A Comparison Among Assemblages in Areas Invaded by Caulerpa taxifolia and C. racemosa on a Subtidal Mediterranean Rocky Bottom
Abstract
Abstract. This study compares the structure of Mediterranean macroalgal assemblages invaded by Caulerpa taxifolia and C. racemosa. Assemblages in areas colonized by the two algae and in reference areas were sampled and analyzed for 2 years. Significant differences were recorded both between reference and invaded areas and between areas invaded by different Caulerpa species. Macroalgal assemblages colonized by C. racemosa were more separated from references than those colonized by C. taxifolia. Differences between assemblages colonized by C. racemosa and the others decreased during the alga's period of vegetative rest and increased at the last sampling date. While erect and turf species showed similar patterns in invaded areas, covers of encrusting algae were lower in C. racemosa areas than in C. taxifolia areas.
Problem
Biological invasions in marine habitats are widely reported and represent a recognized threat to the integrity of native communities (Carlton, 1989; Carlton & Geller, 1993; Cohen et al., 1995; Trowbridge, 1995). In particular, modified benthic assemblage structure and lower biodiversity are described worldwide as a consequence of the spread of alien plants (Critchley et al., 1990; Chambers et al., 1993; Viejo, 1997; Walker & Kendrick, 1998). Knowing the mechanisms and effects of invasions is an important goal if ecologists wish to predict the consequences of this phenomenon (Grosholz & Ruiz, 1995; Abrams, 1996).
In the Mediterranean Sea, more than 60 macroalgal species have been introduced by international shipping, aquaculture and through the Suez Canal (Ribera & Boudouresque, 1995). Although most species have remained confined to restricted areas, others showed invasive traits (Verlaque, 1994). Chlorophyta belonging to Caulerpaceae are considered particularly invasive because of their capacity for vegetative growth and population persistence (Meinesz et al., 1995; Piazzi & Cinelli, 1999). Their spread represents a very serious ecological problem in the Mediterranean basin (Gravez et al., 2001).
Caulerpa taxifolia (Vahl) C. Agardh has occurred along French coasts since 1984 (Meinesz & Hesse, 1991) and thereafter spread along Italian, Spanish and Croatian coasts (Boudouresque et al., 1992; Meinesz et al., 1993, 2001).
Caulerpa racemosa (Forsskål) J. Agardh has been considered a lessepsian migrant (Lipkin, 1972); it long colonized the eastern Mediterranean Sea (Hamel, 1926; Aleem, 1948) and has recently spread in the western part of the basin (Piazzi et al., 1994, 1997; Gambi & Terlizzi, 1998; Modena et al., 2000), where it represents a new introduction (Verlaque et al., 2000, 2003).
Both the Caulerpa are strong competitors; in colonized areas they tend to eliminate native species and often constitute monospecific beds (Verlaque & Fritayre, 1994; Piazzi et al., 2001b). Until now, C. racemosa and C. taxifolia invasions have been studied only separately because of their different distribution. The two Caulerpa came into contact along the Tuscany coast near Leghorn in 1996 (Piazzi et al., 2001a). Spreading characteristics of co-occurring populations have been compared and interactions between the two species have been evaluated in previous studies (Piazzi et al., 2001a; Piazzi & Ceccherelli, 2002).
The aim of this work is to compare the structure of Mediterranean macroalgal assemblages invaded by C. taxifolia and by C. racemosa. Accordingly, macroalgae in areas colonized by the two Caulerpa and in reference areas were sampled and analyzed over a 2-year period. A combination of univariate and multivariate analysis was employed to detect differences among samples.
Material and Methods
The study was carried out along the Tuscany coast (43°28′24′′ N, 10°19′42′′ E) on a very exposed rocky platform at 10 m depth. The bottom was colonized by a macroalgal assemblage dominated by algal turfs (Airoldi et al., 1995). At this location, C. taxifolia and C. racemosa first co-occurred in 1996 (Piazzi et al., 2001b).
