Risso 1816) | A | S2 | Occasional, under the pebbles |
| Palaemon elegans Rathke 1837 | B, C, D, E, F | S4 | Frequent |
| Palaemon longirostris H. Milne-Edwards 1837 | B, C, D, E, F | S2, S4 | Frequent |
| Palaemon serratus (Pennant 1777) | C, D, E, F | S2, S3 | Frequent ♀ ov. |
| Palaemon xiphias Risso 1816 | B, C, D, E, F | S1, S2 | Frequent |
| Lysmata seticaudata (Risso 1816) | A, C | S1, S2 | Occasional |
| Dromia personata (Linnaeus 1758) | B, C, D | S1, S2, S3, S4 | On the rocky walls, ♀ ov. |
| Ilia nucleus (Linnaeus 1758) | A | S3 | One specimen, during night diving |
| Achaeus cranchii Leach 1817 | C | S1, S2, S3, S4 | Occasional |
| Macropodia rostrata (Linnaeus 1761) | E | S3 | One specimen |
| Herbstia condyliata (Fabricius 1787) | B, C, D, E | S2, S3 | Frequent, on the rocky walls, ♀ ov. |
| Herbstia nitida Manning & Holthuis 1981 | B, C | S2, S3 | Under the pebbles |
| Xantho pilipes A. Milne-Edwards 1867 | A | S1, S2, S3, S4 | Frequent |
Palaemon spp., Herbstia condyliata, and Xantho pilipes were the strongly predominant species in the entire periods of investigation. The occasional and not specifically directed observation of the two latter species, did not allow us to investigate any correlation between them and the abiotic data recorded.
Focusing on the Palaemon genus, four species were identified: P. serratus in Winter (S2) and, mainly, in Spring (S3); Palaemon longirostris caught in Winter (S2) and Summer (S4); Palaemon xiphias identified in Autumn (S1) and Winter (S2); and P. elegans found only during Summer (S4). With the exception of P. serratus, this is the first time that the other three Palaemon species have been reported in a submarine cave. All these four Palaemon species are marine, euryhaline, and eurythermal (Köhn & Gosselck 1989; González-Ortegón & Cuesta 2006; González-Ortegón et al. 2006).
Palaemonid specimens were found in large amounts inside the cave during all sampling periods, both during day and night, mainly near the bottom. Overall, abundance values ranged from a few to 14 individuals 10 m−2 (peak of abundance during Summer) (Fig. 2). Palaemon elegans juveniles about 10 mm long occurred during Spring and Summer and large schools of them were observed mainly in the most internal cave sector.
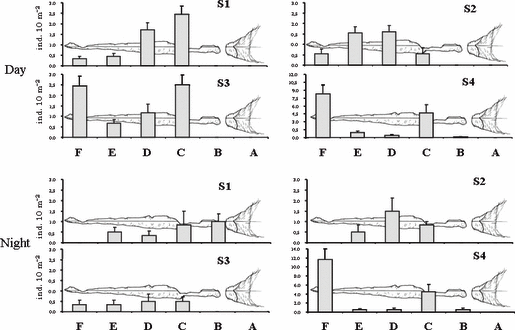
Mean abundances (SE) of Palaemon spp., during both the day and the night, at the different sectors of the cave and in the four seasons. S1 = Autumn 2002; S2 = Winter 2002–2003; S3 = Spring 2003; S4 = Summer 2003.
The ANOVA performed on the diurnal quantitative data revealed the significance of the ‘Season × Sectors’ interaction term (Table 2), indicating that differences in time are not consistent across seasons. The SNK test revealed significant differences among sectors in each season; indeed, the same test shows that significantly lower values occurred in the outermost sectors (A, B) throughout the year, whereas the higher values occurred in the central (C, D) and innermost sectors (E, F) (Table 3). Juveniles appeared in large numbers in Spring (S3) and, mainly in Summer (S4), and they occurred mostly in the innermost and darkest sector of the cave (F) (Fig. 2). Thus, juvenile recruitment appeared to be the most important process influencing temporal changes in the density of palaemonids inside the cave. The number of adult specimens also increased during the same seasons, although their spatial distribution mainly affected the central sector of the cave (C).
| Source of variation | DF | MS | F | P | F versus |
|---|---|---|---|---|---|
| S | 3 | 65.6111 | 2.88 | 0.1030 | T(S) |
| T(S) | 8 | 22.7870 | 5.99 | 0.0000 | Residual |
| Se | 5 | 109.7537 | 8.38 | 0.0000 | Se × T(St) |
| S × Se | 15 | 49.3796 | 3.77 | 0.0004 | Se × T(S) |
| Se × T(S) | 40 | 13.0981 | 3.44 | 0.0000 | Residual |
| Residual | 360 | 3.8065 | |||
| Total | 431 |
| Cochran’s test (Transform = none) | SNK test | |
|---|---|---|
| n.s. | S1 | A = B < E = F < C = D |
| S2 | A = B < C = F < D = E | |
| S3 | A = B < E = F < C = D | |
| S4 | A = B < D = E < C < F | |
- Reported are: T, time; Se, sector; S, season; P < 0.001, n.s., not significant.
