Genetic variability analysis in five populations of the sea cucumber Stichopus (Apostichopus) japonicus from China, Russia, South Korea and Japan as revealed by microsatellite markers
Abstract
The genetic structure of populations of the sea cucumber Stichopus (Apostichopus) japonicus was investigated using 10 microsatellite markers. In all, 152 individuals from five natural populations were collected from Aomori, Japan (JA and JR), Yosu, South Korea (KY), Dalian, China (CD) and Vladivostock, Russia (RV). A total of 145 alleles were found at 10 loci. The number of alleles per locus ranged from 9 at PSC03 to 20 at SCZ06, with an average of 14.5. Average Ho and He ranged from 0.260 (JR) to 0.434 (JA) and from 0.654 (RV) to 0.778 (KY), respectively. No significant differences at A, Ho and He were found, indicating similar genetic diversity in the five populations. A single allele was found at the PSC05 locus in the RV population. Of the 50 loci, 42 significantly deviated from Hardy–Weinberg equilibrium, all showing heterozygote deficiency. The genetic distances were all relatively great, ranging from 0.497 (between JA and KY) to 1.029 (between KY and JR). This suggests the five populations are genetically distinct. Cluster analysis indicated that JA, KY and CD form one branch and RV and JR another in the UPGMA tree. A hypothesis is proposed for the evolution of the Japanese red sea cucumbers and the genetic relationship among the populations.
Introduction
The sea cucumber Stichopus (Apostichopus) japonicus is a commercially important species found off the coasts of North China, Japan, Korea, and Far East Russia in Northeast Asia. Its geographical distribution originally divides this species into four basic populations: Chinese, Japanese, Korean and Far East Russian population. Most sea cucumbers are black, green or brown. The Japanese red sea cucumber was also classified as Stichopus japonicus by Choe & Oshima (1961). But Kanno & Kijima (2003) and Kanno et al (2006) reported that the Japanese red population has a strong population differentiation and reproductive isolation. Therefore, we have divided Stichopus japonicus into five basic populations: four continental populations and the Japanese red population. Ecological and geographical conditions throughout long-term evolutionary history have shaped populations leading to special appearance of them. Although little detailed fossil evidence is available, we could also speculate that speciation and separation of sea cucumber populations might have occurred as an adaptation to the ecological and geographical changes in their evolutionary history.
The Stichopus japonicus populations have different commercial values on the Chinese market based on their different appearance. Increased commercial demand has resulted in a great interest in sea cucumber aquaculture. Unfortunately, the industry is hampered by a lack of understanding of the importance of fundamental genetic background and consequent effective breeding (Chang et al. 2004). Therefore, a number of questions have arisen about the genetics and ecology of the sea cucumbers of the five basic populations. Are there any significant differences in the genetic diversity of the five populations? How can sea cucumbers from the Far East Russian population be identified? Are all the populations in Hardy–Weinberg equilibrium? What are the genetic distances among them? To what extent do geographic distance and ecology shape their genetic structure? Are they geographically independent of each other or is there gene exchange among them? What are the genetic relationships among them?
To answer these questions, 10 microsatellite markers were used to investigate genetic variability and differentiation of the five basic populations of Stichopus japonicus. Although microsatellite markers have been used to investigate the genetic structure in Japanese (Kanno et al. 2005, 2006), Chinese (Chen & Li 2007) and Korean (Kim et al. 2008) populations of Stichopus japonicus, the present study is the first genetic analysis of Stichopus japonicus throughout its distribution range. It increases our understanding of the genetics and ecology of Stichopus japonicus and provides fundamental genetic information necessary for breeding programs.
Material and Methods
Sample collection
In all, 152 sea cucumbers from five natural populations were collected from four representative sites in their distribution areas in 2005. Sample sites covered most of their distribution range (Fig. 1): Aomori, Japan (JA, 40°58′ N, 140°22′ E); Yosu, South Korea (KY, 37°00′ N, 127°30′ E); Dalian, China (CD, 38°55′ N, 121°38′ E) and Vladivostock, Russia (RV, 43°7′ N, 131°54′ E). Japanese red sea cucumbers (JR), representing the fifth population, were also collected from Aomori, Japan.
