Bergmann’s rule in amphibians: combining demographic and ecological parameters to explain body size variation among populations in the common toad Bufo bufoLa règle de Bergmann chez le Amphibiens: combiner les paramètres démographiques et écologiques pour expliquer la variation de la taille corporelle entre les populations de Crapaud commun Bufo bufo.
Abstract
enLarge-scale patterns of body size variation are described by well-known generalizations such as Bergmann’s rule; the generality and underlying causes of these patterns have been much debated. Intraspecific extension of this rule was tested in various ectotherms, and evidence was found for both Bergmann and converse Bergmann clines. In this study, we explored spatial patterns of variation in a widespread amphibian, the Common toad (Bufo bufo), along a 2240 km latitudinal gradient across Europe. We tested for covariation of adult body size, age and growth parameters with latitude, altitude, length of activity period and mean temperature during this period using both original and literature data. We selected 13 European populations, representing a latitudinal range from 43 to 63°N and altitudinal range from 15 to 1850 m a.s.l. The length of activity period (12–33 weeks) and Tmean (6.6–15.6°C) significantly decreased as latitude and altitude of these populations increased. Mean body size decreased as latitude increased (not with altitude), and increased with Tmean (not with length of activity period). Mean and minimal adult age increased with latitude and altitude, longevity increased with altitude only. Age increased as length of activity period decreased (not with Tmean). The growth coefficient (0.32–0.92 in males, 0.18–0.74 in females, available for six populations) decreased as altitude increased, and increased as both length of activity period and Tmean increased; latitudinal trend was non-significant. Our analysis shows that B. bufo clearly exhibited a converse Bergmann cline along latitudinal gradient, but not along altitudinal gradient; the main effect of elevation was on age. The effects of ecological conditions also differed: body size increased with Tmean, while age parameters were related to the length of activity period. This study highlights that, to identify causal factors underlying general ecogeographical rules, we have to take into account different phases of the life cycle, co-variation among life history traits and ecological factors acting on each of these traits. In amphibians with complex life cycles, lack of appropriate demographical or ecological data may affect our understanding of the variety of observed body size patterns.
Résumé
esLes variations à grande échelle de la taille corporelle sont décrites par des généralisations comme la célèbre règle de Bergmann. Son application générale et les causes des variations observées ont été largement débattues. L’extension de cette règle à l’échelle intraspécifique a été testée chez de nombreux ectothermes, avec des résultats contrastés, allant dans le sens ou l’opposé des prédictions de cette règle. Dans cette étude, nous avons exploré les variations observées chez un amphibien à grande aire de répartition, le Crapaud commun (Bufo bufo), le long d’un gradient latitudinal 2240 km en Europe. Nous avons testé la co-variation de la taille corporelle des adultes avec leur âge et des paramètres de la croissance en fonction de la latitude, de l’altitude, de la durée de la période d’activité et de la température moyenne au cours de cette période (Tmoyenne), à l’aide de données originales et de la littérature. Nous avons sélectionné 13 populations en Europe, réparties de 43°N à 63°N de latitude, et de 15 à 1850 m d’altitude. La durée de la période d’activité (12 à 33 semaines) et la Tmoyenne (6.6°C à 15.6°C) observées pour ces populations diminuent quand la latitude et l’altitude augmentent. La taille corporelle moyenne décroît quand la latitude augmente (mais pas l’altitude), et augmente avec la Tmoyenne, (mais pas avec la durée de la période d’activité). Les âges moyen et minimal augmentent avec l’altitude et la latitude, alors que la longévité n’augmente qu’avec l’altitude. L’âge augmente quand la durée de la période d’activité diminue (pas avec la Tmoyenne). Le coefficient de croissance (0.32 à 0.92 pour les mâles, 0.18 à 0.74 pour les femelles, disponibles pour 6 populations) diminue quand l’altitude augmente, et aussi quand la durée de la période d’activité et la Tmoyenne diminuent (relation non-significative le long du gradient latitudinal). Notre analyse montre clairement que la taille corporelle de B. bufo suit l’inverse de la prédiction de la règle de Bergmann le long du gradient latitudinal. La taille corporelle ne varie pas avec l’altitude, contrairement à l’âge des individus. Les effets des conditions écologiques diffèrent également, avec une augmentation de la taille corporelle avec la Tmoyenne, alors que les variations d’âge dépendent de la durée de la période d’activité. Cette étude confirme que l’identification des facteurs de causalité sous-jacents aux règles écogéographiques générales nécessite la prise en compte des différentes phases du cycle vital, de la co-variation des traits d’histoire de vie et des facteurs écologiques agissant sur ces traits. Chez les amphibiens avec un cycle vital complexe, le manque de données démographiques et écologiques pourrait limiter notre compréhension de la variété des patrons de variations de la taille corporelle observés.
Introduction
An important issue in evolutionary ecology is the analysis of intra- and interspecific patterns of variation in body size. One of the most well-known generalizations concerning these patterns (i.e. ‘ecogeographical rules’) is Bergmann’s rule (Bergmann 1847), which states that body size of endothermic species increases from warm to cool climates and thus from low to high latitude or altitude. Ray (1960) proposed that ectothermic species also follow this rule. Initially formulated for interspecific level, it was later extended to intraspecific level (e.g. Mayr 1963).