ANOVA performed on nocturnal samplings (Table 4) indicates that differences across sectors were not the same at all times. Fairly similar temporal trends between diurnal and nocturnal collections were observed (Fig. 2). Also nocturnal quantitative data reflect similar mean abundances found during the diurnal observations, with the exception of S3, when the diurnal mean abundance appears higher than the nocturnal one. The SNK test shows that, at night, specimens (both adults and juveniles) lay inside the cave, and no specimen was ever found at the entrance sector (A).
| Source of variation | DF | MS | F | P | F versus |
|---|---|---|---|---|---|
| S | 3 | 58.6181 | 23.89 | 0.0000 | Residual |
| Se | 5 | 29.7903 | 0.89 | 0.5093 | S × Se |
| S × Se | 15 | 33.3014 | 13.57 | 0.0000 | Residual |
| Residual | 120 | 2.4542 | |||
| Total | 143 |
| Cochran’s test (Transform = none) | SNK test | |
|---|---|---|
| n.s. | S1 | A = F < D = E < B = C |
| S2 | A = B = F < C = E < D | |
| S3 | A = B < C = D = E = F | |
| S4 | A = B = D = E < C < F | |
- Reported are: T, time; Se, section; S, season; P < 0.001, n.s., not significant.
Regarding the abiotic factors measured inside the cave, hydrodynamics displayed dissimilar patterns among sectors and the SNK test reveals that it was significantly higher at the intermediate sector (C) than at the other sectors (A, E) (Fig. 3).
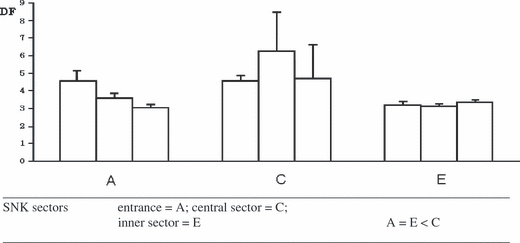
Differences in hydrodynamics (by plaster balls consumption) among three sectors and among areas in each sector. D F: Diffusion Factor; A, C, E: Sectors of the cave as reported also in Figure 1 b.
Discussion
Thirteen species of decapods (Table 2) were recorded in the submarine cave ‘Grotta di Ciolo’ during a year of investigation, but only the palaemonid group was regularly present in numbers sufficiently large to warrant further analysis.
This study provides the first ever data about Palaemon spp. assemblages associated with a coastal submarine cave. Visual counts have only recently been used in marine caves for underwater fish assessment (see Bussotti et al. 2002, 2003) and this method seems suitable to evaluate the abundance of shrimps of the genus Palaemon in marine caves: they were, indeed, easily detectable and did not react too quickly to the SCUBA diver (e.g. they did not flee in front of the lights used by underwater observers).
The distribution pattern of juveniles and adult specimens inside the cave ‘Grotta di Ciolo’ may be explained by two main factors, related to their survival: (i) escape from predators and (ii) food availability.
Escape from predators
Decapod crustaceans are often an important food source for at least 16 fish species (Dauvin & Desroy 2005; Sa′et al. 2006) and mollusc cephalopods (Aguado Giménez & García García 2002). The progressive decreasing presence of predator fishes along the cave axis (Bussotti et al. 2002) and the absence of cephalopods in the inner part of the cave (Authors’ personal observations) could lead Palaemon shrimps to occupy the darkest sectors. They were frequently observed together with the cardinal fish Apogon imberbis (Bussotti et al. 2002, 2003) and, occasionally, with mugilids, Atherina sp. and Oligopus ater (Authors’ personal observation). Juveniles and adults of palaemonid shrimps are not included in the diet of these fish species (Bini 1969; Vizzini & Mazzola 2002). A close investigation reveals that juveniles occupy the inner and confined sector of the cave where abiotic conditions (24-h darkness, low water salinity values, etc.) (Table 1) and long distance from the entrance would reduce the presence of many diurnal and/or stenohaline predators, such as cephalopods (Aguado Giménez & García García 2002) and fish species belonging to Mullidae and Serranidae (Sa′et al. 2006).
