Associational resistance mediates predator--prey interactions in a marine subtidal system
Abstract
There is a growing awareness of the role that indirect interactions play in influencing food webs and ecosystem structure. In this study, the hypothesis that crustose algal epibionts provide gastropods associational resistance from predation was investigated through field surveys and laboratory feeding assays. In rocky low intertidal/shallow subtidal systems in the northeast Pacific, several species of crustose algae (the red alga Peyssonnelia meridionalis and crustose corallines) can colonize the shells of living Tegula brunnea snails. The growth patterns of these epibiontic crustose algae allow them to cover their host’s surface completely, which may, in turn, protect their hosts from predation. A multi-site field survey of T. brunnea revealed that >60% of snails were at least 75% covered with one or more species of crustose algae, with 35% fully covered, indicating that this is common in the field. Laboratory feeding assays revealed that sea stars, a primary predator of T. brunnea, distinguished among snails with different shell coverings; Pisaster consumed nearly three times as many bare (i.e. no crustose algae) snails as those covered with Peyssonnelia, while Pycnopodia consumed four times as many bare snails as those covered with crustose corallines. These results suggest that epibiont crustose algae can benefit their hosts via associational resistance; this finding may have implications for the role of associational resistance in trophic interactions.
Problem
Associational resistance can occur when the mortality risk of one species is reduced when it lives nearby (or in close contact with) another species. Evidence of associational resistance can be found in both marine and terrestrial systems (Wahl & Hay 1995; Agrawal 2004; Poore & Hill 2005), and the impacts of this association can span a range of intensities, from individual populations to overall community structure (Hamback et al. 2000; Hillebrand & Cardinale 2004). Associational resistance has been extensively documented within a trophic level (Stiling et al. 2003; Poore 2004). However, in addition to its effects on intra-guild interactions, associational resistance can also influence the interactions between species at different trophic levels (see review by Laudien & Wahl 2004).
Epibionts can play important roles in cases of associational resistance occurring between trophic levels. Epibionts are sessile species that frequently colonize the surface of other organisms in a (usually) facultative association (Wahl 1989). Many sessile taxa can live as epibionts, and many do so as a refuge from intense competition on primary substrata. Epibiontic associations have been documented in algae (Wahl 1996; Ozolinsh & Kupriyanova 2000; Ballantine et al. 2001), barnacles (Enderlein et al. 2003; Chan & Chan 2005), bryozoans (Stachowicz & Whitlatch 2005), hydroids (Dougherty & Russell 2005), polychaetes (Warner 1997), and sponges (Marin & Belluga 2005), among others. Host organisms that are unable to remove or prevent the settlement of epibionts may find that the presence of these sessile organisms alters their interactions with both other organisms and their surrounding environment (Wahl & Hay 1995; Wahl et al. 1997).
The presence of epibionts can have either positive or negative effects on their hosts. Epibionts can negatively affect their hosts via increased predation pressure as shared doom (Wahl & Hay 1995; Wahl et al. 1997; Enderlein et al. 2003), energy expenditure via drag (Donovan et al. 2003) and decreased buoyancy (McAllen & Scott 2000); see review in Laudien & Wahl (2004) for other examples. In contrast, benefits provided by epibionts to their hosts include an increase in swimming efficiency in fish (Sar & Rosenberg 1987), a decrease in the desiccation rate of intertidal organisms (Penhale & Smith 1977), or, more commonly, decreased host mortality due to reduced predation as associational resistance, including camouflage (Wahl & Hay 1995; Laudien & Wahl 1999, 2004; Marin & Belluga 2005). The sea star Pisaster giganteus, for instance, preferentially consumed Chama clams that had no epibionts over those with epibionts on their shells (Vance 1978).
The ubiquity of sessile organisms in marine systems and high probability of epibiosis in space-limited environments have meant that many marine epibiont studies have focused on sessile hosts (Vance 1978; Manriquez & Cancino 1996; Enderlein et al. 2003). Although slow-moving shelled animals like gastropods also provide suitable habitat for epibionts (Warner 1997; Chan & Chan 2005), the effects of such epibiont–host interactions on gastropod predation risk remains poorly studied. Epiphytic crustose algae can completely cover the shells of their gastropod hosts (Morris et al. 1980), making the impacts of their effects even more intriguing.
