Reproduction of the intertidal barnacle Balanus glandula along an estuarine gradient
Abstract
The barnacle Balanus glandula is predominantly an open coast species in the Northeast Pacific. However, B. glandula densely inhabits estuaries where environmental conditions such as salinity and temperature drastically differ from the open coast. The increased variability of environmental conditions within an estuary can potentially affect spatial patterns of reproduction in B. glandula. I examined gonad maturity, reproductive periodicity and fecundity, and then calculated reproductive output in B. glandula populations distributed along an estuarine gradient. Results indicated that reproductive output of this intertidal barnacle decreased four times over a spatial scale of kilometers, as a transition occurred from a marine to a freshwater habitat along an estuarine gradient. Additionally, a higher proportion of the population had well-developed gonads in the oceanic end of the estuary compared to the riverine end. These results indicate how reproductive pattern can significantly vary over a spatial scale of kilometers, resulting in site-specific contributions of offspring to the larval pool.
Problem
Understanding of temporal and spatial reproductive patterns provides an important foundation for determining the dynamics that regulate the distribution and abundance of marine invertebrate species. Invertebrate organisms display a variety of patterns in their timing of reproduction. At one end of the spectrum, some species are reproductive over a very short time span, whereas at the other end, some species are reproductive year round (Barnes 1989; Hadfield & Strathmann 1996). A reproductive season is not necessarily fixed for a species and can vary with environmental conditions (reviewed in Giese & Kanatani 1987; Sastry 1975). Reproducing across multiple seasons may be an adaptation to increase the probability of successful recruitment in an environment that is highly variable (Hadfield & Strathmann 1996).
Habitat-specific environmental conditions will also influence the abundance of offspring produced. General patterns of fecundity in invertebrates have been shown to vary as a function of depth (Barber et al. 1988), intertidal height (Barnes & Barnes 1968; Qian & Chia 1991; Honkoop & van der Meer 1997; Leslie et al. 2005), and wave exposure (Etter 1989; Bertness et al. 1991). The main focus has been on variation along a vertical gradient with less being known about intra-specific variation in reproductive patterns along a horizontal gradient (Leslie et al. 2005; Phillips 2007).
Some aspects of barnacle reproductive biology have been well studied. Barnacle reproductive patterns are regulated by a complex interaction of multiple environmental variables. Reproduction is impacted by factors such as temperature (Barnes 1963; Crisp & Patel 1969; Barnes & Stone 1973; Hines 1978), salinity (Barnes & Barnes 1968; however, see O’Riordan & Murphy 2000), food availability (Patel & Crisp 1960; Barnes & Barnes 1967, 1975; Page 1983; Bertness et al. 1991), light (Crisp 1959; Barnes et al. 1963; however, see Hines 1978), and population density (Wethey 1984; Leslie 2005). These factors may act simultaneously in regulating reproductive periodicity or fecundity and may interact along environmental gradients.
An estuary presents a unique opportunity for observing reproduction over a horizontal environmental gradient. Over a spatial scale of kilometers, a gradient of environmental conditions occurs well within the observed distribution of an adult barnacle population (Berger et al. 2006). The mouth or ‘oceanic end’ of a typical estuary in the Pacific Northwest, although seasonally variable, is characterized by water temperatures and salinity more similar to the open coast than the rest of the estuary. At the riverine end of an estuary, water temperature is relatively warmer in the summer and cooler in the winter, and the salinity is lower all year due to an input of fresh water. Between the oceanic and riverine ends, mixing occurs, creating natural temperature and salinity gradients along the estuary.
Populations of the barnacle Balanus glandula Darwin, 1854 were used to test the hypothesis that reproductive output will decrease along an estuarine gradient as distance from the oceanic inlet increases. Environmental conditions become less marine-like as distance from the oceanic inlet increases. Balanus glandula is an ideal organism to study reproductive patterns along an environmental gradient. This species is commonly found in estuaries (Morris et al. 1980) where environmental factors can vary over a spatial scale of kilometers. Gonad maturity and reproductive status can easily be determined, as B. glandula is hermaphroditic and broods its offspring for approximately 1 month (Hines 1978; Anderson 1994). Specifically, I measured the percent of the population brooding and fecundity over time to calculate an estimate of reproductive output.