Food availability
Most Palaemonidae are considered detritivorous and can live by feeding directly on decomposed organic matter (Ott & Svoboda 1977; Berglund 1980). Köhn & Gosselck (1989) demonstrated that P. elegans is a generalist, feeding on algae (mostly Enteromorpha intestinalis), small crustaceans and fish larvae, detritus and sand grains. Nevertheless, Guerao (1995) and Guerao & Ribera (1996), by analyzing respectively the stomach contents of P. xiphias and P. serratus, revealed a prevalent carnivorous diet based on planktonic prey such as other malacostracans and copepods. Finally, stomach analysis of P. longirostris showed this species feeds mainly on mesozooplanktonic copepods, although it is also able to adapt its diet to the seasonal availability of benthic or planktonic prey (Dauvin & Desroy 2005).
The ‘Grotta di Ciolo’ offers all these feeding sources, as demonstrated by the presence of a zooplanktonic component, benthic prey, and organic detritus carried from outside by tidal currents and waves (Denitto et al. 2007; Moscatello & Belmonte 2007).
Furthermore, no nocturnal migration to the open sea has been observed, contrary to what has been previously noted for other large Decapoda genera such as Plesionika, Palinurus, Scyllarus, and Homarus, which seasonally live in many submarine caves (Ott & Svoboda 1977). This may suggest that Palaemon spp. can find inside the cave all they need to survive for one or more seasons. Intense feeding activity during both day and night (mainly on the bottom but also on the rocky walls of the cave) has been observed. This is in contrast to many observations regarding Palaemon populations which live in several intertidal areas. Previous work, indeed, demonstrated that P. serratus (Guerao & Ribera 1996), P. longirostris (Fincham & Furlong 1984), and P. elegans (Berglund 1980) show a circadian endogenous activity, with peaks at night. This behaviour can change in particular environmental conditions, such as in cloudy water (e.g. in the Bristol Channel, Great Britain) (Rodriguez & Naylor 1972), or in macrophyte meadows growing on the rock pools of tidal areas (Guerao & Ribera 1996). In these habitats, as observed in the submarine cave, a strong feeding activity could be expected at any time of day with low risk of exposure to visual predators.
The submarine cave ‘Grotta di Ciolo’ appears as a ‘passive collector’ of food coming from the open sea. Such a food source is available all day without any predation risk. The particular configuration of this ‘blinded cave’, with the presence of a wide superficial entrance and air chambers along its longitudinal axis, facilitates the canalisation of great water volumes. The water, moved by tidal currents and sea-storms, transfers organic matter such as fragments of macroalgae (Cystoseira spp.), seagrasses (mainly P. oceanica and Zostera sp.), and fragments of sponges scratched from the coastal rocky bottom by the hydrodynamic action. It is, indeed, possible to observe deposits of this matter floating on the bottom inside the cave. The data analysis confirms the highest density of adults in the cave sections where this potential food source is accumulated. This is also confirmed by the results obtained from studies on the recruitment of new cave benthic assemblages conducted in the same biotope and demonstrating that the highest biodiversity of passive propagules is located in the intermediate section (Denitto & Licciano 2006; Denitto et al. 2007). Finally, the distribution pattern of palaemonids reflects the hydrodynamic values found in the cave, which provide evidence of a high water circulation in all sectors, and even higher in the central sectors (Fig. 3).
The presence of adult ovigerous females (probably belonging to P. serratus) and juveniles suggests that the submarine cave ‘Grotta di Ciolo’ is an important spawning and nursery ground not only for several fish species (Bussotti et al. 2002, 2003) but also for invertebrate vagile species living in this coastal biotope.
The seasonal occurrence of the four species of Palaemon may suggest that the site offers shelter alternatively to the four species which frequent the cave throughout the year. It may be that each Palaemon species spends part of its life cycle outside the cave, allowing other congeneric species to occupy the same spatial niches inside the cave in other seasons.
In conclusion, the present paper provides the first quantitative observations on palaemonid assemblages associated with a submerged shallow marine cave in the Mediterranean and suggests a potentially important ecological role of this habitat for some common littoral palaemonids during different phases of their life history.
Acknowledgements
We are indebted to the speleodivers of the Centro di Speleologia Subacquea ‘Apogon’, Nardò (Italy) for their invaluable attendance during diving in the cave. Thanks to A. Terlizzi (University of Salento, Lecce, Italy) for his help in statistical analysis. Thanks as well to M. Pastore (Institute for Marine Coastal Environment – CNR of Taranto, Italy) for the identification of the decapod species and S. Lignier for the English revision of the manuscript.