Locality of Stichopus japonicus sampling 487 × 318mm (72 × 72 DPI)
DNA extraction and microsatellite genotyping
Genomic DNA was extracted from longitudinal muscle (JA, CD and KY) or tube feet (JR and RV) using the standard phenol-chloroform method (Liu et al. 1998). Ten microsatellite loci reported by Zhan et al. (2006) and Ding et al. (2006) were used (Table 1). PCR amplification was carried out in 25-μl reaction volumes with 50 ng of template DNA, 0.4 μm of each primer, 2 mm MgCl2, 1 × PCR Buffer (50 mm KCl, 10 mm Tris–HCl 0.1% Triton X-100, pH = 8.0), 200 μm dNTP mixture, and 0.75 U of Taq DNA polymerase. The PCR amplification protocol involved initial denaturing for 5 min at 94 °C, followed by 26 cycles of 30 s at 94 °C, 30 s at optimum annealing temperature, and 30 s at 72 °C, and a 10-min final extension at 72 °C. PCR products were analysed by electrophoresis on 8% non-denaturing polyacrylamide gels in 1× TBE buffer and visualized with silver staining (Creste et al. 2001). The images obtained were scanned with an Epson Perfection 2480 scanner and analysed using GEL-PRO ANALYZER (version 4.5).
| Locus | Primer sequences (5′→3′) | T m/°C | GenBank no. | Reference |
|---|---|---|---|---|
| SCZ01 | F:AACATCGACTTCTCACTCCAGG | 64 | AB106646 | |
| R:ATGATACAAGAGTTGGGGCAGG | ||||
| SCZ02 | F:TGCAACGTTGATGTCATGAGC | 62 | AB106638 | |
| R:GAGACCTAGGCACTATAATTCC | ||||
| SCZ03 | F:TTTGGTCAGCTTGCGGCTTTG | 60 | AB106637 | |
| R:ATTGCATCGAAGGAGGCGATC | ||||
| SCZ04 | F:AATTGGAAGTTCCCTGACCCC | 62 | AB106630 | |
| R:GTAAAATTTGCCTCAGCGAGGG | ||||
| SCZ05 | F:CCCTCATTATGGATCTGCCATG | 55 | AB106639 | Zhan et al. (2005) |
| R:TTCTCTCCCTACCTCAACTACCC | ||||
| SCZ06 | F:TTTCCATTCGCTCCTGCAAACC | 50 | AB106633 | |
| R:CCGGCCACAAAACTCTCCTATAAG | ||||
| PSC02 | F:TCTAGGCTAGCCAAACCAAAA | 50 | AF455030 | |
| R:GATCAAAATTGCATCCACCA | ||||
| PSC03 | F:AAATCTCCCACCGAAAAGTGA | 55 | AF455031 | |
| R:TTCGCAAACTATTTGTGGTG | ||||
| PSC05 | F:ACCGCCCTACATCCTCTC | 58 | AF455033 | |
| R:AGACTGGCATTAAAATTAGACAAAC | ||||
| SC057 | F:TGTATGTGATGTTCCCTGTA | 46 | – | Ding et al. (2005) |
| R:AATAGCCTTTGACCCTGA |
Statistical analysis
The observed number of alleles per locus (A), observed (Ho) and expected heterozygosity (He), Chi-squared test for Hardy–Weinberg equilibrium (HWE), Fis and Fst index, Nei’s unbiased measures of genetic distance and genetic identity, were analysed using POPGENE32 software version 1.31 (Yeh et al. 1999). One-way ANOVA was performed with the SPSS 11.0 statistical software to detect the differences of A, Ho and He among populations. A probability level of P < 0.05 was considered statistically significant. On the basis of genetic distance, the UPGMA tree was constructed using MEGA 2 software (Kumar et al. 2001).