The validity of Bergmann’s rule in endothermic species is now well-documented (Ashton et al. 2000; Ashton 2002a; Freckleton et al. 2003). Meiri and Dayan (2003) report that it holds for over 72% and 65% of bird and mammal species respectively. However, the general applicability of this rule (to both ectotherms and endotherms) has been vigorously debated as evidence was found for both Bergmann and converse Bergmann clines, as well as for inconsistent biogeographical patterns, in various groups of ectotherms (Lindsey 1966; Blanckenhorn and Fairbairn 1995; Mousseau 1997; Ashton 2002b; Belk and Houston 2002; Ashton and Feldman 2003; Blanckenhorn and Demont 2004; Olalla-Tárraga et al. 2006).
Evaluating the generality of geographical patterns of body size variation, and understanding the underlying mechanisms requires comprehensive reviews for various groups (Belk and Houston 2002; Laugen et al. 2005), and the number of species studied so far is both taxonomically biased and insufficient (Gaston et al. 2008). Among them, amphibians are particularly interesting (Ashton 2002b; Laugen et al. 2005; Olalla-Tárraga and Rodríguez 2007; Adams and Church 2008), and resolving this question for amphibians is considered an important step in the understanding of body size clines evolution in vertebrates in general (Adams and Church 2008).
Empirical evidence for the prevalence of Bergmann clines in amphibians is still controversial. Ashton’s (2002b) showed that most amphibian species exhibited Bergmann clines with respect to latitude or altitude, although this trend was not significant within anurans. Olalla-Tárraga and Rodríguez (2007) concluded that anurans follow a marked Bergmann’s rule pattern and urodeles the reverse. On the other hand, a recent meta-analysis of Adams and Church (2008) questioned the generality of Bergmann’s rule, since they found no support for it at the class (Amphibia) level.
The aim of this study was to analyse body size variation among populations in the Common toad, Bufo bufo, a species with wide latitudinal and altitudinal distribution in Europe. Ashton’s (2002b) reported results for only three species in family Bufonidae, and two of them followed the inverse of Bergmann’s rule.
Body size is a complex attribute and it is unrealistic to expect a single factor underlying its variation, although the relationship with temperature has always been in the focus (Van Voorhies 1996; Angilletta et al. 2004). The concave pattern of latitudinal body size variation in the Common frog (Laugen et al. 2005) suggests more complex mechanisms than simple correlation with temperature. In amphibians, growth rates can decrease dramatically after the attainment of sexual maturity (e.g. Hemelaar 1988; Miaud et al. 1999), and delayed reproduction can, thus, allow a prolonged growth period and the attainment of larger adult size. As a consequence, interpopulational variation in adult body size can be explained by difference in the age structure.
In this study, we tested for association of body size, age and growth parameters with positional and environmental variables: latitude, altitude, length of activity period (AP) and mean temperature during activity period (Tmean) across the European range of B. bufo. We combined the data on variation in body size and associated life history traits obtained from two populations from the southern part of range (Serbia) (Tomaševic et al. 2008; this study) with the available data from literature. This allowed us to explore the patterns of body size and age variation among 13 populations in Europe, representing a latitudinal range from 43°N (southern France) to 63°N (Norway) and an altitudinal range from 15 m a.s.l. (the Netherlands) to 1850 m a.s.l. (Switzerland).
The analysis of altitude, latitude and environmental factors that might directly influence body size and demography (e.g. temperature and length of the activity period) allowed us to not only to explore the variation patterns in this species (i.e. Bergmann, converse Bergmann or no clines), but also to identify the putative proximate causes of this variation.
Materials and Methods
Study species and study sites
The Common toad, B. bufo, has a wide distribution, from northwest Africa, over all of Europe (except Ireland and some Mediterranean islands) to the District of Irkutsk (108°30′E) in Russia (Frost 2008). In Europe, it occupies both lowland and mountainous habitats (Borkin and Veith 1997). Demographic parameters from populations of various parts of the European range are available (e.g. Hemelaar 1988; Reading 1991, 2007; Grossenbacher 2002), but data from southern part of the continent are still fragmentary (e.g. Cvetković et al. 2003, 2005).
The study sites were the small artificial lake Trešnja (44°36′26.6″N, 20°34′14″E, altitude 222 m a.s.l.) and the pond near Zuce village (44°40′55.9″N, 20°33′7.4″E, altitude 240 m a.s.l.). 240 adult toads (Trešnja, n = 148; Zuce, n = 92) were collected around the breeding sites from 2001 to 2003. Newly metamorphosed froglets (n = 25) were caught near water at Trešnja site in June 2004. (Detailed descriptions of sites and sampling procedures are given in Tomaševic et al. 2008). The impact of the sampling was expected to be small because minimum estimates of adult population sizes were 1396 and 1948 for Trešnja and Zuce respectively (J. Crnobrnja-Isailović, unpublished data).
Body size and associated life history traits
Body size of adults (sexed by the external characteristics and the presence of gonads) and newly metamorphosed froglets was measured as the length from snout to vent to the nearest 0.1 mm using dial caliper. Sexual size dimorphism was quantified by Sexual Dimorphic Index (Lovich and Gibbons 1992): SDI = (mean length of larger sex/mean length of smaller sex) − 1, with the result defined as positive when females are the larger sex.