In this study, I investigated the role of crustose algal epibionts on the turban snail Tegula brunnea in mediating snail–sea star interactions. The shells of these snails can become covered with crustose macroalgae, and there is no evidence that T. brunnea can affect either the settlement or removal of these epibionts. A prior pilot study indicated that the crustose macroalgae on T. brunnea may reduce the snails’ mortality rate due to consumption by predatory sea stars (V. Sehgal, unpubl. data).
I investigated the questions: (i) How frequently are living T. brunnea snail shells covered with algae in the field (i.e. is this a common phenomenon?), and is there a correlation between snail and predator densities?, and (ii) Does the presence/absence of algal shell coverings (specifically Peyssonnelia meridionalis and coralline crusts) influence feeding preferences of the predatory sea stars Pisaster ochraceus and Pycnopodia helianthoides? These findings shed light on epibiont-mediated indirect interactions in marine systems and add to the understanding of the importance of associational resistance in multi-trophic studies.
Material and Methods
Study system
Tegula brunnea is a turban snail commonly found in low intertidal and shallow subtidal rocky habitats along the California coast (Watanabe 1984). Unlike other snail species occurring in the same habitat, including its congeners, living T. brunnea shells may frequently be covered by different species of encrusting or foliose red algae, such as the red crustose algae Peyssonnelia meridionalis and crustose coralline algae like Lithothamnion and Pseudolithophyllum (Morris 1996). Crustose algae generally grow slowly in two dimensions along their substrate (i.e. not upright), are attached firmly to their substrate, and are resistant to herbivory (Dethier 1994). Predators of T. brunnea include the sea stars Pisaster ochraceus and Pycnopodia helianthoides, as well as the crabs Cancer productus and C. magister (Watanabe 1983).
Field surveys
I assessed the density of herbivores and predators in the marine rocky low intertidal/shallow subtidal zone at nine field sites along the northern California coast (see Results) in the spring of 2002. During spring low tides, at each site, I haphazardly placed two 10 m transect lines at ∼30 cm depth and counted all Pisaster, Pycnopodia and Cancer within 1 m of each side of the transect (=20 m2 per site; this large survey area was necessary as these larger predators are patchily distributed). While both sea stars were relatively common, Cancer crabs were not present in any of the survey areas. All areas had rocky bottoms and were thoroughly searched. Because small herbivores like T. brunnea are relatively abundant, I placed a 0.25 m2 quadrat at every meter along the transect (=20 quadrats per site) and collected all gastropods in each quadrat. For each T. brunnea, I recorded the maximum shell diameter (mm) with Vernier calipers and percent cover of each type of algae (primarily Peyssonnelia and coralline crusts) on the shells. Because the exact quantification of snail shell percent cover was very difficult, snails were visually classified into one of the following categories: 0%, 1–25%, 26–50%, 51–75%, 76–99%, and 100% cover, for each epibiont. Crustose algae made up the vast majority of epibiontic organisms, and invertebrate epibionts like bryozoans, slipper snails, and limpets were only rarely (<2%) found on snail shells. Empty T. brunnea shells were rarely encountered, and those occupied by hermit crabs were frequently very worn, thus unfortunately precluding the ability to draw contrasts between algal coverage on living versus dead snail shells.
Because very small (young) T. brunnea snails may not have had the opportunity to be colonized by algae, only snails with a shell diameter of 15 mm or greater (i.e. adult snails as defined by Watanabe 1984) were included in the analyses. However, juvenile snails were very rare in the surveys; excluding juveniles from the analyses did not change the statistical significance of the results. For the chi-square analysis of the frequency of different snail shell covers across sites, it was necessary to exclude three of the nine sites because of the absence of snails. Data from all nine sites were used in a correlation analysis (JMP v 5.1, http://www.sas.com) to assess the relationship between T. brunnea abundance and predatory sea star density (all P. ochraceus and Pycnopodia).