Results
Genetic variability and Hardy–Weinberg equilibrium
A total of 145 alleles were observed in 152 individuals within the 10 loci. The number of alleles per locus ranged from 9 at PSC03 to 20 at SCZ06, with an average of 14.5. Nine unique alleles were found in the CD population but only three in the RV population. Average Ho and He varied from 0.260 (JR) to 0.434 (JA) and from 0.654 (RV) to 0.778 (KY), respectively (Table 2). One-way ANOVA showed no significant differences for A, Ho and He (P > 0.05). The most divergent variability was found at the SCZ06 locus, where A was 6 for the JR population and 11–18 for the other populations. Average Ho at this locus, on the other hand, was only 0.067 for the JR population and 0.533–0.767 for the other populations. At the PSC05 locus, a single allele was observed in the RV population. Positive deviations of the Fis values were observed in all populations for the 10 loci, except RV at SCZ01. Of the 50 population-locus cases (five populations × 10 loci), 42 deviated significantly from HWE (P < 0.05), all of which showed heterozygote deficiency.
| Locus | PSC02 | PSC03 | PSC05 | SC57 | SCZ01 | SCZ02 | SCZ03 | SCZ04 | SCZ05 | SCZ06 | Mean | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| JA | A | 12 | 7 | 7 | 8 | 9 | 8 | 6 | 9 | 11 | 12 | 9 |
| H o | 0.719 | 0.406 | 0.094 | 0.313 | 0.500 | 0.375 | 0.344 | 0.375 | 0.531 | 0.688 | 0.434 | |
| H e | 0.847 | 0.539 | 0.530 | 0.791 | 0.796 | 0.819 | 0.640 | 0.834 | 0.823 | 0.849 | 0.747 | |
| F IS | 0.151 | 0.246 | 0.823 | 0.605 | 0.372 | 0.542 | 0.463 | 0.550 | 0.354 | 0.190 | 0.430 | |
| P | 0.459 | 0.000** | 0.000** | 0.000** | 0.003** | 0.000** | 0.002** | 0.000** | 0.000** | 0.002** | – | |
| CD | A | 18 | 5 | 8 | 10 | 7 | 7 | 8 | 8 | 8 | 18 | 10 |
| H o | 0.833 | 0.400 | 0.333 | 0.533 | 0.433 | 0.100 | 0.467 | 0.233 | 0.300 | 0.533 | 0.417 | |
| H e | 0.913 | 0.644 | 0.678 | 0.888 | 0.590 | 0.751 | 0.747 | 0.813 | 0.750 | 0.903 | 0.768 | |
| F is | 0.088 | 0.379 | 0.508 | 0.400 | 0.267 | 0.867 | 0.375 | 0.713 | 0.600 | 0.409 | 0.407 | |
| P | 0.029* | 0.004** | 0.000** | 0.000** | 0.000** | 0.000** | 0.020* | 0.000** | 0.000** | 0.000** | – | |
| KY | A | 13 | 6 | 8 | 8 | 10 | 4 | 4 | 9 | 8 | 13 | 8 |
| H o | 0.533 | 0.633 | 0.267 | 0.533 | 0.667 | 0.100 | 0.367 | 0.233 | 0.367 | 0.567 | 0.427 | |
| H e | 0.852 | 0.706 | 0.756 | 0.829 | 0.856 | 0.711 | 0.665 | 0.783 | 0.741 | 0.881 | 0.778 | |
| F is | 0.374 | 0.102 | 0.647 | 0.357 | 0.221 | 0.859 | 0.449 | 0.702 | 0.505 | 0.357 | 0.457 | |
| P | 0.000** | 0.098 | 0.000** | 0.003** | 0.012* | 0.000** | 0.003** | 0.000** | 0.000** | 0.010* | – | |
| RV | A | 11 | 3 | 1 | 10 | 8 | 7 | 4 | 9 | 6 | 11 | 7 |
| H o | 0.767 | 0.000 | 0.000 | 0.300 | 0.767 | 0.333 | 0.033 | 0.467 | 0.033 | 0.767 | 0.347 | |
| H e | 0.849 | 0.427 | 0.000 | 0.837 | 0.748 | 0.798 | 0.459 | 0.808 | 0.754 | 0.858 | 0.654 | |
| F is | 0.097 | 1.000 | – | 0.642 | -0.025 | 0.582 | 0.927 | 0.423 | 0.956 | 0.106 | 0.523 | |
| P | 0.264 | 0.000** | – | 0.000** | 0.250 | 0.000** | 0.000** | 0.000** | 0.000** | 0.362 | – | |
| JR | A | 17 | 6 | 6 | 5 | 11 | 6 | 9 | 12 | 8 | 6 | 9 |
| H o | 0.800 | 0.267 | 0.133 | 0.033 | 0.567 | 0.133 | 0.267 | 0.300 | 0.033 | 0.067 | 0.260 | |
| H e | 0.904 | 0.599 | 0.742 | 0.693 | 0.827 | 0.522 | 0.716 | 0.766 | 0.824 | 0.682 | 0.727 | |
| F is | 0.116 | 0.555 | 0.820 | 0.952 | 0.315 | 0.744 | 0.628 | 0.608 | 0.960 | 0.902 | 0.660 | |
| P | 0.453 | 0.008 | 0.000** | 0.000** | 0.0037* | 0.000** | 0.000** | 0.000** | 0.000** | 0.000** | – | |