Age was assessed by skeletochronology (e.g. Miaud 1992; Cvetković et al. 2005). As endosteal resorption can affect the accuracy by removing the first and sometimes even the second line of arrested growth (LAG) (e.g. Frétey and Le Garff 1996), the degree of resorption was estimated by osteometrical analysis (Tomaševic et al. 2008; Sagor et al. 1998).
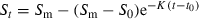
Growth rates at metamorphosis (Rinit) and at the minimum age of maturation (Rmat) were calculated using equation for age specific growth rate, derived from the von Bertalanffy equation: r = dS/dt = K (Sm–St), where St = S0 and Smat = average body size at maturation. The von Bertalanffy growth model was fitted to the average growth curves using the least square procedure.
The average number of growing seasons to be experienced before Smat is reached is the average minimum age of maturation (Amat). The potential reproductive lifespan (PR) was defined as the difference between maximal and minimal recorded age of breeding adults; it represents the number of potential reproductive seasons.
Literature study and climatic variables
We compiled our field results and literature data on body size, age and growth patterns from 11 populations of B. bufo (Gittins et al. 1980, 1982; Hemelaar 1988; Kuhn 1994; Frétey and Le Garff 1996; Schabetsberger et al. 2000). These references were selected because they were comparable with respect to variables analysed and applied methods (e.g. age assessed by skeletochronology), given that populations were widely scattered geographically.
Climatic data (annual and monthly mean, minimum and maximum temperature, annual and monthly precipitations) were obtained from the Woldclim data set (http://www.worldclim.org), at a resolution of 2.5 × 2.5 min (Hijmans et al. 2005). The lengths of B. bufo activity period (AP) vary according to latitude and altitude. These AP were available in or were estimated from data in the original papers (Table 1). We recalculated the mean values of climatic variables for the specific months of the AP recorded in each of studied populations.
| Location | Latitude (°N) | Altitude (m) | AP (weeks) | T mean (°C) | Body size (mm) | Age (years) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ♂ | ♀ | SDI | min♂ | min♀ | max♂ | max♀ | mean♂ | mean♀ | PR♂ | PR♀ | |||||
| 1. Nor | 63.38 | 150 | 17 | 10.8 | 58.9 | 72.1 | 0.224 | 4 | 6 | 10 | 11 | 7.3 | 8.8 | 6 | 5 |
| 2. GB | 52.23 | 304 | 30 | 10.7 | 59.5 | 73.6 | 0.237 | 2 | 3 | 5 | 8 | 3.4 | 4.8 | 3 | 5 |
| 3. Neth | 51.78 | 15 | 29 | 13.7 | 52.8 | 64.7 | 0.225 | 2 | 3 | 7 | 8 | 4.3 | 5.2 | 5 | 5 |
| 4. Ger1 | 51.75 | 25 | 29 | 13.8 | 56.8 | 68.4 | 0.206 | 3 | 4 | 7 | 8 | 4.4 | 5.4 | 4 | 4 |
| 5. Ger2 | 48.13 | 560 | 27 | 13.1 | 90.1 | 4 | 8 | 5.6 | 4 | ||||||
| 6. Fra1 | 48.10 | 39 | 32 | 13.2 | 66.9 | 77.7 | 0.161 | 2 | 3 | 12 | 9 | 5.3* | 5.2* | 10 | 6 |
| 7. Ger3 | 47.92 | 720 | 27 | 12.6 | 80.9 | 3 | 6 | 4.4 | 3 | ||||||
| 8. Aus1 | 47.56 | 1102 | 16 | 11.9 | 66.0 | 81.4 | 0.233 | 5 | 6 | 11 | 15 | 6.6 | 9.9 | 6 | 9 |
| 9. Aus2 | 47.55 | 1282 | 16 | 12.1 | 72.4 | 89.1 | 0.231 | 4 | 5 | 9 | 11 | 6.6 | 7.2 | 5 | 6 |
| 10. Swi | 46.65 | 1850 | 12 | 6.6 | 62.5 | 73.9 | 0.183 | 6 | 8 | 11 | 12 | 8.5 | 10.0 | 5 | 4 |
| 11. Ser1 | 44.68 | 240 | 30 | 15.6 | 70.4 | 96.3 | 0.368 | 3 | 4 | 10 | 9 | 5.5 | 5.9 | 7 | 5 |
| 12. Ser2 | 44.61 | 222 | 30 | 15.6 | 67.2 | 92.8 | 0.380 | 3 | 4 | 9 | 11 | 5.0 | 5.4 | 6 | 7 |
| 13. Fra2 | 43.47 | 500 | 33 | 13.9 | 69.1 | 87.4 | 0.265 | 3 | 4 | 9 | 10 | 5.2 | 6.8 | 6 | 6 |
- AP, length of activity period (in weeks); Tmean, mean temperature during the AP (°C); SDI, index of sexual size dimorphism; min, max, mean, minimum, maximum and mean age of adults estimated by skeletochronology; PR, potential reproductive life span; Nor, Norway (Hemelaar 1988); GB, Great Britain (Gittins et al. 1980, 1982); Neth, the Netherlands (Hemelaar 1988); Ger1, Germany (Hemelaar 1988); Ger2 and Ger3, Germany (Kuhn 1994); Aus1 and Aus2, Austria (Schabetsberger et al. 2000); Swi, Switzerland (Hemelaar 1988); Fra1, France (Frétey and Le Garff 1996); Fra2, France (Hemelaar 1988); Ser1, Serbia (Zuce), this study; Ser2, Serbia (Trešnja), this study.
