Skeletal linear extension rates of the foliose scleractinian coral Merulina ampliata (Ellis & Solander, 1786) in a turbid environment
Abstract
Skeletal linear extension rates of a foliaceous, IndoPacific, skiophilous, heterotrophic, scleractinian Merulina ampliata (Ellis & Solander 1786) were obtained along a sediment/nutrient load gradient at the southern islands of Singapore. Measurements were made during November 1999– November 2000 using the alizarin red-S staining technique. Suspended particulate matter concentration (r2adj = 0.76), turbidity (r2adj = 0.59), the organic content of suspended sediments (r2 = 0.50), and nitrite-nitrate concentration (r2adj = 0.50) were significant predictors of the skeletal linear extension rate of M. ampliata. Maximum linear extension growth rates of M. ampliata (mean ± SD: 1.43 ± 0.67–3.26 ± 0.59 cm·year−1) were comparable to 15-year-old accounts at the same research sites, indicating adaptation to low-light, high-sediment waters.
Problem
Human activities on land (e.g. agriculture, deforestation, coastal development) and at sea (e.g. dredging, aquaculture, municipal/industrial sewage disposal) alter the quality of coastal waters worldwide through the increases in sediments, nutrients, pollutants, and pathogens (GESAMP 2001; Hassan et al. 2005). In tropical and subtropical regions, skeletal growth of zooxanthellate scleractinian corals (linear extension, bulk density or calcification) has been used as a promising sublethal indicator of reef health (Dodge et al. 1974; Bak 1978; Tomascik & Sander 1985). In fact, coral skeletons can provide a record of the environmental conditions at the time of growth (Scoffin et al. 1989), while their growth integrates a number of physiological processes through the scope for growth (Brown & Howard 1985). However, following short-term or prolonged change in environmental conditions, some scleractinian coral species showed change in some but not all growth characteristics (Dodge & Brass 1984); divergent changes among growth characteristics (Edinger et al. 2000; Koop et al. 2001), or no change in any of their growth characteristics, despite evidence of marked changes in coral cover and diversity (Hudson et al. 1982; Brown et al. 1990; Chansang et al. 1992). It seems that the ‘sensitivity’ of scleractinian corals’ growth to environmental alteration varies with species and location and that ‘… A considerable amount of work is needed before we can begin to understand and, hopefully, predict which growth characteristic of which species is likely to respond to a given environmental change in a particular location…’ (Lough & Barnes 1997).
The variability in the response of skeletal growth of scleractinian corals to increases in terrestrially derived material, nutrients and sediments in particular, may reflect the differential importance of the four agents affecting coral growth: (i) dissolved inorganic nutrients; (ii) particulate organic matter; (iii) sedimentation; and (iv) turbidity, along with spatial, physical, hydrodynamic and biological properties of coral reefs, sediment type/quality, and frequency of sediment impact (reviews by Brown & Howard 1985; Pastorok & Bilyard 1985; Rogers 1990; Brown 1997; Fabricius 2005; Kruzic & Benkovic 2008). Furthermore, we now know that scleractinian corals can acclimatize/adapt to increased sediment and nutrient loads through morphological (e.g. changes in coral shape, form, and orientation, Todd 2008), physiological (e.g. changes in photosynthetic apparatus, Titlyanov 1981) and feeding plasticity (e.g. change in dependence on heterotrophy, Anthony & Fabricius 2000) or interactions among them (Anthony & Hoegh-Guldberg 2003; Hoogenboom et al. 2008) in order to optimize energy acquisition. Finally, up to date the bulk of anthropogenic impact studies on coral growth involved only a few massive (mainly of the genus Porites in the Indo-Pacific and of Montastrea annularis in the Caribbean) and branching species (mainly of the genus Acropora), whose abundance/cover distinguishes them as primary reef builders on reefs. The interplay of the aforementioned factors impedes the identification of widely applicable, exclusive, and ‘sensitive’ coral species and growth parameters when assessing the effects of increased nutrients and sediments. Consequently, we still have only a tentative speculation on threshold limits to coral growth under sediment and nutrient stress (Hawker & Connell 1989).
The reefs of the southern islands of Singapore are used as a case study to assess and evaluate the chronic effects of increase in sediment and/or nutrient loads on skeletal linear extension of the scleractinian coral Merulina ampliata (Ellis & Solander, 1786). Merulina ampliata, a potentially large (1–2 m in diameter) foliaceous species, is a widely distributed scleractinian on many Indo-Pacific coral reefs (Veron & Stafford-Smith 2000). The objectives of the study were to (i) determine whether the skeletal linear extension rate of M. ampliata varies among stations along a sediment/nutrient load gradient and (ii) relate skeletal linear extension rate to environmental variables along the gradient, particularly those generally thought to affect coral skeletal growth.