Three different conditions were selected: areas colonized by C. racemosa, areas colonized by C. taxifolia and areas without Caulerpa species. The study areas were sampled five times in 2 years: October 1999, April 2000, October 2000, April 2001 and October 2001. October and April were chosen based on the vegetative cycle shown by the two Caulerpa species in previous studies; these months represent the end of vegetative growth and the end of the rest period, respectively (Piazzi et al., 2001a). Two sites were randomly chosen for each combination of condition and date. In each site two replicated samples were collected by scraping off 400 cm2 surface. Materials were preserved in 4% formalin seawater and observed under a microscope to identify macroalgal species and to evaluate cover of each species. Cover was estimated as surface covered in vertical projection by each species and expressed as percentage of the sampling surface (Boudouresque, 1971).
For each sample, species number was recorded and the total percent cover of algae (Caulerpa excluded) was calculated as the sum of percent cover of all species found. Percent cover of turf, encrusting and erect layers was also calculated (Verlaque & Fritayre, 1994; Airoldi et al., 1995).
Species number, total percent cover and percent cover of vegetation layers were analyzed by three-way ANOVA, with condition (C. racemosa versus C. taxifolia versus references) as fixed factor, date (five levels) as random factor orthogonal to condition and site (two levels) as random factor nested in date and condition. Homogeneity of variances was checked by the Cochran C-test. The Student-Newman-Keuls (SNK) test was used for a posteriori multiple comparison of means (Underwood, 1997).
Similarity in species composition and abundance among samples was analyzed using Bray-Curtis similarity coefficients. Data were double square root transformed before calculation of Bray-Curtis coefficients. For graphical representation of the data, a two-dimensional non-metric multidimensional scaling (nMDS) ordination was carried out. One-way pairwise analysis of similarity (ANOSIM) was performed to test differences among conditions. The SIMPER method was used to examine taxa that contributed to the patterns shown by MDS analysis (Clarke & Warwick, 1994).
Results
A total of 98 macroalgal species was identified: 11 Chlorophyta, 15 Fucophyceae and 72 Rhodophyta (Table 1). Halimeda tuna, Flabellia petiolata, Laurencia obtusa and Cladophora prolifera were the most abundant species in the erect layer. Turf was dominated by Womersleyella setacea, but Acrothamnion preissii, Jania rubens and Haliptilon virgatum were also common. The encrusting layer was characterized by encrusting coralline algae and Peyssonneliaceae.
| C. racemosa | C. taxifolia | reference areas | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O 99 | A 00 | O 00 | A 01 | O 01 | O 99 | A 00 | O 00 | A 01 | O 01 | O 99 | A 00 | O 00 | A 01 | O 01 | |
| Chlorophyta | |||||||||||||||
| Acetabularia acetabulum (Linnaeus) P. C. Silva | − | + | − | − | − | − | + | − | − | − | − | − | − | − | − |
| Bryopsis cupressina J. V. Lamouroux | − | − | − | + | − | − | − | − | − | − | − | − | − | + | + |
| Chaetomorpha linum (O. F. Müller) Kützing | + | + | + | − | + | + | + | + | − | + | + | + | − | + | + |
| Cladophora dalmatica Kützing | − | + | − | − | − | − | + | − | + | − | − | − | − | − | − |
| Cladophora prolifera (Roth) Kützing | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Codium bursa (Linnaeus) C. Agardh | − | − | − | − | − | + | − | − | − | + | − | − | − | − | − |