- *As mean ages were not available for this population, they were estimated using a regression equation from age min and age max (multiple regression, F2,19 = 124.9, p < 0.0001, r2 = 0.93).
Statistical analyses
We used the analysis of covariance (ancova) to evaluate the variation of activity period and temperature with latitude and altitude. Variations in adult body size were also analysed with ancova. We included sex as a fixed factor and latitude, altitude, AP, temperature and age as covariates. In the first analysis, we used geographic features (altitude and latitude) as independent variables, to evaluate body size geographic variation along both gradients. Subsequently, we used climatic parameters (temperature and AP) as independent variables. As climatic parameters strongly depend on geographic features (see Results), this analysis was therefore performed a posteriori, to evaluate how the climatic features mediate the effect of geographic parameters.
Similarly, we performed multivariate analysis of covariance (mancova) to evaluate the effects of altitude and latitude on age parameters, as well as the effects of temperature and AP on the same parameters. Multivariate analyses were followed by univariate analyses to evaluate the effect of independent variables on each dependent parameter.
In all parametric tests, residuals were normally distributed (Kolmogorov–Smirnov tests, all p > 0.1). We used the Variance Inflation Factor (VIF) to evaluate issues because of collinearity among independent variables. Values of VIF > 5 indicate that collinearity can cause issues in the results of the analyses (Bowerman and O’Connell 1990). Statistical tests were performed using statistical packages Statistica v. 6 (Statsoft Inc., Tulsa, OK, USA) and spss 13.0. (SPSS Inc., Chicago, IL, USA).
Results
Field study
Body size and age structure
Body size and age data for Trešnja and Zuce populations are presented in Table 1 (Trešnja = Serbia 2; Zuce = Serbia 1). Males were significantly smaller than females in both populations (t-test, p < 0.0001). The SDI was 0.380 and 0.368 for Trešnja and Zuce populations respectively. Body size of metamorphosed froglets averaged 8.6 ± 0.1 mm (range: 7.2–9.8 mm).
Skeletochronological analysis was performed on 187 individuals. LAGs in phalangeal cross sections were sharp and easily distinguishable. Endosteal resorption was often present, but did not affect the accuracy of age estimation (Tomaševic et al. 2008). The youngest age at which toads came to breed in both populations was 3 years for males and 4 years for females (Fig. 1). The oldest recorded ages were 9 and 10 years for males and 11 and 9 years for females, in Trešnja and Zuce populations respectively. Age distributions did not differ significantly between populations, neither for females nor for males (Fig. 1, Kolmogorov–Smirnov two-sample test, p > 0.10).
Age structures of Bufo bufo populations in (a) Trešnja and (b) Zuce (Serbia) assessed by skeletochronology. Closed bars: males; open bars: females; X-axis: age in years; Y-axis: number of individuals
Body length and age were significantly correlated only in males from population Trešnja: rs = 0.385, p = 0.002 (Trešnja females: rs = 0.206, p = 0.16; Zuce males: rs = −0.037, p = 0.84; Zuce females: rs = 0.056, p = 0.71).
Patterns of growth
The growth of Common toad was well described by the von Bertalanffy growth model in Trešnja population (growth coefficient for Zuce population was not computed because of insufficient number of metamorphosed froglets found at this site). Females had a larger asymptotic size (Sm = 95.4) than males (Sm = 70.9), but the growth coefficients K were similar (males: 0.79 ± 0.11; females: 0.74 ± 0.08).
The growth rates were notably higher at metamorphosis (Rinit) than at maturation (Rmat): for males Rinit = 49.32 and Rmat = 8.55; for females Rinit = 64.44 and Rmat = 12.54. In other words, Rmat is reduced to about 17% and 19% of the initial growth rates in males and females respectively. The calculated values of Amat (2.5 years for males and 2.7 years for females) were somewhat lower than the recorded age of the youngest adults captured in both analysed populations.
Minimum recorded body size of breeding individuals was 55.9 mm and 60.4 mm in males and 72.2 mm and 78.3 mm in females, for Trešnja and Zuce populations respectively.
Literature survey
Climatic variables and the length of AP
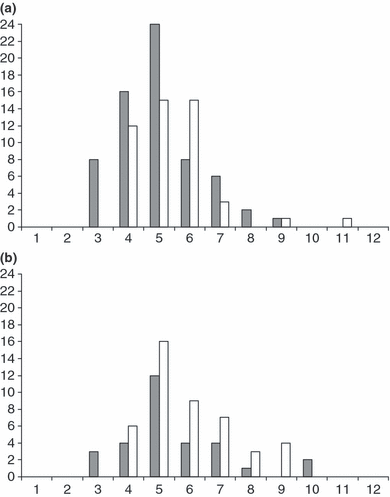
The locations of studied populations (n = 13) are given in Table 1 and Fig. 2.
Locations of the European populations of Common toad Bufo bufo selected for this study. Numbers refer to studied populations (see details in Table 1)
First, we evaluated the relationship among climatic variables: the maximum (Tmax) and mean temperature (Tmean) during the AP were positively correlated (rs = 0.92, p < 0.001). On the other hand, the minimum temperature (Tmin) during the AP was not correlated with either Tmax or Tmean (rs = 0.07, p = 0.81 and rs = 0.25, p = 0.41). Tmin was similar in all populations, suggesting that about 3°C was the threshold minimum value over which the Common toad activity can start. The annual precipitation was correlated with the mean monthly precipitation during the AP (rs = 0.97, p < 0.001). The mean monthly precipitation was strongly correlated with Tmean (rs = −0.79, p = 0.001).