| All pops | A (total) | 19 | 9 | 15 | 13 | 15 | 12 | 10 | 17 | 15 | 20 | 14.5 |
| A (mean) | 14 | 5 | 6 | 8 | 9 | 6 | 6 | 9 | 8 | 12 | 9 | |
| H o | 0.730 | 0.342 | 0.165 | 0.342 | 0.586 | 0.211 | 0.296 | 0.322 | 0.257 | 0.526 | 0.378 | |
| H e | 0.914 | 0.801 | 0.823 | 0.891 | 0.890 | 0.863 | 0.690 | 0.870 | 0.876 | 0.888 | 0.850 | |
| F st | 0.046 | 0.273 | 0.341 | 0.094 | 0.142 | 0.165 | 0.065 | 0.079 | 0.111 | 0.060 | 0.136 | |
- Sample abbreviations are as follows: Number of alleles observed per locus (A); observed (Ho) and expected (He) heterozygosities; analogue of Wright’s Fis (Fis); and single-locus estimates of Fst over all samples (Fst); P-value of Chi-squared test for HWE; the asterisk (*) indicates significant deviation from HWE, the double asterisk (**) indicates highly significant deviation from HWE.
Genetic differentiation among populations
F st ranged from 0.100 (between KY and JA) to 0.196 (between RV and JA), with an average of 0.136 (Table 2). The genetic distances were all relatively great, ranging from 0.497 (between JA and KY) to 1.029 (between KY and JR), suggesting they have low genetic identities (Table 3).
| pop | JA | CD | KY | RV | JR |
|---|---|---|---|---|---|
| JA | ***** | 0.122 | 0.100 | 0.196 | 0.164 |
| CD | 0.641 | ***** | 0.124 | 0.159 | 0.133 |
| KY | 0.497 | 0.692 | ***** | 0.149 | 0.158 |
| RV | 0.969 | 0.677 | 0.642 | ***** | 0.164 |
| JR | 1.007 | 0.777 | 1.029 | 0.723 | ***** |
- Nei’s genetic identity (above diagonal) and genetic distance (below diagonal).
The UPGMA tree based on genetic distances shows two distinct clusters: JA, KY and CD form one cluster and RV and JR another (Fig. 2). JA and KY form a sub-cluster.
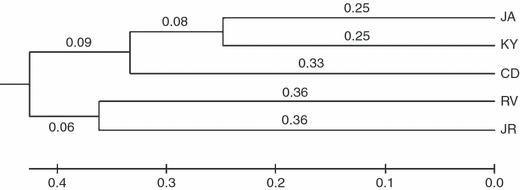
UPGMA tree for the five populations constructed from Nei's genetic distance based on 10 microsatellite loci.
Discussion
Genetic variability and Hardy–Weinberg equilibrium
Kim et al. (2008) compared their analysis of Ho and He with those of Kanno et al. (2005) and Chen & Li (2007). They concluded that the pattern of genetic diversity of the Korean population was lower than that of the Japanese but higher than that of the Chinese. In the present study, however, we found no significant difference among Chinese (CD), Japanese (JA) and Korean (KY) populations at A, Ho and He (P > 0.05). This suggests there is no significant difference in genetic diversity among the three populations. Our independent study with a statistical analysis enhanced the reliability of the results. In addition, the RV and JR populations did not show any significant difference at A, Ho and He, indicating that all five populations did not differ in genetic diversity. The most divergent variability was found at the SCZ06 locus, where A and Ho of JR was obviously lower than that of other populations, suggesting some different genetic variability of the JR population at this locus.
The Far East Russia population of Stichopus japonicus, which has a thicker body wall with six lines of papillae that are denser and longer than those of other populations, has a high commercial value. Identification and protection of its germplasm might be of major assistance in artificial breeding and hybridization studies to promote sea cucumber aquaculture. In the present study, a single allele at the PSC05 locus was observed in the RV population. Further studies should be done to confirm that the single allele at this locus is unique to the Far East Russian population. If so, a population-detecting method might be established to identify the Far East Russia population of Stichopus japonicus.