| Derbesia tenuissima (Moris et De Notaris) P. L. & H. M. Crouan | − | − | − | − | − | − | − | − | − | − | + | − | − | + | + |
| Flabellia petiolata (Turra) Nizamuddin | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Halimeda tuna (J. Ellis et Solander) J. V. Lamouroux | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Pseudochlorodesmis furcellata (Zanardini) Børgesen | + | − | − | + | + | + | + | + | + | − | + | + | + | + | + |
| Valonia macrophysa Kützing | − | + | − | + | − | − | − | − | − | − | − | − | − | + | − |
| Fucophyceae | |||||||||||||||
| ‘Aglaozonia parvula (Greville) Zanardini’ stadium sporophyte of Cutleria multifida (Smith) Greville | − | − | − | − | − | − | − | − | − | + | + | − | − | − | − |
| Cladosiphon sp. | − | − | − | − | − | + | − | − | − | − | − | + | − | − | − |
| Cladostephus spongiosus (Hudson) C. Agardh | − | − | + | − | − | − | − | − | − | + | − | − | − | − | + |
| Dictyopteris polypodioides (A. P. De Candolle) J. V. Lamouroux | − | + | − | − | − | − | + | − | − | − | − | − | − | − | − |
| Dictyota dichotoma (Hudson) J. V. Lamouroux | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Dictyota fasciola (Roth) J. V. Lamouroux | − | + | − | − | − | − | − | + | − | − | − | − | − | − | − |
| Dictyota linearis (C. Agardh) Greville | + | + | + | − | − | + | − | − | − | + | + | − | − | + | + |
| Ectocarpus sp. | − | − | + | − | − | − | − | − | − | − | + | − | − | − | − |
| Halopteris filicina (Grateloup) Kützing | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Nereia filiformis (J. Agardh) Zanardini | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − |
| Padina pavonica (Linnaeus) Thivy | − | + | − | − | − | − | + | − | + | − | + | + | − | − | + |
| Sphacelaria cirrosa (Roth) C. Agardh | + | + | + | + | + | + | + | + | + | + | + | + | − | + | + |
| Sphacelaria plumula Zanardini | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − |
| Stypocaulon scoparium (Linnaeus) Kützing | + | + | + | + | + | + | + | − | − | + | − | + | − | + | + |
| Zanardinia typus (Nardo) G. Furnari | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − |
| Rhodophyta | |||||||||||||||
| Acrodiscus vidovichii (Meneghini) Zanardini | − | + | − | + | − | + | + | − | + | − | − | − | − | − | + |
| Acrosorium venulosum (Zanardini) Kylin | + | + | + | + | + | − | − | + | + | + | − | + | + | + | + |
| Acrosymphyton purpuriferum (J. Agardh) G. Sjösted | − | − | − | + | − | − | − | − | + | − | − | − | − | + | − |
| Acrothamnion preissii (Sonder) Wollaston | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Aglaothamnion tenuissimum (Bonnemaison) Feldmann-Mazoyer | + | − | − | + | − | − | − | − | + | − | + | − | − | + | − |
| Aglaothamnion tripinnatum (C. Agardh) Feldmann-Mazoyer | − | − | − | − | − | + | − | − | − | − | − | − | + | + | − |
| Amphiroa rigida J. V. Lamouroux | + | + | + | − | + | + | + | + | − | + | + | + | + | + | + |
| Anotrichum barbatum (Smith) Nägeli | − | − | − | − | − | − | − | − | + | − | + | − | − | + | − |
| Antithamnion cruciatum (C. Agardh) Nägeli | + | + | + | + | + | + | − | + | + | + | − | − | + | + | + |
| Antithamnion tenuissimum (Hauck) Schiffner | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − |
| Apoglossum ruscifolium (Turner) J. Agardh | + | − | + | − | − | + | − | − | − | − | − | − | − | − | − |
| Bonnemaisonia asparagoides (Woodward) C. Agardh | − | − | − | − | − | − | − | − | + | − | − | − | − | + | − |
| Botryocladia botryoides (Wulfen) Feldmann | + | + | + | + | − | + | + | + | + | + | − | + | + | + | + |