Material and Methods
Study sites
Coral reefs off the main island of Singapore (latitudes 1o09′ N and 1o29′ N, longitudes 103o36′ E and 104o25′ E; ≈ 3.5 million people; Fig. 1) have been subjected to increased sediment load during the last 40 years mainly due to reclamation and dredging activities. The sources of sediment load in the waters surrounding the reefs of the southern islands of Singapore are multiple, mobile and extend their effects at the spatial scale of a few kilometres. They have led to an increase in sediment load and, possibly, eutrophication, and a decrease in water clarity and light penetration (Dikou & van Woesik 2006). In the last 30 years a 30-fold increase in primary productivity has been found despite levels of nutrients remaining largely unaltered (Gin et al. 2000).
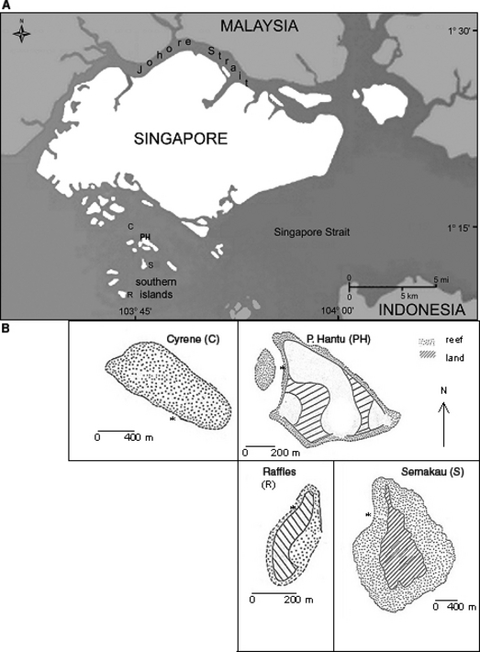
Map of the Republic of Singapore, the southern islands and studied reef sites. C, 01° 15′ 26′′ N, 103° 45′ 12′′ E; S, 01° 12′ 10′′ N, 103° 45′ 16′′ E; PH, 01° 13′ 35′′ N, 103° 44′ 48′′ E; R, 01° 13′ 36′′ N, 103° 44′ 53′′ E.
In the present work we studied four reefs at increasing distances from the main island of Singapore (Fig. 1). The Cyrene patch reef (C, 4.5 km distance) is situated in the middle of a fairway of maritime vessels proceeding to and from the nearby port, with petro-chemical and industrial facilities; ship groundings have been reported. The Pulau Hantu fringing reef (PH, 7.5 km distance) was reclaimed in 1974, losing most of its reef flat and obtaining a rock wall; today it is used by recreational SCUBA divers. The Semakau fringing reef (S, 8 km distance), which was inhabited till the mid-1970s, has functioned as earth spoils and solid waste dumping ground since 1990s; it is also used for subsidence fishing and reef collecting. The Raffles lighthouse fringing reef (R, 15 km distance), was reclaimed in 1976; most of its original reef flat was covered and a rock wall was build. No Acanthaster planci outbreaks have been reported on these reefs. Bleaching events occurred during the summers 1998 and 2002. Sampling sites of environmental variables, growth rates and benthic cover were uniformly set along the west coast of the four reefs studied (Fig. 1).
Environmental monitoring
The studied reefs are under the influence of the southwest monsoon from May to September and of the northeast monsoon from November to March (Chia 1974; Watts 1974). To assess the influence of the monsoons on the main physical seawater variables of the reefs, 10 seawater variables were sampled twice a month (when possible) from April 1999 to November 2000, along the west coast of the four reef sites at 4 m depth. They included physic-chemical factors such as salinity (SAL), temperature (T), and dissolved oxygen (DO) concentration. The sediment load on the studied reefs was assessed through suspended particulate matter (SPM) concentration, the downward flux of suspended particulate matter (DF-SPM) and the light extinction coefficient (K). To evaluate eutrophication, nitrite + nitrate (NO2 + NO3), phosphate (PO4-P) and chlorophyll a (CHLA) concentrations were determined. The volatile portion of suspended particulate matter was used to assess the proportion of suspended sediment that is organic, i.e. the particulate organic matter (POM) concentration. Salinity was measured with an ATAGO hand refractometer. Turbidity was measured with a Secchi disk. The depth (D) of the Secchi disk in metres was used to calculate the light extinction coefficient K (= 1.7/D). Temperature and dissolved oxygen were measured with a portable YSI, model 57, oxygen meter at ≈ 4 m depth. A 2 l-volume Van Dorn sampler was used to collect seawater at ≈ 4 m depth. On deck, the seawater was sub-sampled for dissolved inorganic nutrients (nitrite + nitrate and phosphate), suspended particulate matter, and chlorophyll a. Samples for nutrients were temporarily stored in a cooler box during field trips and subsequently stored in −20 °C until laboratory analysis. Concentrations of nutrients were assessed with a flow injection analyser, model Technicon TRAACS 800 (Technicon Instruments Corporation, Tarrytown, NY, USA). One litre of each seawater sample was used for assessing total suspended solids and 1 l was used to assess chlorophyll a concentration. Suspended particulate matter was measured by filtering 1 l of seawater (n = 2) through a pre-combusted, pre-washed, and pre-weighed Whatman GF/C filter (42.5 mm Ø), drying the filter at 110 °C for at least 1.5 h and weighing. Combustion of the filter at 550 °C for 20 min gave the organic content of total suspended solids (Greenberg et al. 1985). Chlorophyll a concentration was measured following the standard procedure of Parsons et al. (1984). The downward flux of suspended particulate matter was measured with sediment traps (5 cm height × 13 cm diameter) secured on metal rods approximately 20 cm above the substrate (four sets of three traps in each site) at ≈ 4 m depth. The traps were retrieved monthly (when possible), and the sediment was filtered through a pre-weighed Whatman qualitative filter (150 mm Ø), dried at 60 °C for 2–3 days and subsequently weighed.