Material and Methods
Collection and preparation
Adult Balanus glandula were sampled at three sites distributed along an estuarine gradient in the South Slough Estuary, Charleston, OR, USA (see Berger et al. 2006). Sites were located at the oceanic end, mid-estuarine, and riverine end of a salinity gradient within the estuary. Monthly, between April 2002 and July 2003, 50 adult B. glandula were collected from wooden pilings at each of these sites. Specimens were collected by placing a 10 × 10 cm quadrat with a removable clear acetate sheet containing 50 dots over the piling. The barnacle nearest to a dot was collected. Hines (1976) reported B. glandula larger than 5 mm were consistently mature, therefore, only specimens with a rostro-carinal diameter >5 mm were collected. Additional criteria included collecting specimens that were not crowded or hummocked, but were adjacent to another individual so copulation could occur. Specimens were collected at an intertidal height with equivalent submergence times (Berger et al. 2006). As a reference, the collection height at the oceanic site was 1.41 m above mean low water.
Following collection, the specimens were returned to the lab and dissected. Presence or absence of yolky ovarian tissue, a sperm-filled seminal vesicle, and egg lamellae were recorded. Egg lamellae were placed in a 1.0% (w/v) protease solution (Sigma, P-5380) for approximately 3 h at room temperature to separate the individual embryos and then fixed in a 4% formalin solution. Total number of embryos per individual was estimated by diluting the fixed embryos in 50 ml of seawater and then counting the number of embryos in eight 1-ml subsamples. The prosoma was removed without ovarian tissue, mantle tissue, or opercular muscles and dried to a constant weight at 60 °C for approximately 10 days.
Physical site characteristics
Salinity and water temperature were continuously monitored throughout the sampling period at the oceanic, mid-estuarine, and riverine site. Salinity and water temperature were recorded with a YSI model 6600 data logger at the oceanic and riverine site, and with a YSI model 6000 data logger at the mid-estuarine site.
Reproductive output
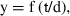
where y is the estimated number of broods, f is the proportion of the population with broods during April 2002 through April 2003 (oceanic = 0.39; mid-estuarine = 0.24; riverine = 0.13), t is the time period being examined (395 days), and d is the development time for a brood (Hilgard 1960; Lewis & Chia 1981; Page 1984). An estimate of 30 days for a brood cycle was used (Hines 1978).
Analysis
This study was observational, not experimental, in design and was meant as a baseline for future experimental field and laboratory research. The experimental design used in this study incorporated replication at the level of individual barnacles; subsamples of 50 individual barnacles were collected at each study site. Taking subsamples within a site allows differences within a site and between study sites to be determined (Quinn & Keough 2002). Because replication was at the level of individual barnacles, inferences are directed towards barnacles examined in this study. Multiple sites at each estuarine level, which were not part of the experimental design, are necessary to make inferences regarding barnacle reproduction at the scale of an estuarine gradient. In an effort to overcome some of the limitations imposed by the experimental design used in this study, sampling was repeated monthly, over a period of 15 months.
To test the overall effect of location within the estuary on the proportion of Balanus glandula brooding, samples from each site were pooled over the entire sampling period (April 2002–July 2003). A chi-squared analysis on multiple proportions was performed to test the null hypothesis that the proportions of B. glandula brooding between sites were not different (Zar 1984). To determine which sites were different, a post-hoc test to compare multiple proportions was performed (Zar 1984). Identical analyses were performed to test the effect of estuarine location on (i) the proportion with filled seminal vesicles and (ii) the proportion with yolky ovaries.