| Callithamnion corymbosum (J. E. Smith) Lyngbye | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − |
| Ceramium circinatum (Kützing) J. Agardh | + | + | + | + | + | + | − | − | + | + | − | − | + | + | + |
| Ceramium codii (H. Richards) Feldmann-Mazoyer | + | − | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Ceramium diaphanum (Lighfoot) Roth | − | − | − | − | − | − | − | − | − | − | + | − | − | + | − |
| Ceramium flaccidum (Kützing) Ardissone | − | + | + | + | − | + | − | − | + | + | − | − | − | + | + |
| Champia parvula (C. Agardh) Harvey | + | + | + | + | + | + | + | − | − | + | − | + | + | + | + |
| Chondria capillaris (Hudson) M. J. Wynne | + | − | − | + | + | + | − | − | + | − | + | − | − | + | − |
| Chondria dasyphylla (Woodward) C. Agardh | + | − | − | + | − | + | − | − | − | + | − | − | − | + | + |
| Chrysymenia ventricosa (J. V. Lamouroux) J. Agardh | − | + | − | + | − | − | + | − | + | + | − | + | − | + | − |
| Contarinia squamariae (Meneghini) Denizot | − | − | + | + | − | − | + | + | − | − | − | − | − | + | + |
| Corallina elongata J. Ellis & Solander | + | + | − | + | + | + | + | + | + | + | + | − | − | + | + |
| Crouania attenuata (C. Agardh) J. Agardh | + | − | − | + | − | + | − | − | + | + | − | − | − | + | − |
| Dasya corymbifera J. Agardh | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − |
| Dasya ocellata (Grateloup) Harvey | + | − | − | − | − | + | − | − | − | − | − | − | − | − | + |
| Dasya rigidula (Kützing) Ardissone | + | + | + | + | − | + | − | + | + | + | + | + | + | + | + |
| Dipterosiphonia rigens (C. Agardh) Falkenberg | − | + | + | − | − | − | − | + | − | − | − | − | − | + | − |
| Eupogodon cervicornis (J. Agardh) Kützing | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| Eupogodon planus (C. Agardh) Kützing | + | + | + | − | + | − | + | + | − | − | + | − | + | − | + |
| ‘Falkenbergia rufolanosa (Harvey) F. Schmit’stadium sporophyte of Asparagopsis armata Harvey | + | − | + | + | − | + | − | + | + | + | − | − | + | + | + |
| Feldmannophycus rayssiae (Feldmann & Feldmann-Mazoyer) Augier et Boudouresque | + | + | + | + | + | + | + | + | + | + | − | + | + | + | + |
| Gelidiella lubrica (Kützing) Feldmann & Hamel | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − |
| Gelidium bipectinatum G. Furnari | + | + | − | + | + | − | − | − | + | − | − | + | + | + | + |
| Gelidium pusillum (Stackhouse) Le Jolis | + | − | − | + | − | − | − | − | − | − | − | − | − | + | − |
| Haliptilon virgatum (Zanardini) Garbary & H. W. Johansen | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Halydiction mirabile Zanardini | − | − | − | − | − | − | − | + | − | − | − | − | − | − | + |
| Halopitys incurva (Hudson) Batters | + | + | + | − | + | + | + | − | − | − | − | − | − | − | + |
| Haraldia lenormandii (Derbès & Solier) Feldmann | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − |
| Herposiphonia secunda (C. Agardh) Ambronn | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Heterosiphonia crispella (C. Agardh) M. J. Wynne | + | − | + | + | + | + | − | + | + | + | − | − | + | + | + |
| Hypoglossum hypoglossoides (Stackhouse) Collins & Hervey | + | + | + | + | + | + | + | + | + | + | − | + | + | + | + |
| Jania rubens (Linnaeus) J. V. Lamouroux | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Laurencia obtusa (Hudson) J. V. Lamouroux | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Lomentaria chylocladiella Funk | − | − | − | + | − | − | − | − | + | − | − | − | − | + | − |
| Meredithia microphylla (J. Agardh) J. Agardh | + | + | + | + | + | − | + | + | + | + | − | + | + | + | + |