Skeletal linear extension monitoring
The growth of a number of specimens of M. ampliata was followed along the west coast of four reefs from April 1999 to November 2000 through SCUBA diving. In the field, colonies of M. ampliata were collected along the upper reef slope, at 3–4 m below MSL, fixed to concrete slabs (10 × 25 × 2.5 cm) with underwater cement (epoxy resin), tagged and repositioned at the four research sites and at the same depth of 3–4 m below MSL. To measure linear extension rate, alizarin red-S stain (12 mg·l−1) was used in situ as a date marker following Dustan’s (1975) method. There were four staining periods (P1–P4): P1 – April–May 1999; P2 – June–November 1999; P3 – November 1999–March 2000; P4 – March–November 2000, Table 1). Staining periods P1 and P2 were test trials.
| Staining periods | Reef sites | |||
|---|---|---|---|---|
| Cyrene | Pulau Hantu | Semakau | Raffles | |
| P1 | 7.4.99–2.6.99 (56) | 7.4.99–2.6.99 (56) | – | 7.4.99–1.6.99 (55) |
| P2 | 2.6.99–18.11.99 (136) | 2.6.99–17.11.99 (135) | 15.6.99–19.11.99 (124) | 1.6.99–16.11.99 (135) |
| P3 | 18.11.99–30.3.00 (122) | 17.11.99–4.3.00 (124) | 19.11.99–18.3.00 (134) | 16.11.99–20.3.00 (141) |
| P4 | 30.3.00–10.11.00 (225) | 4.3.00–17.11.00 (258) | 18.3.00–8.11.00 (233) | 20.3.00–7.11.00 (230) |
After corals were removed from the sea, they were left for 2 days inside plastic buckets containing freshwater and bleaching solution so that the dead tissue could easily be removed with a water jet. They were then re-tagged for convenience and their initial and final dimensions were recorded. Colonies were left to dry under the sun for approximately 2 months. A total of 17, 24, 20 and 31 coral specimens were collected from Cyrene, Pulau Hantu, Semakau and Raffles reef sites, respectively, during the mensurative experiment, but only 5, 11, 12, and 23 coral specimens were used for the extraction of linear skeletal extension measurements (Appendix I). Specimens that showed obvious signs of stress during the experiment, such as bleaching or partial mortality (see also Dodge et al. 1984), were excluded and substituted with new specimens. Thus, specimens carried a maximum of four and a minimum of two alizarin stain-rings at the end of the experiment (Fig. 2).

Underside view of a typical specimen of M. ampliata (15.3 cm max. diameter) retrieved from the sea at the end of the mensurative experiment, bleached to remove tissue and dried under the sun. Pink, diffuse cycles are alizarin red stain-rings corresponding to periods of staining P1, P2, P3 and P4 (from the centre of specimen and outwards). Red lines indicate positions of maximum skeletal linear extension, and blue lines indicate randomly selected positions to obtain measurements for the calculation of mean skeletal linear extension. The white colour at the centre of the specimen shows epoxy resin used to attach the specimen on a concrete slab.
Maximum (n = 1 measurements on each specimen) and mean (n = 5 measurements on each specimen) skeletal linear extension measurements of M. ampliata were taken under a magnifying lens using a drafting compass. The position of maximum skeletal linear extension was easy to detect as all colonies were laminar and flat. The positions of the five independent measurements for the assessment of the mean skeletal linear extension rate were determined by generating random numbers between 1 and 360, which correspond to the 360° of a cycle. The compass was used to measure with increased accuracy the distance between two successive alizarin marks and between the last alizarin ring and the edge of the colony on the same specimen. The compass was then placed on the top of a ruler and the distance between the end-points of the compass was recorded in mm.
To compare and contrast patterns of skeletal linear extension rates with patterns of M. ampliata colony size, the benthos of the four reef sites was surveyed from September 2000 to January 2001 using SCUBA to record the size (as areal cover in cm2) of M. ampliata colonies encountered under randomly positioned 1-m2 quadrats (n = 12), along the reef crest at ≈ 4 m depth and at the same localities that seawater variables and skeletal linear extension monitoring took place.