Feeding trials
I collected the predators Pisaster ochraceus and Pycnopodia helianthoides, as well as T. brunnea, from low rocky intertidal/shallow subtidal sites at and around Horseshoe Cove at the Bodega Marine Laboratory (BML), Bodega Bay, CA during the summer of 2002. Snails collected for the feeding trials fell into one of the following three categories: (i) bare shells, (ii) shells with 100% cover of Peyssonnelia meridionalis, or (iii) shells with 100% cover of crustose coralline algae (mostly Lithothamnion and Pseudolithophyllum spp.). Predators of both species were observed consuming T. brunnea in the field (C. Thornber, pers. obs.).
Predator selectivity was analyzed through a series of paired choice feeding assays. Each assay consisted of one predator and two of the three different T. brunnea prey choices (hereafter referred to as bare, coralline, or Peyssonnelia shells). Each predator was only used for one feeding assay. A total of six different assays were conducted (three different assays for each of two predator species). Approximately 20 replicates were conducted for each assay in flow-through seawater tanks at BML on a 12 h light:12 h dark cycle. Flow-through seawater tanks were approximately 40 l for each Pycnopodia, 20 l for each Pisaster, and had mesh lids to prevent snails from escaping. In each replicate, four snails were initially added (two of each of two shell types) with one predator. Replicates were checked five to six times daily at regular intervals, and the approximate time of both the start (sea star touching snail) and end (empty snail shell released from sea star) of each predation event were recorded. After the first snail was consumed, I added another snail of the same shell type to the tank to ensure that two snails of each shell type were always present. Replicates were concluded when the first two snails had been consumed (generally less than 10 days). The first two (not one) snails consumed were counted, because individual predators frequently can, and did, eat two snails at once (it was often impossible to determine which snail was selected first). Any replicates in which predators did not eat two snails within 10 days were removed and are not included here (this occurred <5%). Because of the discrete nature of these experiments, consumption preferences were analyzed using chi-square tests for each assay by comparing the number of each snail type consumed, with the Yates correction factor for one degree of freedom, and the multistage correction for multiple P-values (Rice 1990).
Results
Field survey
Coverage of T. brunnea shells by crustose algae is common in the field (Fig. 1). Although there are significant differences in the frequency of shell covering types among sites (Pearson’s chi-square15 = 54.846, P < 0.0001), at each site >60% of living adult T. brunnea snails were at least half covered with Peyssonnelia, crustose coralline algae, or a mixture of the two (Fig. 1). There was a significant inverse relationship between T. brunnea abundance and the density of their sea star predators (Fig. 2, r2 = 0.82, P = 0.0008). When analyzed separately for each predator, this relationship appeared to be primarily driven by the abundance of P. ochraceus (r2 = 0.77, P = 0.0017), as densities of Pycnopodia were low (r2 = 0.15, P = 0.29). Statistical comparisons of relative algal abundances on snails at sites with high versus low sea star abundances were not possible, unfortunately, as there were no snails at sites where predatory sea stars were abundant (Fig. 2).
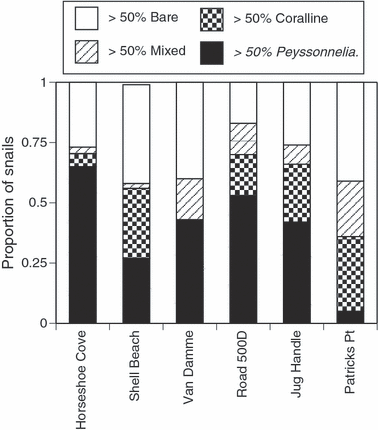
Shell coverings of T. brunnea snails in the field. Each vertical bar represents the proportion of snails at each site with over half of their shells either bare, Peyssonnelia covered, coralline algae covered, or mixed (Peyssonnelia plus coralline algae). Sites are listed from south to north and are all located in California; three additional sites sampled had no T. brunnea and are not included here (see Fig. 2).
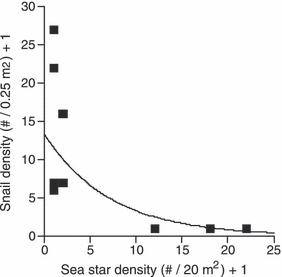
Log–log correlation between the density of T. brunnea and the density of predatory sea stars (Pisaster ochraceus and Pycnopodia helianthoides) in the low intertidal/shallow subtidal, at the same field sites used in Fig. 1, as well as Coleman Beach, Indian Beach, and Pinnacle Gulch, which had no T. brunnea. Data are displayed as site means +1, to allow for logarithmic transformation as many sites had either zero snails or zero sea stars.