| Monosporus pedicellatus (J. E. Smith) Solier | + | + | + | + | + | + | + | + | + | + | − | + | + | + | + |
| Nitophyllum punctatum (Stackhouse) Greville | + | + | + | + | − | + | + | + | + | + | + | − | + | + | + |
| Osmundea pelagosae (Schiffner) Nam | − | − | + | − | − | − | + | − | + | + | − | − | − | − | − |
| Peyssonnelia rubra (Greville) J. Agardh | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Peyssonnelia squamaria (S. G. Gmelin) Decaisne | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + |
| Peyssonnelia stoechas Boudouresque & Denizot | − | − | + | − | − | + | + | − | − | + | − | − | − | − | − |
| Phyllophora crispa (Hudson) P. S. Dixon | − | + | − | − | − | − | − | − | − | − | + | − | − | − | − |
| Plocamium cartilagineum (Linnaeus) P. S. Dixon | + | + | + | + | − | + | − | + | + | + | + | + | + | + | − |
| Polysiphonia elongata (Hudson) Sprengel | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − |
| Polysiphonia furcellata (C. Agardh) Harvey | + | + | + | + | − | − | + | − | + | + | + | + | − | + | + |
| Polysiphonia subulifera (C. Agardh) Harvey | + | + | + | + | + | − | − | + | + | − | − | − | − | − | + |
| Polysiphonia tripinnata J. Agardh | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + |
| Pterothamnion plumula (J. Ellis) Nägeli | + | + | + | − | − | + | − | − | − | + | − | − | − | − | − |
| Ptilothamnion pluma (Dillwyn) Thuret | − | − | − | + | − | − | − | − | − | − | − | − | − | + | + |
| Radicilingua reptans (Kylin) Papenfuss | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − |
| Rhodymenia sp. | − | − | − | − | − | − | + | − | − | − | − | − | + | − | − |
| Rodriguezella strafforellii F. Schimtz ex Rodriguez | − | + | + | − | + | − | + | + | + | + | − | − | − | + | + |
| Seirospora sp. | − | − | + | + | − | + | − | − | + | − | − | − | − | + | − |
| Spermothamnion flabellatum Bornet | − | − | − | − | − | − | − | − | − | − | − | − | + | − | + |
| Sphaerococcus coronopifolius Stackhouse | + | + | + | + | + | − | − | + | + | + | − | − | + | + | + |
| Spyridia filamentosa (Wulfen) Harvey | + | + | + | − | − | − | − | − | − | − | − | − | − | − | − |
| Tricleocarpa fragilis (Linnaeus) Huisman & R. A. Townsend | − | − | − | − | − | − | − | − | + | − | − | − | − | + | + |
| Womersleyella setacea (Hollenberg) R .E. Norris | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Wrangelia penicillata (C. Agardh) C. Agardh | + | + | + | + | − | + | − | + | + | + | + | + | + | − | + |
| encrusting Corallinaceae | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
Both C. taxifolia and C. racemosa cover showed strong seasonal variations, with lower values in April and higher values in October. In October 2001, the percent cover of C. taxifolia and C. racemosa was 100 ± 0 and 98.3 ± 1.6 (mean ± SE, n = 4), respectively, while the minimal values were shown by C. taxifolia (2.7 ± 2.1) and by C. racemosa (3.5 ± 0.9) in April 2000 (Fig. 1).
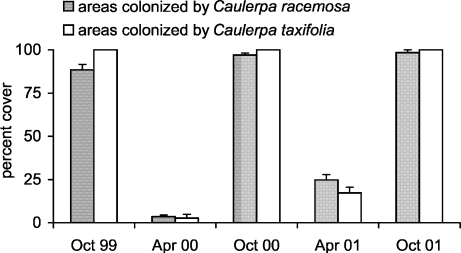
Temporal fluctuation of Caulerpa taxifolia and C. racemosa percent cover in invaded areas (means ± SE, n = 4).
Total percent cover in reference areas ranged between 171 ± 21.1 in October 1999 and 202.7 ± 11.9 in April 2001; in C. racemosa areas, values ranged between 62.2 ± 7.1 in October 2000 and 130.6 ± 5.5 in April 2000, in C. taxifolia areas between 107.7 ± 17.4 in October 1999 and 195.7 ± 23.7 in April 2000 (Fig. 2A). ANOVA detected significant differences between conditions and dates (Table 2/I). Values in reference areas were higher than in invaded areas, and in C. racemosa areas values were lower than in C. taxifolia areas.