Data analysis
Data analysis of both environmental variables and skeletal linear extension rates of M. ampliata involved only time periods P3 and P4, which correspond to a year’s cycle with convenient distinction between the two monsoons and maximum possible number of specimens for the four studied reefs. Differences in environmental variables and in skeletal linear extension rates among the four reef sites and two staining periods were evaluated through two-factor repeated measures analysis of variance (ANOVA, random effects or Model I). Prior to ANOVA, all datasets were tested for the basic assumptions of normality and homogeneity of variance with the Shapiro-Wilk W statistic and Levene’s test, respectively. To satisfy the assumptions of normality and homogeneity of variance, nitrite-nitrate concentration (NO2 + NO3), phosphate concentration (PO4-P), and light extinction coefficient (K) required a logarithmic, a logarithmic, and a power (λ = −0.315) transformation, respectively. The rest of the environmental variables were analyzed with the non-parametric equivalent to ANOVA, the Kruskal–Wallis test. The Kruskal–Wallis test was also employed to test whether there were statistically significant differences in median colony size of M. ampliata in the field among the four studied reef sites.
To determine whether the skeletal linear extension rate of M. ampliata was independent of colony size, simple linear regression analysis was employed using measurements of linear extension rate of M. ampliata specimens from Raffles during the first trial staining period (P1; 7.4.99–1.6.99). To evaluate whether the staining technique interfered with coral growth during periods of study P3 and P4, four (one for each research site) two-way (factors: number of stainings, time) repeated measures ANOVAs were employed.
Simple linear regressions (n = 4 reefs × 2 time) of mean skeletal linear extension rates of M. ampliata with mean values of the 10 environmental variables were determined using Model I regression. Their performance in terms of variance explained was compared to corresponding logarithmic, quadratic, power and exponential regressions (n = 4 reefs × 2 time) to test for non-linear relationships and threshold responses. Also, the fit of the same models, i.e. linear, logarithmic, quadratic, power and exponential, were tested for the relationships between distance from the main island of Singapore (D) and mean annual values of the skeletal linear extension rate and of the 10 environmental variables (n = 4 reefs).
Results
Seawater monitoring indicated a turbid environment (Fig. 3). There were significant differences among the four reef sites in Box-Cox transformed (λ = −0.315) mean values of the light extinction coefficient (two-factor ANOVA, Fsite = 19.6241, P < 0.05), median chlorophyll a concentration (Kruskal–Wallis test, H3,138 = 7.9112, P < 0.05), median downward flux of suspended particulate matter (Kruskal–Wallis test, H3,231 = 116.6037, P < 0.01), and median suspended particulate matter concentration (Kruskal–Wallis test, H3,152 = 12.817, P < 0.01).
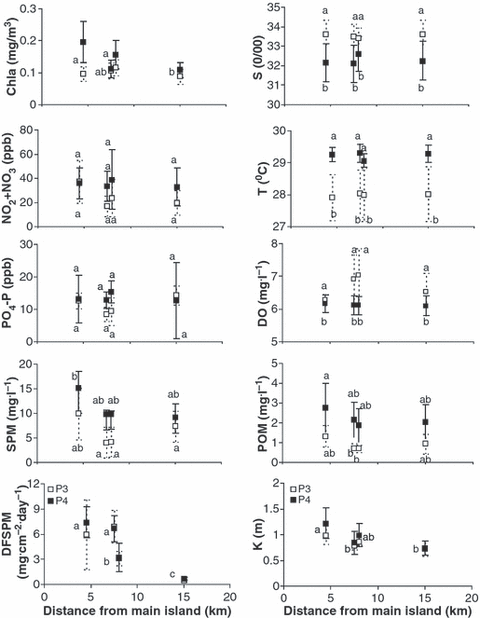
Variation (mean ± 95% confidence intervals) in seawater variables corresponding to periods P3 (November 1999–April 2000) and P4 (May 2000–November 2000) along the west coast of four reef sites (Cyrene 4.5 km; Pulau Hantu 7.5 km; Semakau 8 km; Raffles 15 km) at the southern islands of Singapore (∼ 4 m depth). Chla, chlorophyll a concentration; DF-SPM, downward flux of suspended particulate matter; DO, dissolved oxygen concentration; PO4-P, phosphate concentration; SAL, salinity; K, light extinction coefficient; T, temperature; POM, particulate organic matter concentration; SPM, suspended particulate matter concentration; NO2 + NO3, nitrite-nitrate concentration. Letters on top of error bars indicate significant differences.