The length of AP at each location was strongly correlated with the mean annual temperature (rs = 0.92, p < 0.001) and with the mean temperature during the AP (rs = 0.675, p = 0.01). The AP in 13 studied populations varied from 12 to 33 weeks and Tmean during this period varied from 6.6 to 15.6°C (Table 1). Altitudes and latitudes of populations were not correlated (rs = −0.44, p = 0.13). The ancova showed that AP was significantly shorter in high altitude and high latitude populations (latitude, F1,10 = 26.45, p = 0.0004; altitude, F1,10 = 73.30, p < 0.0001). Similarly, Tmean significantly decreased as latitude and altitude increased (ancova: latitude, F1,10 = 13.41, p = 0.004, altitude, F1,10 = 29.76, p = 0.0003).
Variation in body size with latitude, altitude, Tmean and AP
An overview of life history traits for 13 European B. bufo populations is given in Table 1. Mean adult body size varied from 52.8 to 72.4 mm and from 64.7 to 96.3 mm in males and females respectively. Body size was the parameter with the lowest coefficient of variation (CV = 9.7 and 12.3 %, in males and females respectively). Mean female body size was always larger than mean male size, the SDI ranging from 0.161 to 0.380 (CV = 27.9%).
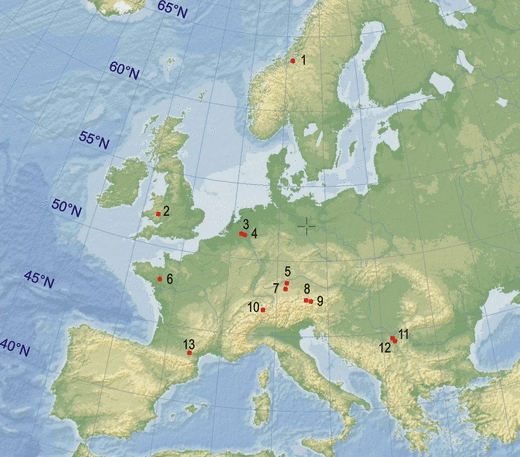
Adult body size decreased as the latitude increased (ancova, F1,12 = 10.16, p = 0.005; Fig. 3), but did not show a similar trend with respect to altitude (F1,12 = 0.223, p = 0.642; Fig. 3). The significant sex effect confirmed that females were larger than males in all populations (F1,12 = 25.68, p < 0.0001). Finally, the age effect was not significant (F1,12 = 0.543, p = 0.47), and thus the observed latitudinal pattern of body size variation cannot be explained by a latitudinal variation in population age structure.
Body length variation among Common toad Bufo bufo populations varying in latitude, altitude, duration of the activity period and mean temperature during the activity period, empty dots indicate females and filled dots indicate males
The analysis of body size variation with AP and Tmean (Fig. 3) showed that body size increased as Tmean increased (ancova, F1,12 = 5.08, p = 0.036), but did not vary significantly with AP (F1,12 = 0.14, p = 0.711).
The SDI did not vary significantly with latitude or altitude (ancova, p ≥ 0.15 for both variables), nor with AP or Tmean (ancova, p > 0.06 for both variables).
Variation in age with latitude, altitude, Tmean and AP
The minimum age of adults was 2 years in males and 3 years in females (CV = 38.2 and 34.3% respectively), and longevity ranged from 5 to 12 years in males and from 6 to 15 years in females (CV = 22.8 and 23.3% respectively, Table 1).
Age parameters (mean, minimal and maximal age, and potential reproductive lifespan) significantly varied with altitude, latitude and AP. No effect of Tmean on age was observed (Table 2). Mean and minimal age increased as latitude and altitude increased and as AP decreased (Table 2). Longevity (age max) significantly increased with altitude and decreased with AP but not with latitude (Table 2). The potential reproductive lifespan did not vary significantly with latitude, altitude, AP nor Tmean, and sex had a significant effect on age min, confirming that females mature later than males (Table 2).