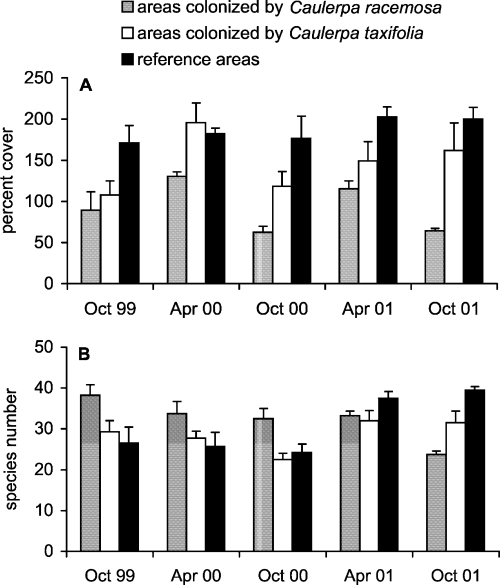
The total percent cover (A) and the species number of macroalgal assemblages (B) in reference areas and areas invaded by Caulerpa taxifolia and C. racemosa (means ± SE, n = 4).
| source of variation | df | (I) total percent cover | (II) species number | ||
|---|---|---|---|---|---|
| MS | F | MS | F | ||
| condition = c | 2 | 44, 604.46 | 22.84 | 68.60 | 0.50 |
| date = d | 4 | 5541.30 | 4.15 | 101.18 | 5.40 |
| sites (c × d) | 15 | 1336.44 | 0.97 | 18.73 | 0.74 |
| c × d | 8 | 1953.09 | 1.46 | 138.5 | 7.40 |
| residual | 30 | 1376.00 | 25.30 | ||
| SNK test | ra > Ct > Cr | d1: Cr > ra = Ct d2: Cr = ra = Ct | |||
| d3: Cr > ra = Ct d4: Cr = ra = Ct | |||||
| d5: ra > Ct > Cr | |||||
| Cochran's C-test | C = 0.212 | C = 0.168 | |||
- Significant values in bold. ra: reference areas; Cr: C. racemosa areas; Ct: C. taxifolia areas; d1–d5: date 1–5 (Oct 99, Apr 00, Oct 00, Apr 01, Oct 01).
The number of species in reference areas ranged between 24.3 ± 2.1 in October 2000 and 39.5 ± 0.9 in October 2001, in C. racemosa areas between 23.7 ± 0.8 in October 2001 and 38.2 ± 2.5 in October 1999, in C. taxifolia areas between 22.5 ± 1.5 in October 2000 and 32 ± 2.4 in April 2001 (Fig. 2B). ANOVA detected a significant interaction between conditions and dates (Table 2/II). At the end of the study period, values in C. racemosa areas were lower than those of C. taxifolia, and in both the invaded areas values were lower than in reference areas; this pattern is opposite that of the first sampling dates. Differences between conditions were not significant in April.
The percent cover of the encrusting layer in reference areas ranged between 66.5 ± 13 in October 1999 and 102.7 ± 15.3 in April 2001 (Fig. 3A). Values in reference areas were significantly higher than in invaded areas, and in C. taxifolia areas values were higher than in C. racemosa areas (Table 3/I).
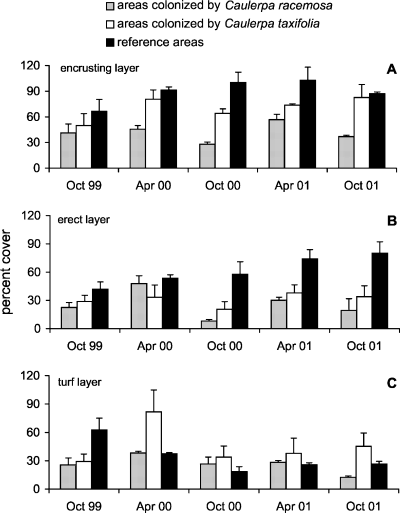
The percent cover of encrusting (A), erect (B) and turf (C) layers in reference areas and in areas invaded by Caulerpa taxifolia and C. racemosa (means ± SE, n = 4).
| source of variation | df | (I) encrusting layer | (II) erect layer | (III) turf layer | |||
|---|---|---|---|---|---|---|---|
| MS | F | MS | F | MS | F | ||
| condition = c | 2 | 11637.69 | 25.70 | 8331.26 | 11.67 | 0.68 | 1.23 |
| date = d | 4 | 1097.11 | 4.04 | 926.11 | 2.20 | 0.95 | 2.77 |
| sites (c × d) | 15 | 271.38 | 0.68 | 420.47 | 1.81 | 0.34 | 1.27 |
| c × d | 8 | 452.90 | 1.67 | 713.69 | 1.70 | 0.55 | 1.61 |
| residual | 30 | 399.23 | 232.01 | 0.27 | |||
| SNK test | ra > Ct > Cr | ra > Ct = Cr | |||||
| Cochran's C-test | C = 0.232 | C = 0.192 | C = 0.255 | ||||
| transformations | ln(x+1) | ||||||
- Significant values in bold. ra: reference areas; Cr: C. racemosa areas; Ct: C. taxifolia areas.