There were also significant differences between the two staining periods P3 and P4 in log(x) transformed mean values of PO4-P (two-factor ANOVA, Ftime = 8.3880, P < 0.01), median dissolved oxygen concentration (Kruskal–Wallis test, H1,57 = 8.3970, P < 0.01), median salinity (Kruskal–Wallis test, H1,68 = 14.0650, P < 0.01), median suspended particulate matter concentration (Kruskal–Wallis test, H1,152 = 7.9290, P < 0.01), median temperature (Kruskal–Wallis test, H1,65 = 23.4297, P < 0.01) and median particulate organic matter concentration (Kruskal–Wallis test, H1,167 = 14.2460, P < 0.01) (Fig. 3).
The mean skeletal linear extension rate of M. ampliata was not dependent on colony size over the size range studied [t(9) = 0.5831, P = 0.5741]. Also, additional numbers of stain-rings did not retard growth at any of the four sites (2-way ANOVA Cyrene: Fstainings = 0.8162, P = 0.4329; Semakau: Fstainings = 14.9170, P < 0.01) but mean linear extension rate of colonies with two stain rings was smaller than that of colonies with three stain rings (Pulau Hantu: Fstainings = 0.3888, P = 0.69; Raffles: Fstainings = 0.66, P = 0.52).
Spatial and temporal variation in the maximum and mean skeletal extension rate of M. ampliata along the west coast of the southern islands of Singapore is presented in Fig. 4. There were significant differences in mean skeletal extension rate of M. ampliata among sites and between times (2-way ANOVA Fsite = 8.0784, P < 0.01; Ftime = 69.3801, P < 0.01). There was also a statistically significant interaction between the factors site and time (Fsite × time = 16.7183, P < 0.01).
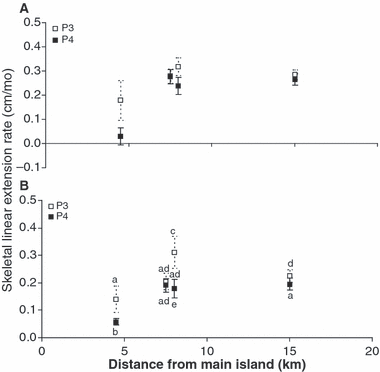
Maximum (A) and average (B) skeletal linear extension rates of M. ampliata along the west coast of the southern islands of Singapore (3–4 m depth below MSL) and at increasing distances from the main island of Singapore (Cyrene 4.5 km, n = 5; Pulau Hantu 7.5 km, n = 11; Semakau 8 km, n = 12; Raffles 15 km, n = 23) during periods P3 (November 1999–March 2000, dry season) and P4 (April 2000–November 2000, wet season). Error bars = ± 95% confidence intervals.
Simple linear regressions of mean skeletal linear extension rates of M. ampliata on mean environmental variables are presented in Table 2. Suspended particulate matter concentration was the best univariate predictor of skeletal linear extension rates. The corresponding linear regression indicated a coefficient of growth of 0.018 cm·mo−1 per mg·l−1 for the suspended particulate matter concentration range 4.07–15.22 mg·l−1. The light extinction coefficient, nitrite-nitrate concentration and particulate organic matter concentration showed weaker significant relationships with the skeletal linear extension rate of M. ampliata. The corresponding linear regressions indicated coefficients of growth of 0.345, 0.006, and 0.072 cm·mo−1 per unit change of the light extinction coefficient, nitrite-nitrate concentration and particulate organic matter concentration, respectively, for their respective range of values. Although linear models provided the best fit to the data for suspended particulate matter and particulate organic matter concentrations, the exponential and the logarithmic models were a better fit to the data for the light extinction coefficient (GR = 1.709e−2.568K, r2adj =0.72, P = 0.0049) and nitrite–nitrate concentration [GR = −0.175Ln(NO2 + NO3)+ 0.777], r2adj = 0.53, P = 0.0238), respectively. The distance from the main island of Singapore was shown to be an effective surrogate predictor of the mean annual skeletal linear extension rate of M. ampliata and of environmental variables such as suspended particulate matter, particulate organic matter and dissolved oxygen concentrations, the downward flux of suspended particulate matter and the light extinction coefficient (non-linear responses), albeit replication was small and, thus, regressions were marginally significant (Table 3).