In all, 85% of population-locus cases (five populations × 10 loci) in all five populations deviated significantly from HWE (P < 0.05). Both the positive Fis value and the finding of He > Ho suggest a heterozygote deficiency at these deviated loci. Potential causes of heterozygote deficiency might be the Wahlund effect (Addison & Hart 2004; Perez et al. 2007), null alleles (Foltz 1986; Zhan et al. 2008), and the release of artificially cultivated sea cucumbers (Kim et al. 2008).
Genetic differentiation among populations
In the present study, the genetic distances between the populations were large. The average Fst value also showed a moderate differentiation among populations (Wright 1978). This indicates these populations are territorially independent. In the analysis of genetic differentiation of Korean populations of Stichopus japonicus,Kim et al. (2008) reported no significant genetic differentiation between Yeosu and Taean (Korea) populations but a significant differentiation between Yeosu and Gangneung populations. Our results and the study by Kim et al. (2008) support the opinion that there is gene exchange among nearby populations with simple hydrographic conditions (Zhan et al. 2008) and that geographic isolation of genetic structure happens over great distances (Liang et al. 2005). In the present study, a single allele at the PSC05 locus (A = 15) in the RV population confirms a small gene exchange among the five populations. Current patterns are a major factor affecting genetic distance among populations of marine animals (Addison & Hart 2004; Kenchington et al. 2006; Zhan et al. 2008). However, this factor may be interrupted or reduced because of hydrographic complexity and variability (Zhan et al. 2008). In the present study, a great distance might explain poor gene exchange among populations.
In the UPMGA tree, JA, KY, and CD populations form a cluster. This is partly supported by the similarity of their growth and color. Japanese red population (JR), which has an obvious color difference compared with the other four populations, was beside the cluster formed by CD, KY and JA. This result agrees with Kanno et al. (2006) who found that red Stichopus japonicus forms a cluster, and black and green specimens another. Kanno & Kijima (2001, 2003) and Kanno et al. (2006) systematically investigated Japanese red sea cucumbers and gave them separate species status. Our experimental results supported this opinion. However, the present study shows that the Far East Russian population (RV) of Stichopus japonicus, which has more developed body wall and papillae development than the other four populations, forms a cluster with the Japanese red population (JR) in our UPGMA tree. As the ecology, evolution and genetic background of Stichopus japonicus, especially the Far East Russian population, remain largely unknown, little direct supporting evidence is available to explain why the RV and JR populations have a closer genetic relationship despite obviously different body phenotypes and geographic distribution. Logically, genetic differentiation in their evolutionary history could be responsible for genetic distance among populations. As a special population, the origin of the Japanese red sea cucumbers is unknown. In the present study, a great genetic distance was observed between the JA and JR populations collected at the same site. This indicates that the speciation of Japanese red sea cucumbers could not have happened locally. The study by Kanno et al. (2006) indicated that the speciation of the red sea cucumbers occurred relatively recently on the evolutionary time scale. It is interesting to note that a geographic change of the Sea of Japan happened in the last glacial period, when the sea was isolated to form the North Pacific, and the sea level, dropped greatly (Liu et al. 2004). These relatively recent geographic and climatic changes provide the possibility of an immigration and the speciation of the Japanese population of the red cucumber. To address this issue, we hypothesize that some sea cucumbers immigrated from Far East Russian to Japan in the last glacial period and that the speciation of the Japanese red population happened at that time. This hypothesis provides a better understanding of the evolution and ecology of sea cucumbers. However, due to a lack of data on paleo-oceanography and paleontology of sea cucumbers, we should be aware that it may be too simplistic to relate the evolution of sea cucumbers to geographical changes in the last glacial period or even to establish this link. Further study should be done to test the hypothesis and clarify the detailed evolutionary history of Stichopus japonicus.
Acknowledgements
This work was supported by Chinese National Project 863 (2006AA10A411) and National Programs for Science and Technology Development (2006BAD09A01). We are very grateful to Prof. John Lawrence from the University of South Florida, for providing editorial suggestions and reviewing the manuscript.




