On the systematics of the fungus gnat subfamily Mycetophilinae (Diptera): a combined morphological and molecular approach
Zur Systematik der Pilzmücken Mycetophilinae (Diptera); ein kombinierter morphologisch-molekularer Ansatz
Abstract
enThe phylogenetic relationships within the fungus gnat subfamily Mycetophilinae (Diptera) are addressed using a combined morphological and molecular approach. Twenty-four species, representing nine genera of the tribe Mycetophilini and 15 genera of the tribe Exechiini, were included in the study. Analyses include nucleotide sequences of mitochondrial (cytochrome oxidase I and 16S), and nuclear (18S and 28S rDNA) genes, in addition to 65 morphological characters. A combined parsimony analysis, including all characters, supports the monophyly of the subfamily Mycetophilinae and two of its tribes, Exechiini and Mycetophilini. There is also statistical support for a Mycetophila-group and a Phronia-group within the tribe Mycetophilini. The Phronia-group includes the genera Phronia, Macrobrachius and Trichonta. The Mycetophila-group includes the genera Mycetophila, Epicypta, Platurocypta, Sceptonia and Zygomyia. A Bayesian analysis based on the nucleotide sequences alone also support these clades within Mycetophilini except for the position of Dynatosoma which is recovered as the sister taxon to the Phronia-group. A somewhat different pattern, however, is observed for the tribe Exechiini – neither molecular data nor the combined data set support unambiguously any intergeneric relationships within Exechiini.
Zusammenfassung
deDie phylogenetischen Verwandtschaftsbeziehungen innerhalb der Pilzmücken der Unterfamilie Mycetophilinae (Diptera) wurden mit einem kombinierten morphologischen und molekularen Ansatz untersucht. Vierundzwanzig Arten aus 9 Gattungen des Tribus Mycetophilini und 15 Gattungen des Tribus Exechiini wurden in die Untersuchungen einbezogen. Die Ergebnisse einer kombinierten kladistischen Analyse von 65 morphologischen Merkmalen und den Nukleotidsequenzen der mitochondrialen Cytochrom Oxidase I und 16S Gene sowie der 18S und 28S Gene des Kerngenoms stützen die Monophylie der Unterfamilie Mycetophilinae sowie der beiden Tribus Exechiini und Mycetophilini. Weiterhin hatten die Mycetophila- und die Phronia-Gruppe innerhalb des Tribus Mycetophilini hohe statistische Unterstützung. Die Phronia-Gruppe schlieβt die Gattungen Phronia, Macrobrachius und Trichonta und die Mycetophila-Gruppe die Gattungen Mycetophila, Epicypta, Platurocypta, Sceptonia und Zygomyia ein. Die Gattung Dynatosoma gruppierte ebenso in der Mycetophila-Gruppe. Die Bayesische Analyse der Nukleotidsequenzen stützt ebenfalls die Monophylie der oben genannten Gruppen innerhalb des Tribus Mycetophilini. Ein anderes Bild ergab sich für den Tribus Exechiini. Weder die Analysen der molekularen Daten alleine noch in Kombination mit den morphologischen Daten ergaben für die einebezogenen Gattungen zweifelsfreie phylogenetische Verwandschaftsbeziehungen mit hoher statistischer Unterstützung.
Introduction
The Mycetophilinae is the most species-rich and abundant subfamily within the fungus gnat family Mycetophilidae. The subfamily includes the two tribes Mycetophilini and Exechiini as originally suggested by Edwards (1925). With about 1460 recognized species in 14 genera, the tribe Mycetophilini is more species-rich than the tribe Exechiini, with approximately 640 species in 19 genera (data from internal compilation, Natural History Museum, Oslo).
Most species of Mycetophilinae have larvae that live in fleshy sporophores of various fungi, while the adults are frequently found in dark and humid environments (e.g. Yakovlev and Zaitsev 1990; Kurina 1994). In general, the biology and life strategies of Mycetophilinae remain insufficiently known. Several species, especially within Exechiini, are known to hibernate as adults in sheltered places such as caves, crevices, hollow trees or even umbelliferous stems (Väisänen 1981; Kjærandsen 1993). The two tribes also display an interesting pattern of distribution: according to the latest comprehensive compilation of fungus gnat distributions (Bechev 2000), 14 of 16 Exechiini genera are recorded from the Holarctic, while only 10 are represented in one or more of the Afrotropical, Neotropical or Australian regions. For the Mycetophilini, with 14 genera, the corresponding numbers are nine and 12 genera.
Although Mycetophilinae is a relatively homogeneous subfamily with respect to morphology, there are differences between the two tribes. Mycetophilini is characterized by the presence of an occipital furrow, setae on the anepimeron and dorsal setae on the distal median plate of the wing base. Exechiini is characterized by a narrow frontal tubercle and hind tibia with incomplete apical brush. For a more comprehensive discussion of the morphological differences between the two tribes, see Rindal and Søli (2006). The genera within the Mycetophilini are better delimited than those within Exechiini, and relatively easier to identify by clear morphological autapomorphies.