| Simple regressions | r2adj | F test | P |
|---|---|---|---|
| 1. GR = −0.018*SPM + 0.343 | 0.76 | 21.661 | 0.003 |
| 2. GR = −0.345*K + 0.496 | 0.59 | 10.979 | 0.016 |
| 3. GR = −0.006* NO2 + NO3 + 0.380 | 0.50 | 7.906 | 0.031 |
| 4. GR = −0.072*POM + 0.302 | 0.50 | 1.701 | 0.030 |
| 5. GR = −1.308*CHLA + 0.348 | 0.33 | 4.385 | 0.081 |
| 6. GR = 0.114*DO − 0.541 | 0.26 | 3.399 | 0.115 |
| 7. GR = 0.052*SAL − 1.529 | 0.13 | 2.047 | 0.204 |
| 8. GR = −0.014*PO4–P + 0.368 | 0.09 | 1.701 | 0.239 |
| 9. GR = −0.050*T + 1.611 | 0.08 | 1.594 | 0.255 |
| 10. GR = −0.007*DFSPM + 0.217 | −0.09 | 0.454 | 0.530 |
| Best fit regression model | r2adj | F test | P |
|---|---|---|---|
| 1. GR = −0.005*D2 + 1.051*D | 0.98 | 97.866 | 0.071 |
| 2. DO = −0.010*D2 + 0.206*D | 0.98 | 92.190 | 0.073 |
| 3. SPM = 0.174*D2 − 3.835*D + 26.716 | 0.97 | 57.504 | 0.093 |
| 4. POM = 0.022*D2 − 0.490*D + 3.932 | 0.96 | 35.461 | 0.118 |
| 5. DFSPM = −0.252Ln(D) + 27.532 | 0.88 | 22.385 | 0.042 |
| 6. K = 1.834*e−0.348D | 0.79 | 12.009 | 0.074 |
| 7. CHLA = 0.257*e−0.352D | 0.56 | 4.733 | 0.162 |
| 8. NO2 + NO3 = −6.973Ln(D) + 45.193 | 0.30 | 2.315 | 0.267 |
| 9. T = 0.001Ln(D) + 28.657 | −0.25 | 0.391 | 0.596 |
| 10. PO4-P = 11.792*e0.007D | −0.29 | 0.314 | 0.632 |
| 11. SAL = 32.682*e0.002D | −0.40 | 0.142 | 0.742 |
Although median colony size (in cm2) of M. ampliata in the field did not differ significantly among the four sites (Kruskal–Wallis test, H3,22 = 5.8520, P = 0.1190) there was a larger range of colony sizes at Pulau Hantu (13.8–811.4 cm2), Semakau (57.2–1068.5 cm2) and Raffles (26.4–293.3 cm2) than at Cyrene (7.7–70.8 cm2) (Bartlett Chi-square test(2) = 8.589, P = 0.0136).
Discussion
Environmental conditions close to the main island of Singapore adversely affected skeletal linear extension rates of M. ampliata. Mean and maximum rates at the site closest to the main island of Singapore (Cyrene, mean ± SD: 1.02 ± 0.31 cm·year−1; max ± SD: 1.43 ± 0.67 cm·year−1) were 2.0 and 2.5 times, respectively, lower than those at the furthest site from the main island of Singapore (Raffles, mean ± SD: 2.46 ± 0.61 cm·year−1; max ± SD: 3.26 ± 0.59 cm·year−1). Lane (1991) reported a comparable maximum annual skeletal linear extension rate of 1.1 and 3.7 cm·year−1 for M. ampliata (extrapolated from measurements within a 6-month interval) at Cyrene and Raffles, respectively, 15 years prior to this study. Furthermore, a larger colony size range was found at Raffles than at Cyrene in this study. Indeed, colonies larger than 1-m diameter of M. ampliata with apparent signs of fission were encountered at Semakau and Raffles during field assessment.
The effect of the factor time on the skeletal linear extension rate of M. ampliata depended on the reef site studied. Thus, M. ampliata at Cyrene and Semakau had significantly lower mean skeletal linear extension rates during period P4 (March–November) than during period P3 (November–March). Period P4 contains the inter-monsoon periods and the southwest monsoon (May–September), which is rainy with less sunshine and stronger currents compared to the northeast monsoon (November–March). Thus, average values of suspended particulate matter concentration, light extinction coefficient, particulate organic matter concentration, and nitrite-nitrate concentration for the four reef sites were respectively 1.7, 1.1, 2.4 and 1.4 times higher during period P4 compared to period P3. Increase in sediment load increases turbidity and reduces the light available for photosynthesis (Te 1997) but at the same time provides extra organic food that may compensate for the light reduction (Anthony & Fabricius 2000), at least for the mixotrophic coral species (Lewis 1977; Sebens 1987). Strong currents keep sediments in suspension inhibiting their settlement on corals and subsequent fission and/or smothering of corals and facilitate the removal of mucus nets trapping sediments. Similarly, skeletal linear extension of the branching scleractinian Acropora formosa was found to be approximately double during the dry northeast monsoon compared to the wet southwest monsoon at Phuket Island, Thailand (Charuchinda & Hylleberg 1984).