Feeding assays
Sea stars generally preferred bare-shelled T. brunnea to algae-covered snails. Pisaster ochraceus was nearly three times as likely to consume bare T. brunnea than Peyssonnelia covered snails (Fig. 3A, chi-square1 = 8.82, P(corrected) = 0.003, n = 25 sea stars), but did not distinguish between bare snails and coralline covered snails (Fig. 3A, chi-square1 = 0.735, P(corrected) = 0.391, n = 17 sea stars), or between the snails covered in coralline versus Peyssonnelia (Fig. 3A, chi-square1 = 0.265, P(corrected) = 0.607, n = 17 sea stars).
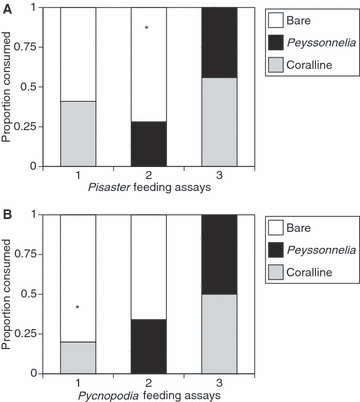
Predation assays on T. brunnea with differing shell coverings. Assay 1 tested bare versus coralline covered snails, Assay 2 tested bare versus Peyssonnelia covered snails, and Assay 3 tested Peyssonnelia versus coralline covered snails. (A) Pisaster ochraceus feeding assays. (B) Pycnopodia helianthoides assays. *Significant difference between snail types (P < 0.05). All data are proportions of snails consumed (two snails consumed in each replicate of each experiment).
Pycnopodia consumed twice as many bare T. brunnea as those with Peyssonnelia crusts (Fig. 3B, chi-square1 = 2.7, P(corrected) = 0.1003, n = 15 sea stars) and four times as many bare T. brunnea as those with coralline crusts (Fig. 3B, chi-square1 = 9.63, P(corrected) = 0.0019, n = 15 sea stars). As was found for Pisaster, Pycnopodia also did not significantly distinguish between the two algal shell covers (Fig. 3B, chi-square1 = 0.036, P(corrected) = 0.8501, n = 14 sea stars).
Discussion
Tegula brunnea are abundant herbivores in low intertidal and shallow subtidal rocky systems along the west coast of the USA (Morris et al. 1980; Watanabe 1984; Byrnes et al. 2006). The experiments presented here indicate that algal epibionts may benefit Tegula brunnea by conferring associational resistance against their sea star predators. Tegula brunnea in the field are often covered by one or more species of encrusting algae (Fig. 1), with a significant inverse correlation between snail density and sea star abundance. In fact, living snails (as well as empty or hermit-crab occupied shells) were absent at sites with high sea star densities (Fig. 2), thus precluding potential comparisons between the frequencies of shell coverings at sites across the natural range of sea star densities.
Although epibiont cover was not always effective at significantly reducing predation in the laboratory (i.e. Pisaster with coralline-covered shells versus bare-shelled snails), there was a trend of preference for bare snails in all cases. Thus, snails with crustose algal coverings generally experienced lower sea star predation relative to bare-shelled snails (Fig. 3A and B). Algal epibionts may also camouflage T. brunnea against visually searching predators (octopus, fish, crabs), as sites were frequently covered with foliose or crustose red algae the same colors as Peyssonnelia and/or coralline algae (C. Thornber, pers. obs.). Although I did not encounter crabs such as Cancer magister in the survey transects, they are present in this system and can prey on T. brunnea (Byrnes et al. 2006). Experimental tests of predation by visual predators such as Cancer would be a useful next step, however, Watanabe (1984) cautions that consumption of T. brunnea by visual predators is fairly low.