To quantify site-specific differences in individual fecundity, the total number of embryos per individual was normalized to dry body mass (Barnes & Barnes 1968). Because the number of embryos increases linearly as a function of barnacle body mass (Barnes & Barnes 1956), normalization to body mass is necessary and appropriate when comparing individuals that vary in size. Samples were pooled from monthly collections during April 2002–April 2003 for each site. A one-way ANOVA was performed to test for site-specific effects of fecundity, followed by a Tukey post-hoc test to examine specific differences between sites. Data were natural log transformed prior to analysis to meet the assumption of homoscedasticity. Although the assumption of normality was violated, ANOVAs are robust to departures from normality (Sokal & Rohlf 1995; Underwood 1997).
Results
Physical site characteristics
Site-specific and seasonal patterns of salinity and water temperature, due to riverine runoff, defined distinct habitats within the South Slough Estuary over the 15 months of this study. During April 2002 through July 2002 salinity decreased along the estuarine gradient with distance from the oceanic inlet (Fig. 1). During August through November of 2002, the estuary had a strong marine influence with relatively high salinity at all sites. A strong salinity gradient emerged as distance from the oceanic inlet increased in December 2002 and remained until the study ended in July 2003.
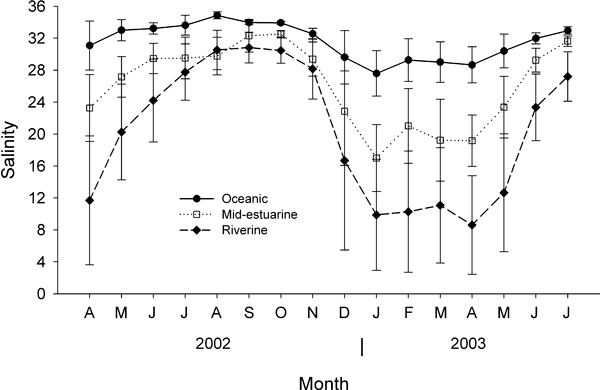
Mean monthly salinity profiles at the collection sites along an estuarine gradient. Error bars are ±1 sd.
Water temperature followed a seasonal pattern with warmer temperatures for the mid-estuarine and riverine sites in the late spring through summer and cooler temperatures in the fall and winter relative to the oceanic site (Fig. 2). The annual variation in water temperature was higher at the mid-estuarine and riverine sites than at the oceanic site. The highest mean monthly water temperatures were 17 °C and 20 °C in June at the mid-estuarine and riverine sites, respectively, and 13 °C at the oceanic site. In late fall and winter (November 2002–March 2003), the water temperature at all three sites was relatively similar. For example, in December, the mean monthly water temperature was 11.4 °C, 10.1 °C, and 9.5 °C at the oceanic, mid-estuarine, and riverine sites, respectively.
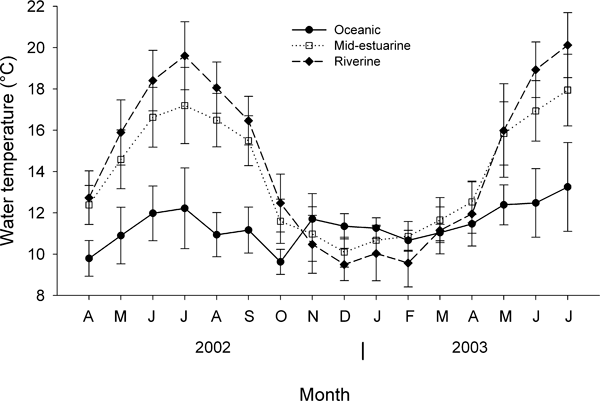
Mean monthly water temperature profiles at the collection sites along an estuarine gradient. Error bars are ±1 sd.