| (a) Multivariate test | ||||
|---|---|---|---|---|
| Effect | Pillai’s trace | F-value | df | p |
| Latitude | 0.552 | 7.386 | 3, 18 | 0.002 |
| Altitude | 0.766 | 19.640 | 3, 18 | <0.0001 |
| Sex | 0.372 | 3.547 | 3, 18 | 0.035 |
| AP | 0.616 | 9.619 | 3, 18 | 0.0005 |
| T mean | 0.184 | 1.352 | 3, 18 | 0.289 |
| Sex | 0.438 | 4.679 | 3, 18 | 0.014 |
| (b) Univariate post hoc tests | ||||
|---|---|---|---|---|
| Source | Dependent variable | df | F-value | p |
| Latitude | Age mean | 1, 20 | 5.669 | 0.027 |
| Age min | 1, 20 | 6.417 | 0.020 | |
| Age max | 1, 20 | 0.003 | 0.957 | |
| PR | 1, 20 | 1.309 | 0.266 | |
| Altitude | Age mean | 1, 20 | 27.573 | <0.0001 |
| Age min | 1, 20 | 46.948 | <0.0001 | |
| Age max | 1, 20 | 4.457 | 0.048 | |
| PR | 1, 20 | 0.451 | 0.510 | |
| Sex | Age mean | 1, 20 | 3.123 | 0.092 |
| Age min | 1, 20 | 9.131 | 0.007 | |
| Age max | 1, 20 | 0.449 | 0.510 | |
| PR | 1, 20 | 0.381 | 0.544 | |
| AP | Age mean | 1, 20 | 22.625 | 0.0001 |
| Age min | 1, 20 | 28.845 | <0.0001 | |
| Age max | 1, 20 | 8.494 | 0.009 | |
| PR | 1, 20 | 1.086 | 0.310 | |
| T mean | Age mean | 1, 20 | 0.008 | 0.929 |
| Age min | 1, 20 | 0.095 | 0.761 | |
| Age max | 1, 20 | 1.596 | 0.221 | |
| PR | 1, 20 | 2.514 | 0.129 | |
| Sex | Age mean | 1, 20 | 5.258 | 0.033 |
| Age min | 1, 20 | 13.706 | 0.001 | |
| Age max | 1, 20 | 0.726 | 0.404 | |
| PR | 1, 20 | 0.375 | 0.547 | |
- AP, length of the activity period; Tmean, average temperature during the AP; PR, potential reproductive lifespan.
Variation in growth with latitude, altitude, Tmean and AP
Growth parameters (minimum age of maturation Amat, growth rate at maturation Rmat, growth rate at metamorphosis Rinit, and growth coefficient K) were available for a smaller subset of six populations (Table 3). Average minimum age of maturation varied from 2.1 to 5.9 and from 2.7 to 8.0 years in males and females respectively. The growth coefficient ranged from 0.32 to 0.92 in males and from 0.18 to 0.74 in females. The ratio Rmat/Rinit varied from 0.17 to 0.24 in males and from 0.19 to 0.28 in females.
| Location | ♂ | ♀ | ||||
|---|---|---|---|---|---|---|
| A mat | R mat/Rinit | K (95% CI) | A mat | R mat/Rinit | K (CI) | |
| Nor | 4.4 | 0.21 | 0.42 (0.40–0.44) | 5.8 | 0.26 | 0.27 (0.24-0.29) |
| Neth | 2.1 | 0.20 | 0.92 (0.86–0.98) | 3.0 | 0.22 | 0.56 (0.47–0.65) |
| Ger1 | 2.4 | 0.21 | 0.86 (0.82–0.91) | 3.3 | 0.21 | 0.58 (0.54–0.62) |
| Swi | 5.9 | 0.21 | 0.32 (0.30–0.35) | 8.0 | 0.28 | 0.18 (0.15–0.21) |
| Ser2 | 2.5 | 0.17 | 0.79 (0.68–0.90) | 2.7 | 0.19 | 0.74 (0.66–0.82) |
| Fra2 | 3.6 | 0.24 | 0.47 (0.43–0.51) | 4.8 | 0.26 | 0.32 (0.26–0.36) |
The growth coefficient significantly decreased as altitude increased (ancova, F1,8 = 13.72, p = 0.006), and the same, although non-significant, trend was observed with latitude (F1,8 = 3.85, p = 0.085); males tended to have higher K (F1,8 = 4.187, p = 0.075). In populations for which K was available, the strong correlation between AP and Tmean caused multicollinearity issues (VIF = 7.7) and prevented the performance of a global analysis including these factors together. When analysed separately, K was positively related to both Tmean and AP (ancova with sex as controlling factor: Tmean, F1,9 = 14.32, p = 0.004; AP, F1,9 = 7.85, p = 0.021).
Discussion
Patterns of variation with latitude
Controversial results for latitudinal body size variation in amphibians are available (Ashton 2002b; Olalla-Tárraga and Rodríguez 2007; Adams and Church 2008). Among Bufonidae, four of five species showed a converse Bergmann cline: Bufo viridis (Nevo 1972; Castellano and Giacoma 1998), B. calamita (Leskovar et al. 2006), B. hemiophrys (Eaton et al. 2005) and B. woodhousii (Kellner and Green 1995). In B. bufo, the pioneering study of Hemelaar (1988), which took into account the age of the individuals, showed that older age and larger maximal size where reached at northern than at southern latitudes. The exception was the southernmost population (43.47°N, France), and Hemelaar (1988) supposed it might belong to a subspecies B. bufo spinosus. However, more recent studies found little support for genetic differentiation of B. bufo spinosus (Lüscher et al. 2001; Kutrup et al. 2006).
Our analysis, which included 13 populations, did not confirm Hemelaar’s (1988) results: the Common toad exhibits a converse Bergmann-type response to latitude (Fig. 3), as observed in other Bufo species. Both sexes follow the same trend and, although the largest values were observed in the southernmost populations, the sexual size dimorphism did not vary significantly with latitude.