How do T. brunnea obtain their epibionts? Algal spores will, in general, opportunistically settle upon any hard substrate available, including other living organisms (Santelices 1990). Although the obligate green algal epibiont Cladophora conchopheria settles preferentially in areas of new growth on Turbo coronatus shells (Yamada et al. 2003), I did not observe any similar trends for T. brunnea. Juvenile T. brunnea typically live in the same habitats as adults, preferring the shallow subtidal, but are also found in the low intertidal (Watanabe 1984). Most of the crustose algal species found on T. brunnea have vertical distributions that span this range as well (Abbott & Hollenberg 1976). However, I have found no published studies examining if T. brunnea is capable of influencing algal epibiont settlement or growth. In addition, why some snails have algae and others do not is unknown. If behavioral differences among snails mediate their likelihood of being covered by algae (i.e. by living in different sub-habitats), then the impacts of predation measured here may also reflect behavioral variability in the snails, as opposed to simple settlement variability of the algae.
There are several potential mechanisms for the likely associational resistance with T. brunnea and its epibionts against sea stars. Epibionts may possess physical and/or chemical properties capable of repelling host predators (Wahl 1989; Stachowicz & Hay 1999), by increasing predator handling time and thus reducing their capture efficiency (Pitcher & Butler 1987) or by deterring predation via chemical cues (Laudien & Wahl 1999). In contrast to some upright (foliose) algal species (e.g. Stachowicz & Hay 1999), most crustose algal species (including those in the study system) are generally believed to have a few chemical defenses, due to the tough thallus of the alga providing a defense against herbivory. However, one coralline crustose alga, Lithophyllum yessoense, does release chemical cues that deter settlement by other algal spores (Kim et al. 2004).
In my experiments, I observed both Pisaster and Pycnopodia capturing and subsequently releasing algal-covered snails (as opposed to just ignoring them). This suggests that the sea stars can recognize algal-covered snails as food and that reduced capture efficiency may not be an issue. The actual mechanism(s) responsible for decreased predation on algal-covered snails may vary between Peyssonnelia and crustose corallines, but probably include chemical and/or tactile (such as increased handling time) mechanisms. Further analyses are difficult, because removing thin, tough crustose algae from snail shells for chemical analysis frequently kills the algae (potentially liberating chemical metabolites prior to analyzing the tissue) and usually damages the host’s shell.
Although T. brunnea appear to benefit from the presence of epibionts, it is unknown whether the reverse is true. Field surveys found that most T. brunnea shells possess at least one crustose algal species (Fig. 1), and that most of the available shell surface areas were covered with crustose algae. It is also suggestive that, although Peyssonnelia is frequently found on T. brunnea (Abbott & Hollenberg 1976), none of the algal species in this study is known to be obligate epiphyte and can occur on rocks as well. However, T. brunnea shells rarely had upright (foliose) algal species attached to them (only 10 of 208 snails were <25% covered with foliose algae). Foliose algae could probably increase the drag experienced by snails and thus increase their rate of dislodgement from rocks (C. Thornber, pers. obs.), potentially resulting in mortality (as “shared doom”) of both the snail and the algae (Wahl & Hay 1995).
Gastropods such as Tegula brunnea play key roles in rocky intertidal and subtidal marine communities along the west coast of North America (Paine 1969; Watanabe 1984; Byrnes et al. 2006). The trophic structure and linkages of these communities, including subtidal kelp forests, have been the focus of numerous ecological studies (Tegner & Dayton 1991; Wootton 1992; Sala & Graham 2002). Sea stars are important predators of gastropods in these systems (Paine 1969; Watanabe 1983), and changes in the strength of predation, such as those suggested here, have the potential to structure herbivore–algal interactions (Steinberg 1988; Byrnes et al. 2006).
Acknowledgements
M. Graham, L. Levin, E. Preisser, J. Stachowicz, and anonymous reviewers provided thoughtful comments towards improving this manuscript. K. Rudolph and M. Nydam provided field and laboratory help. Research funding and seawater space were obtained from UC Davis and the Bodega Marine Laboratory. Funding was provided by the University of Rhode Island to C. S. T. and grant #OCE 82049 from the National Science Foundation to J. Stachowicz. This is contribution no. 2382 of the Bodega Marine Laboratory, University of California at Davis.