Reproductive patterns along an environmental gradient
During the period April 2002 through July 2003, estuarine location had a significant effect on the percent of the B. glandula population brooding (Fig. 3A, χ2 = 153.8, df = 2, P < 0.001), with sperm-filled seminal vesicles (Fig. 3B, χ2 = 147.04, df = 2, P < 0.001), and with ripe yolky ovaries (Fig. 3C, χ2 = 433.20, df = 2, P < 0.001). The percent of all three reproductive indices decreased along the estuarine gradient as distance from the oceanic inlet increased. Specifically, percent brooding was significantly higher at the oceanic site (40%) than at the mid-estuarine site (28%) (q0.05, ∞, 3 = 14.52, P < 0.001), and percent brooding at the riverine site (12%) was significantly lower than at the mid-estuarine site (q0.05, ∞, 3 = 21.9, P < 0.001). The percent with filled seminal vesicles was significantly higher at the oceanic site (92%) than at the mid-estuarine site (75%) (q0.5, ∞, 3 = 25.71, P < 0.001) and the percent with filled seminal vesicles was significantly lower at the riverine site (67%) than at the mid-estuarine site (q0.05, ∞, 3 = 10.35, P < 0.001). The percent with yolky ovaries was significantly higher at the oceanic site (65%) than at the mid-estuarine site (28%) (q0.5, ∞, 3 = 41.93, P < 0.001), which was significantly higher than the riverine site (16%) (q0.05, ∞, 3 = 16.63, P < 0.001).
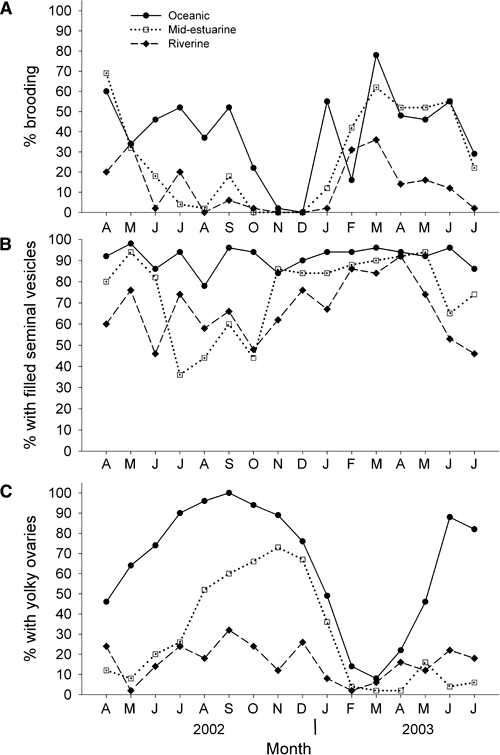
Monthly reproductive patterns of (A) percent brooding, (B) percent with filled seminal vesicles, and (C) percent with yolky ovaries in Balanus glandula along an estuarine gradient. Each data point represents 50 individual barnacles.
As mean monthly water temperature increased, percent brooding decreased. Water temperature explained only the 14% of the variation in percent brooding at all sites over the 15-month duration of this study (linear regression, R2 = 0.138, n = 45, t = −2.63, P = 0.012). Salinity did not significantly explain variation in percent brooding (linear regression, R2 = 0.003, n = 45, t = −0.33, P = 0.74).
Fecundity
When samples were pooled from April 2002 to April 2003, location in the estuary had a significant effect on the fecundity of Balanus glandula (ANOVA, F2,271 = 12.25, P < 0.001). Specifically, mass-specific fecundity per individual was approximately 28% higher at the oceanic site than at the mid-estuarine (Fig. 4, Tukey HSD, P < 0.001) and riverine sites (Tukey HSD, P = 0.002). There was no significant difference in mass-specific fecundity between the mid-estuarine and riverine sites (Tukey HSD, P = 0.86).