The percent cover of the erect layer in reference areas ranged between 41.8 ± 7.8 in October 1999 and 86.4 ± 13.4 in October 2001, remaining higher than in invaded areas throughout the study period (Table 3/II). In invaded areas, erect species increased in April but showed low cover in October (Fig. 3B).
The percent cover of the turf layer varied in reference areas from 18.5 ± 5.2 in October 2000 to 62.6 ± 12.5 in October 1999 (Fig. 3C). Differences were not significant both for conditions and dates (Table 3/III).
In October, MDS ordination showed that C. taxifolia and C. racemosa areas constituted two distinct groups with closed samples, while samples of references were more dispersed. In April, reference and invaded samples were close to one another, except for C. racemosa samples in April 2000 (Fig. 4).
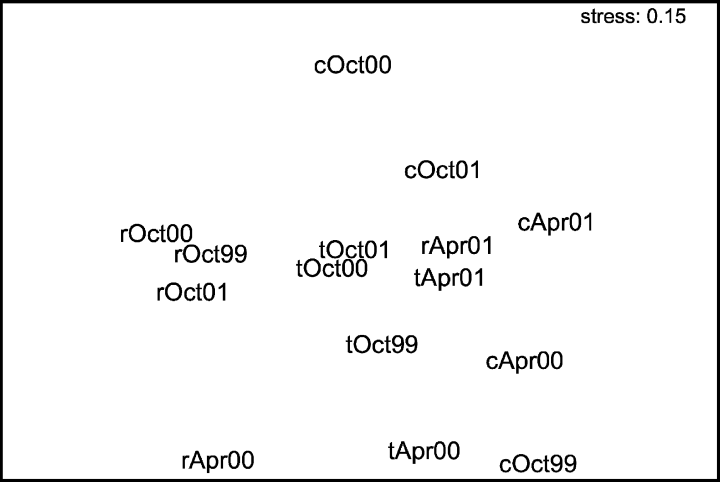
MDS ordination of all replicate samples taken in reference areas (c) and in areas invaded by Caulerpa taxifolia (t) and C. racemosa (r).
The ANOSIM test showed that, in October, C. taxifolia and reference areas were close together, while C. racemosa samples were well separated from the others. The distance between C. racemosa areas and the others decreased in April (Table 4).
| global R | significance (%) | |
|---|---|---|
| October | ||
| C. racemosa areas versus C. taxifolia areas | 0.567 | P < 0.01 |
| C. racemosa areas versus reference areas | 0.740 | P < 0.01 |
| C. taxifolia areas versus reference areas | 0.172 | P < 0.05 |
| April | ||
| C. racemosa areas versus C. taxifolia areas | 0.421 | P < 0.01 |
| C. racemosa areas versus reference areas | 0.477 | P < 0.01 |
| C. taxifolia areas versus reference areas | 0.292 | P < 0.01 |
The SIMPER test showed that, in October – the period of high vegetative growth for Caulerpaceae – encrusting Corallinaceae mostly contributed to separate C. racemosa samples from the others, while the Chlorophyta Flabellia petiolata and Halimeda tuna were important to discriminate between reference and invaded areas. The abundance of Womersleyella setacea was different in areas colonized by C. racemosa and C. taxifolia (Fig. 5).
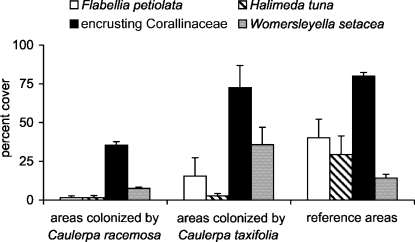
The percent cover of Flabellia petiolata, Halimeda tuna, Womersleyella setacea and encrusting Corallinaceae in invaded and reference areas in October 2001 (means ± SE, n = 4).