Merulina ampliata, however, is a shade-tolerant species normally found in deep waters (Maragos 1974; Sheppard 1980) under low-light environments (mean % subsurface irradiance of 0.23%, Dinesen 1983) or even below Acropora hyacinthus tables on reef flats (Sheppard 1981). Foliose corals, such as M. ampliata, are well-adapted to low levels of light because their large surface-to-volume ratio increases their ability to trap light energy for photosynthesis (Barnes 1973; Dustan 1975; Jokiel & Morrissey 1986). Sorokin (1982), however, estimated that the photosynthesis to respiration ratio was 0.9 for M. ampliata, whereas 52% of respiration requirements were covered through heterotrophy (predator feeding; feeding on DOM; feeding on bacteria). Apparently, M. ampliata is a shade-tolerant or even a skiophilous scleractinian species because it may depend heavily on heterotrophy to adequately cover its energy requirements. This interplay of morphological and feeding plasticity may be quite effective as their fast skeletal linear extension rates make them superior overtopping competitors compared to corals with other life forms (Lang & Chornesky 1990), and efficient space competitors that often dominate the reef slopes (Dai 1990, 1993). Furthermore, their dense skeletons (Hughes 1987) help them survive under strong currents. Also, mucus secretion and sloughing by M. ampliata was observed in the field. Although production of mucus nets is at the cost of metabolic energy (Riegl & Branch 1995) and may increase an inclination to infection (Hodgson 1990), it may be used by the meandroid M. ampliata for sediment rejection (Hubbard & Pocock 1972; Stafford-Smith 1993; Sofonia & Anthony 2008), as an energy source (Anthony & Fabricius 2000; Wild et al. 2004) or both (Brown & Bythell 2005). Finally, mass-coral spawning events, including mature colonies of the hermaphroditic broadcast spawner M. ampliata (5.0–10.0 cm in diameter for Southern Taiwan, Fan & Dai 1998), take place on reefs of Singapore in March (Guest et al. 2002).
Linear regression models indicated that the mean skeletal linear extension rate of M. ampliata at the southern islands of Singapore was significantly and negatively affected primarily by alteration in suspended particulate matter concentration. Specifically, for every mg·l−1 increase in suspended solids concentration there is a 0.216 cm·year−1 (= 0.018 cm × 12 mo) reduction in skeletal linear extension rate of M. ampliata for the range 4.07–15.22 mg·l−1 of suspended particulate matter concentration. For the same increase in suspended particulate matter concentration there was a 0.283 cm·year−1 reduction in skeletal linear extension rate of the facultative heterotroph the massive scleractinian M. annularis, measured in the range 4.26–7.11 mg·l−1 suspended particulate matter concentration along a eutrophication gradient in Barbados (regression calculated based on raw data from Tables 2 and 7 of Tomascik & Sander 1985). On the other hand, average annual skeletal linear extension rates (range: 11.7–16.2 mm·year−1) did not relate significantly with suspended particulate matter concentration (range: 4.49–28.91 mg·l−1) for the massive scleractinian Porites lobata from 13 polluted (by sediments and nutrients) and unaffected sites in Indonesia (Edinger et al. 2000) and for the massive Porites lutea (range: 0.62–1.54 mm·month−1) and the branching Acropora formosa (range: 3.0–7.6 mm·month−1) on reefs affected by sedimentation and tin dredging (range: 2.5–10.5 mg·l−1) along the southwest coast of Phuket Island, Thailand (Chansang et al. 1992). The quadratic regression model indicated that distance from the main island of Singapore was an almost perfect predictor of annual skeletal extension rate of M. ampliata, dissolved oxygen, suspended particulate matter and particulate organic matter concentrations, and revealed threshold responses.
Either because individual growth rates are impaired or because of partial mortality resulting in colony fission (Hughes & Jackson 1985), M. ampliata finds it difficult to attain a large size at the reef sites surveyed. The mean coral colony size of M. ampliata in the field based on maximum diameter (mean: 9.5 cm, range: 3.2–23.0 cm at the four reef sites) was comparable to the mean of maximum colony length of the same species found in shaded environments at the Great Barrier Reef (mean: 12.9 cm, range: 3.0–63.0 cm; Dinesen 1983). Finally, the growth form of M. ampliata in the field was laminar, not convoluted as found in better lit environments (Hughes 1987). Nonetheless, M. ampliata has retained comparable linear extension growth rates under highly turbid waters during the last 15 years (Lane 1991). Coral reefs of the southern islands of Singapore have experienced chronic exposure to increased sediment load, which has led to a dramatic decline in live coral cover since the 1970s, a dominance of benthic space by dead corals covered with sediment and filamentous algae, and survival of only sediment/turbidity-tolerant scleractinian taxa (Dikou & van Woesik 2006) just as Sanders & Baron-Szabo (2005) predicted (Fig. 4B and 5,6 drawing in their paper) for scleractinian assemblages under chronic sediment input. Acquisition of skeletal linear extension rates of M. ampliata from reference (unaffected) sites may help clarify whether this is another example of normal coral growth rates on dying reefs (Edinger et al. 2000) or not.
Conclusion
Suspended particulate matter concentration was the best predictor of skeletal linear extension rate of the foliaceous, skiophilous, heterotrophic, scleractinian M. ampliata in the turbid waters of the southern islands of Singapore. A 0.018 cm·month−1 reduction in skeletal linear extension rate was predicted per mg·l−1 increase in the suspended particulate matter concentration over the range 4.07–15.22 mg·l−1.