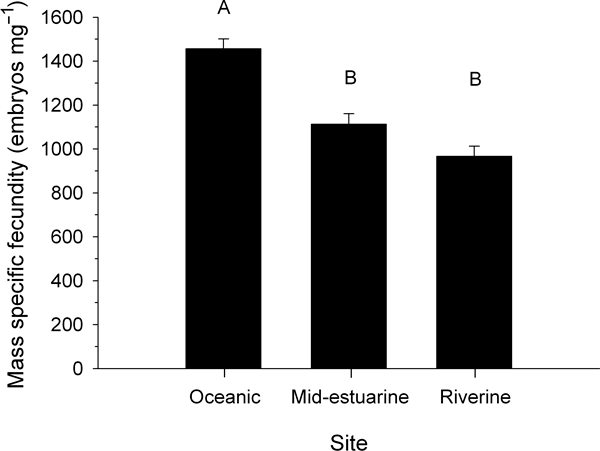
Mass-specific fecundity per individual in Balanus glandula collected at three sites along an estuarine gradient. Samples were collected in April 2002–April 2003 and then pooled. Each bar represents the mean of replicate samples; n = 221 at the oceanic site; n = 119 at the mid-estuarine site; n = 68 at the riverine site. Error bars are ±1 se. Different letters above bars represent significant differences from a Tukey post-hoc analysis (P < 0.05).
Reproductive output
Reproductive output decreased as distance from the oceanic inlet increased (Table 1). In the most physiologically benign habitat along the horizontal gradient at the mouth of the estuary (oceanic site), the barnacle population at the oceanic site annually produced two times as many offspring compared to the mid-estuarine population 4 km upstream and four times as many offspring compared to the riverine population 6 km upstream.
| estuarine location | mass-specific fecundity | estimated number of broods | reproductive output |
|---|---|---|---|
| oceanic | 1457 | 5.1 | 7431 |
| mid-estuarine | 1113 | 3.2 | 3562 |
| riverine | 966 | 1.7 | 1642 |
Discussion
This study has demonstrated that reproductive output in Balanus glandula can vary over a spatial scale of kilometers along a horizontal gradient. Similar spatial variation in reproductive output of barnacles (Leslie et al. 2005) and mussels (Phillips 2007) has been observed over a scale of tens of kilometers. This and other studies indicate that production of offspring is not spatially homogeneous, suggesting that not all sites contribute equally to a larval pool.
There was a decoupling between growth and reproduction along the observed horizontal estuarine gradient. The highest rates of growth were observed at the mid-estuarine site compared to the oceanic or riverine site (Berger et al. 2006). In comparison, the highest reproductive output occurred at the oceanic site. In contrast to the results of this study, sites with high invertebrate growth rates typically have a higher reproductive output compared to sites with lower growth rates (Bertness et al. 1991; Phillips 2007). Although growth rates were highest at the mid-estuarine site, resources appear to be directed toward growth and not reproduction.
The observed decoupling between growth and reproduction warrants further experimentation. This decoupling was potentially a result of a complex interaction between multiple site-specific environmental variables such as temperature, salinity, and food availability (Vernberg & Vernberg 1972; Sastry 1975; Vernberg 1981; Barnes 1989). High temperatures can delay the timing of reproduction or reduce the reproductive output in barnacles with boreo-arctic to temperate distributions (Barnes 1963; Tighe-Ford 1967; Crisp & Patel 1969; Hines 1978; Page 1984). For example, the percentage of populations of B. glandula and Elminius modestus brooding was lower at warmer sites than at cooler sites (Hines 1978; O’Riordan & Murphy 2000). Further, in a laboratory experiment the percentage of individuals brooding was higher in B. glandula maintained at 11 °C than at 20 °C (Hines 1978). Balanus glandula has the potential to reproduce at relatively higher temperatures observed at field sites in this study; however, relatively higher temperatures at the mid-estuarine and riverine sites may contribute to a reduced reproductive output over the duration of this study.
Page (1983) attributed the decrease in reproduction in the barnacle Pollicipes polymerus above a critical temperature to a reallocation of resources due to an increase in metabolism. Multiple balanomorph barnacle species display at a minimum a doubling of cirral beating rate, a correlate for metabolic rate, over a 10 °C increase in temperature (Southward 1955, 1962). Potentially, a similar metabolic increase in B. glandula at higher temperatures, coupled with physiological stress in an estuarine environment, could result in limiting resources for reproduction. Although there is no absolute certainty in assigning causality to temperature, an increase in water temperature was correlated with a reduction in the percent of the population brooding in this study.