Discussion
Caulerpa taxifolia and C. racemosa showed a similar vegetative cycle, as previously described in other studies (Komatsu et al., 1997; Piazzi & Cinelli, 1999). Their decrease in cover in April was higher than elsewhere in the Mediterranean (Meinesz et al., 1995; Piazzi et al., 2001b). This disagreement probably reflects the exposition of the study location to storms that may remove the two Caulerpa species after the end of the vegetative period when they are more sensitive to disturbance.
Univariate and multivariate analysis detected significant differences both between reference and invaded areas and between C. taxifolia and C. racemosa areas. In April, differences between C. racemosa areas and the others decreased, following the vegetative cycle of the alga, while they tended to increase at the last sampling date.
The structure of invaded communities was apparently related to the invader. While erect and turf species showed similar patterns in the invaded areas, the cover of encrusting algae was lower in C. racemosa areas than in C. taxifolia areas. These results agree with previous studies carried out in separated locations (Verlaque & Fritayre, 1994; Piazzi et al., 2001b). Differences may be related to the different morphology and competition mechanisms exhibited by the two species. C. taxifolia is a canopy species that can interfere with both erect algae and seagrasses such as Posidonia oceanica (L.) Delile (Villèle & Verlaque, 1995) and Cymodocea nodosa (Ucria) Ascherson (Ceccherelli & Cinelli, 1997). C. racemosa, although smaller, quickly form extensive mats of stolons and trapped sediments that typically affect the lowest vegetation layers.
Differences between references and invaded assemblages were less evident than those observed in other Mediterranean locations both for C. taxifolia (Verlaque & Fritayre, 1994) and for C. racemosa (Piazzi et al., 2001b). One potential explanation is the strongly reduced cover of the two species in the cold period in a very exposed location. Persistence also during the rest period in more sheltered locations, as reported in other studies (Meinesz et al., 1995; Ceccherelli & Cinelli, 1998; Piazzi et al., 2001b), might hinder the spread of native algae, while the decrease of Caulerpa cover might allow native species to reconstitute their populations. However, differences between invaded and reference areas tended to increase in the third year of study, especially for C. racemosa.
Moreover, the presence of other introduced algae in the studied assemblage, such as Womersleyella setacea and Acrothamnion preissii, might modify the interaction between the two Caulerpa species and native algae, differently affecting the populations. In fact, the structure of macroalgal assemblages might already have been changed by the presence of invasive turfs (Airoldi et al., 1995), modifying the effect of Caulerpa invasion. A possible explanation for the high species number observed in 1999–2000 in C. racemosa areas is the competition between this alga and turfs: C. racemosa might initially affect W. setacea, permitting other turf species that are normally outcompeted by the Rhodophyta to spread; after the first phases of colonization, however, the species number decreases, as C. racemosa probably also eliminates other species. The lack of a formal BACI design (Underwood, 1994) did not permit us to verify this assertion. Experimental studies that isolate the different factors will be needed to test this model.
In general, differences in the macroalgal assemblage between invaded and reference areas were stronger in the present study in C. racemosa areas than in C. taxifolia areas. In a previous study carried out in the same zone, C. racemosa showed a higher invasiveness (growth ability) than C. taxifolia (Piazzi et al., 2001a). The present data also indicate that C. racemosa invasion here is more serious than that of C. taxifolia. Finally, the patterns observed in our study suggest that the effects of Caulerpa may change in relation to different environmental and biological factors. As invasion effects are difficult to predict, appropriate long-term studies are necessary to achieve this objective.
Summary
In this study, significant differences in the composition and structure of Mediterranean macroalgal assemblages were detected between areas invaded by introduced Caulerpa species and reference sites. Differences between C. racemosa and C. taxifolia areas were also evident, as vegetation layers were differently affected. The high exposition of the location and the presence of introduced turf species seem to partially modify the interaction between Caulerpa and native algae if compared with results of previous studies. Further investigations are necessary to elucidate these ecological aspects.
Acknowledgement
This work was supported by SINAPSI research project (MURST – CNR program).