The first question to be asked is if the studied gradients are representative of the species range. Insufficient sampling of gradients may lead to erroneous conclusions, e.g. of simple trends where complex patterns exist (Gaston et al. 2008). Considering continental Europe, B. bufo is present from Finland (about 68°N) to southern Spain and Greece (about 37°N). Our study included populations from 43.47°N to 63.38°N (a 2240 km gradient, Fig. 2), thus representing the largest part of this range. Testing Bergmann’s rule with Rana temporaria along a 1600 km latitudinal gradient across Scandinavia, Laugen et al. (2005) found that body size decreased in the northernmost populations (65–67°N). In B. bufo, data are scarce for high latitudes, but mean body size of males was 65.8 mm (n = 47, SE = 0.82) at 64.10°N (J. Merilä, unpublished data), which is higher than the size observed in Norway at 63.38°N (Table 1). The Common toad clearly exhibits a converse Bergmann cline along the latitudinal gradient, but more data are needed to verify this trend at high latitudes.
Despite the body size variation along the latitudinal gradient, age (at maturity, mean age and longevity) did not vary significantly and growth did not differ among populations. A similar pattern was observed in R. temporaria (Laugen et al. 2005) and Rana septentrionalis (Leclair and Laurin 1996).
Patterns of variation with altitude
Bufo bufo from high altitude grew slower, and tended to reach older age and larger maximal size than lowland populations (Hemelaar 1988). Our review included populations from 15 to 1850 m a.s.l., rather close to the altitudinal limit (2300 m in the Alps, K. Grossenbacher, pers. comm.). Body size of both sexes did not vary significantly with altitude up to 1850 m; this observation was confirmed by the size of adults at 2110 and 2180 m, which was similar to the size of lowland individuals (K. Grossenbacher, unpublished data). Minimum and mean adult age significantly increased with altitude (this study), and Grossenbacher (2002) showed that the main effect of altitude on B. bufo life history traits was a strong influence on age (i.e. increased longevity). As adult body size did not increase with altitude while age did, growth was slowed down in highland populations: a low value of K (which decreased with increasing altitude) is associated with delayed maturation. Such strong delay in age at maturity, increased longevity and decrease of growth with altitude was observed in other amphibians as well (Tilley 1980; Berven 1982; Miaud et al. 1999, 2000).
Mechanisms
Several factors (i.e. climatic conditions, trophic resources, metabolic properties, interspecific competition and predator–prey interactions) may explain differences in body length and age among populations. Since these causes are not mutually exclusive, it is difficult to determine their individual influence on the optimal size for species or for particular location (Kozlowski 1992). Also, considerable variation in body size could be observed between neighbouring populations of amphibians (Reading 1988; Bruce and Hairston 1990; Augert and Joly 1993; Miaud et al. 2001).
Our study combined the analysis of geographic gradients with the study of biologically relevant climatic variables, and of demographic features of populations. Despite complex causes of size variation, our approach identified mechanisms that could influence the observed pattern of geographic variation – by coping with temperature conditions and length of activity period.
How can we explain the pattern of body size variation over the latitudinal gradient observed in B. bufo? The pattern of adult size variation could result directly from variation in population age structure, older individuals being larger. This was not the case with B. bufo, since age did not significantly increase with latitude. Variation in size among individuals of the same age may result from variation in size at metamorphosis (Berven 1990; Miaud et al. 1999; Morrison et al. 2004), differences in growth rates before maturity (Halliday and Verrell 1988; Ryser 1988; Miaud et al. 2000), and following maturity (Ryser 1989; Smirina 1994; Kellner and Green 1995).
Size at metamorphosis can vary with development temperature and a lower development temperature resulted in larger body size in numerous ectotherms (the temperature rule, Atkinson 1994) and, specifically, in amphibians (Laugen et al. 2005; Ficetola and De Bernardi 2006). In R. temporaria, higher developmental rates of northern tadpoles were promoted by selection stemming from seasonal time constraints rather than from low ambient temperature per se (Laugen et al. 2003). Data on size at metamorphosis among B. bufo populations are lacking and how adult size can be influenced by size at metamorphosis remains to be tested. However, if the recorded Tmean reflects mean water temperature of developing tadpoles, this temperature decreased as the latitude increased (this study) and the temperature rule cannot explain the observed correlative adult body size decrease.
A higher growth rate before maturity can lead to a larger size at the same age. The growth coefficient decreased as altitude increased and the same, although non-significant trend was observed with latitude. Moreover, this growth parameter increased as both Tmean and AP increased. Growth variation can, thus, partly explain adult body size variation among B. bufo populations.
It is clear that, generally, body size might also be strongly tied to environmental factors other than temperature or AP duration, such as precipitation and humidity (Ashton 2002b), or food availability (Miaud et al. 2001; Chown and Klok 2003; Meiri et al. 2007). The strong correlation between precipitation and temperature makes it difficult to perform analyses including the effects of each factor. In the newt Taricha granulosa, adults were larger in cooler, drier areas (Nussbaum and Brodie 1971), the growth of the salamander Salamandra lanzai strongly depended on rain levels (Miaud et al. 2001), and the toad Bufo viridis was larger in warmer, drier areas (Castellano and Giacoma 1998). Moisture in the environment can be extremely important, larger individuals having greater desiccation tolerance because of the relative decrease in surface area (Duellman and Trueb 1994). However, there was no significant correlation between B. bufo body size and the amount of precipitation (mean monthly precipitation during the AP, range 56–167 mm, males: r = 0.23, p = 0.50 and females: r = 0.01, p = 0.96).