Food availability is another factor driving offspring production. A delay in ovarian maturation, the formation of egg lamellae, and subsequent brooding occurs when food is limited (Patel & Crisp 1960; Barnes & Barnes 1967, 1975; Page 1983). A delay in reproduction can decrease the number of broods produced per year (Hines 1978), which will ultimately affect the total reproductive output. Reproductive output in barnacles is typically higher in habitats with high primary productivity compared to habitats with low primary productivity (Bertness et al. 1991; Leslie et al. 2005). In the South Slough Estuary, Roegner & Shanks (2001) demonstrated that oceanic water entering the estuary is generally higher in chlorophyll-a than estuarine water. Therefore, higher reproductive output at the oceanic site may in part be due to more abundant food, particularly in comparison with the riverine site. The decrease in the percent of the populations with yolky ovarian tissue along the estuarine gradient also suggests that food may have been a limiting factor as distance from the oceanic end increased. When food is abundant, ovarian tissues are built up (Barnes et al. 1963; Barnes & Barnes 1967; Wu & Levings 1978). Hines (1978) estimated that ovarian tissue in B. glandula stored enough yolky material for at least three broods. In summary, the effect of temperature and food availability may act together to drive the reproductive patterns observed in this study. Further laboratory experiments and field sampling are necessary to determine how the relationship between temperature and food availability affects barnacle reproductive patterns.
No correlation between salinity and the percent of the population brooding was observed. Examples describing the effect of salinity on barnacle reproduction are limited; however, they suggest that exposure to reduced salinity can decrease the number of offspring produced and reduce reproductive output in barnacles (Barnes & Barnes 1968; Lewis & Chia 1981; however, see O’Riordan & Murphy 2000).
Offspring produced in the riverine end of the estuary also have to contend with being immersed and released into low-salinity water. Peak reproduction in B. glandula at the riverine end of the estuary occurred during February and March 2003, when mean monthly salinity values were approximately 10 and reached values as low as zero. Salinity below 16 has been demonstrated to lyse developing B. glandula embryos (Bergen 1968). Similarly, at salinities from 15 to 20, normal development did not occur in a number of barnacle species closely related to B. glandula (Barnes & Barnes 1974). Interestingly, egg lamellae removed from B. glandula at the riverine site had intact and viable embryos (Berger M.S. personal observation), suggesting that behaviorally mediated closure of the opercular valves by the adult reduced exposure of embryos to low salinity. Once released in the estuary, larvae are potentially exposed to low salinity, which has been demonstrated to cause larval mortality in numerous Balanus spp. (Barnes 1953; Cawthorne 1978). Alternatively, offspring could be opportunistically released during a spring high tide when salinity could be tolerated. Therefore, at the riverine site during periods of low salinity, if larvae are not released during a spring tide with relatively high salinity levels, larval production may be ‘wasted’ and would not be a significant contribution to the larval pool.
Although the spatial sampling of this study limits the capacity to infer differences along the estuarine gradient, the results do demonstrate a decrease in barnacle reproduction. The site-specific inequality in reproductive output was potentially driven by interactions between site-specific environmental conditions. Further laboratory and field work are necessary to determine the mechanisms that influenced the reproductive patterns observed in this study.
Acknowledgements
I thank S. Rumrill, S. Powell, and the South Slough National Estuarine Research Reserve for access to water quality data. Earlier drafts of this manuscript were improved by comments by R. Emlet, P. Phillips, A. Shanks, N. Terwilliger, and three anonymous reviewers. This research was supported by NSF grant OCE-9911682 to R. Emlet and ERD-OCRM-NOAA grant NA17OR1172 to M. Berger. This research was performed in partial fulfillment of a Ph.D. in the Department of Biology, University of Oregon.