Acknowledgements
I thank researchers of the Tropical Marine Science Initiative, postgraduate students of the National University of Singapore, and volunteer divers for valuable assistance in the field. The study was funded with a research scholarship to the author by the National University of Singapore.
Appendix
Appendix I
Staining periods (P1–P4) and initial coral colony size (surface area = 3.14 × max. length × max. width) for specimens of M. ampliata from Cyrene (C), Pulau Hantu (PH), Semakau (S) and Raffles (R) reef sites used for the extraction of skeletal linear extension rates at the end of the mensurative experiment.
| Specimen i.d. | Staining period | Coral colony size | |||||
|---|---|---|---|---|---|---|---|
| P1 | P2 | P3 | P4 | Length max. (cm) | Width max. (cm) | Surface area (cm2) | |
| CM4 | + | + | 16.5 | 14.5 | 751.2 | ||
| CM6 | + | + | 13.0 | 9.0 | 367.4 | ||
| CM8 | + | + | + | + | 8.5 | 7.5 | 200.2 |
| CM10 | + | + | + | + | 5.9 | 3.4 | 63.0 |
| CM11 | + | + | 6.1 | 5.7 | 109.2 | ||
| HM6 | + | + | + | 8.5 | 7.4 | 197.5 | |
| PHM7 | + | + | + | + | 18.5 | 13.0 | 755.2 |
| PHM8 | + | + | + | 8.5 | 7.5 | 200.2 | |
| PHM9 | + | + | + | 5.3 | 5.2 | 86.5 | |
| PHM12 | + | + | + | + | 15.1 | 11.2 | 531.0 |
| PHM13 | + | + | + | 7.3 | 5.5 | 126.1 | |
| PHM14 | + | + | 5.3 | 5.1 | 84.9 | ||
| PHM18 | + | + | 5.5 | 5.0 | 86.4 | ||
| PHM19 | + | + | 14.0 | 10.0 | 439.6 | ||
| PHM20 | + | + | + | + | 8.5 | 8.0 | 213.5 |
| PHM24 | + | + | + | + | 10.2 | 8.2 | 262.6 |
| SM1 | + | + | + | 10.0 | 9.2 | 288.9 | |
| SM2 | + | + | + | 5.1 | 5.0 | 80.1 | |
| SM3 | + | + | 6.3 | 4.2 | 82.1 | ||
| SM4 | + | + | + | 14.0 | 12.0 | 527.5 | |
| SM5 | + | + | + | 10.5 | 6.5 | 214.3 | |
| SM6 | + | + | + | 19.5 | 13.0 | 796.0 | |
| SM9 | + | + | 5.8 | 5.0 | 91.1 | ||
| SM10 | + | + | + | 8.8 | 4.4 | 121.6 | |
| SM11 | + | + | + | 8.0 | 6.5 | 163.3 | |
| SM13 | + | + | + | 9.5 | 9.0 | 268.5 | |
| SM16 | + | + | + | 11.5 | 10.8 | 390.0 | |
| SM17 | + | + | 3.2 | 2.8 | 27.2 | ||
| RM2 | + | + | + | + | 12.5 | 11.0 | 431.8 |
| RM3 | + | + | + | + | 13.3 | 12.2 | 509.5 |
| RM5 | + | + | + | + | 6.5 | 5.8 | 118.4 |
| RM6 | + | + | + | + | 7.5 | 7.0 | 164.9 |
| RM7 | + | + | + | + | 7.8 | 7.0 | 171.4 |
| RM9 | + | + | + | + | 8.3 | 7.5 | 195.5 |
| RM11 | + | + | + | + | 8.1 | 7.8 | 198.4 |
| RM12 | + | + | + | + | 8.0 | 6.5 | 163.3 |
| RM13 | + | + | 9.3 | 5.5 | 160.6 | ||
| RM14 | + | + | 11.0 | 11.0 | 379.9 | ||
| RM15 | + | + | + | + | 7.5 | 6.0 | 141.3 |
| RM16 | + | + | + | 7.0 | 5.0 | 109.9 | |
| RM17 | + | + | + | 6.0 | 5.0 | 94.2 | |
| RM18 | + | + | + | + | 7.2 | 6.5 | 147.0 |
| RM19 | + | + | 8.0 | 6.5 | 163.3 | ||
| RM20 | + | + | + | 6.0 | 5.3 | 99.9 | |
| RM21 | + | + | + | 11.5 | 10.0 | 361.1 | |
| RM22 | + | + | + | 5.8 | 5.2 | 94.7 | |
| RM23 | + | + | + | 4.5 | 5.0 | 70.7 | |
| RM25 | + | + | + | 19.0 | 17.0 | 1,014.2 | |
| RM26 | + | + | + | 14.1 | 9.5 | 420.6 | |
| RM27 | + | + | + | + | 12.4 | 9.3 | 362.1 |
| RM28 | + | + | + | 23.0 | 18.0 | 1,300.0 | |