The impact of ecological conditions along the altitudinal gradient differed from that observed along the latitudinal gradient: body size did not vary, but age of adulthood was strongly delayed as altitude increased, i.e. the growth coefficient decreased (flattened growth curve). Both AP and Tmean significantly decreased with increasing altitude, as well as with latitude. The difference in life history traits along these two gradients indicates that global ecological measures such as Tmean and AP are not sufficient to fully reflect differences between high latitudes and altitudes relevant for population biology. Ecological conditions obviously differ between high latitudes and altitudes (Bliss 1956): the amount of heat accumulated during the growing season is higher in temperate alpine environment than in the arctic, local contrasts (e.g. difference between exposed and non-exposed slopes) are lower in the arctic, with less incident energy and lower evapotranspiration (Billings 1973; Körner 2003).
Our analysis revealed that adult body size increased with Tmean during the active season, while age parameters were linked to the length of the activity season rather than to Tmean. This complex pattern can arise because amphibian body size depends on proximate factors acting on different phases of the life cycle (from eggs via maternal effects, tadpoles, to juvenile and adult life), and optimal factors (e.g. thermal optima for growth efficiency and growth rate) can vary as individuals grow (Angilletta and Dunham 2003). Moreover, such temperature thresholds can be different for development rate and for growth rate (Walters and Hassall 2006). Finally, adult body size is often correlated with fitness through fecundity and mating success, maturation depending on ecological conditions mediated by seasonal time constraints (Cabinita and Atkinson 2006).
Relations with other life history traits
With respect to patterns for allocating resources over the lifetime of amphibians (Bernardo 1994), B. bufo exhibited a typical S-shaped growth curve, i.e. decreased, but continuous growth after maturation. Growth and maturation are under the constraint of an annual environmental cycle, and an optimum growth rate would be a compromise between the benefit of attaining particular size in a given time and the cost incurred for rapid growth per se (Sibly and Atkinson 1994). This compromise would be observed as a trade-off between growth rate and other fitness traits (e.g. fecundity and juvenile mortality). Unfortunately, data on life history traits other than age and body size are sparse in B. bufo, as in many other amphibian species, and Bergmann’s clines have been rarely explained in the context of life-history traits so far.
Sexual size dimorphism in many amphibians follows the pattern of many other lineages of ectothermic vertebrates, i.e. female being larger than males, pattern usually explained by fecundity selection (Kupfer 2007). If latitude and altitude via correlated ecological conditions constrain growth, the effect could be larger on females that have to invest in fecundity more than males. Females were larger than males in all populations, and the sexual size dimorphism did not vary with latitude, altitude, AP or Tmean. There was insufficient data to analyse fecundity and egg size variation along latitudinal and altitudinal gradients. The number of eggs per female seems to be higher in southern populations (mean fecundity was more than 7000 egg/female/year (range: 2118–15050) in Serbia, Tomaševic et al. 2008), compared with Great Britain (range 425–4796, Gittins et al. 1984; 700–5000, Reading 1986), the Netherlands (1859–6305, Van Gelder 1995) and Germany (750–8100, Kuhn 1994), but comparisons must be treated with caution because of heterogeneity of studies. Fecundity variation probably results directly from female size variation, since body size is often associated with increased fecundity in amphibians (e.g. Gibbons and McCarthy 1986).
Another way to limit investment in fecundity is to reproduce, for example, biannually. There is strong evidence of intermittent breeding in female Common toads (e.g. Kuhn 1994; Schmidt et al. 2002; Frétey et al. 2004; K. Grossenbacher, pers. obs.), but it is not clear whether breeding frequency is lower in e.g. high altitude or latitude populations or in females compared with males. If these patterns exist, losing reproductive seasons with biannual reproduction can easily be counterbalanced by the strong increase in longevity in highland populations (Grossenbacher 2002; this study).
The question whether amphibians follow Bergmann’s rule remains particularly interesting because it requires identification of proximate causes acting on species with complex life cycles. If the rule appears to be a general trend for anurans when comparing between species (Lindsey 1966; Olalla-Tárraga and Rodríguez 2007), it is no longer the case when comparing body size variation within species (Ashton 2002b; this study). The relationship between intra- and interspecific patterns of spatial variation appears as one of the major open questions in the field (Gaston et al. 2008). Although it has often been assumed that patterns at both levels are similar (implying common underlying mechanisms), the differences may exist, as our study confirmed.
At the interspecific level, mean body size of anurans was negatively correlated with evapotranspiration, supporting the heat balance hypothesis (greater thermoregulatory abilities allow anurans to reach larger sizes in low energy areas, Olalla-Tárraga and Rodríguez 2007). As a converse pattern was clearly observed among populations of the Common toad, this hypothesis does not hold for intraspecific level. Studies are needed now that examine spatial patterns in different types of traits and their interactions (Gaston et al. 2008). The significant relation between B. bufo body size and mean temperature and duration of the activity period (and the varying patterns observed between latitude and altitude) prompts us to select precise ecological variables acting on each phase of the life cycle to infer causes of body size variation in anurans along gradients.
Acknowledgements
This work was approved and supported by Ministry of Science and Nature Protection of Republic of Serbia (grant No. 143040). The authors thank Thierry Frétey, Kurt Grossenbacher and Juha Mërila who kindly gave us access to unpublished data, Alexander Kupfer and Wolf Blanckenhorn for constructive comments, and Jean-Noël Avrillier for composing Fig. 2.