There is today good evidence for the monophyly of the tribe Exechiini, which is well supported by both morphological characters (Rindal and Søli 2006) and by nucleotide sequence data (Rindal et al. 2007). Despite recent efforts (Kjærandsen 2006; Rindal and Søli 2006; Rindal et al. 2007), the inter-generic relationships within Exechiini remain unclear. Analyses of morphological characters and molecular data have not yielded well supported phylogenies, and little consensus can be found between different approaches. Based on an interpretation of branch lengths within the Bayesian tree and biogeographical distribution patterns, Rindal et al. (2007) suggested the lack of phylogenetic resolution could be explained by the genera of Exechiini originating within a short period of time.
In contrast to the Exechiini, the intergeneric relationships within the Mycetophilini have received little attention except for assigning species groups. Tuomikoski (1966) argued that the tribe Mycetophilini might be a paraphyletic assemblage of three groups of genera, viz. the Mycetophila-group, the Phronia-group and a monogeneric group consisting of Pseudalysiina. This point of view was rejected by Rindal and Søli (2006) based on morphological characters. They provided evidence for the tribe Mycetophilini and the Mycetophila-group being monophyletic. However, despite some morphological characters supporting the postulated Phronia-group, this group was rendered paraphyletic, with Macrobrachius as a sister to the Mycetophila group and a group consisting of Phronia and Trichonta. The genus Pseudalysiina was not included in the study of Rindal and Søli (2006), and it is reasonable to question even the inclusion of this genus in Mycetophilinae. When originally described it was considered closely related to the genus Dziedzickia in the subfamily Gnoristinae (Tonnoir 1929). The systematic position of Dynatosoma also varies between authors: Tuomikoski (1966) included the genus in the Phronia-group, whereas Rindal and Søli (2006) placed it together with Mycetophila and its allies. For the purpose of the current study we define the Mycetophila-group as consisting of the genera Mycetophila, Epicypta, Platurocypta, Sceptonia and Zygomyia, and the Phronia-group as consisting of Phronia, Macrobrachius and Trichonta, while Dynatosoma is still incertae sedis.
The present study is aimed at resolving the phylogeny of Mycetophilinae and addresses in particular (1) the monophyly of the tribe Mycetophilini, (2) the monophyly of the Phronia-group and (3) the intergeneric relationships within the tribes Mycetophilini and Exechiini.
Materials and Methods
We applied a combined approach, using both morphological and molecular characters. For this purpose, we established a combined data set for Mycetophilinae species representing 24 genera that consisted of morphological data from Rindal and Søli (2006), molecular data from Rindal et al. (2007) and newly sequenced nucleotide sequences of the nuclear 18S and 28S rDNAs, and the mitochondrial 16S rDNA and the cytochrome oxidase I (coxI) genes.
Sampling
The specimens included in the molecular study were collected at 10 localities in Norway and Sweden and at one locality in Korea (Table 1) using sweep nets and Malaise traps with 80% ethanol as fixative.
| Taxa1 | Collections number | GenBank accession numbers | |||
|---|---|---|---|---|---|
| NHM, Oslo | 28S | 18S | 16S | coxI | |
| Tribe Exechiini | |||||
| Anatella lenis Dziedzicki, 1923 | NHM_MYC_ER_125 | EU219582 | DQ787911 | DQ787936 | DQ787886 |
| Allodia sp. | NHM_MYC_ER_018 | EU219584 | DQ787912 | DQ787937 | DQ787887 |
| Allodiopsis rustica (Edwards, 1941) | NHM_MYC_ER_079 | EU219593 | DQ787913 | DQ787938 | DQ787888 |
| Brachypeza bisignata Winnertz, 1863 | NHM_MYC_ER_090 | EU219596 | DQ787919 | DQ787944 | DQ787894 |
| Brevicornu improvisum Zaitzev, 1992 | NHM_MYC_ER_028 | EU219587 | DQ787915 | DQ787940 | DQ787890 |
| Cordyla sp. | NHM_MYC_ER_024 | EU219586 | DQ787904 | DQ787929 | DQ787879 |
| Exechia frigida (Boheman, 1865) | NHM_MYC_ER_004 | EU219575 | DQ787906 | DQ787931 | DQ787881 |
| Exechiopsis sagittata Lastovka & Matile, 1974 | NHM_MYC_ER_100 | EU219577 | DQ787908 | DQ787933 | DQ787883 |
| Notolopha cristata (Staeger, 1840) | NHM_MYC_ER_093 | EU219598 | DQ787918 | DQ787943 | DQ787893 |
| Pseudobrachypeza helvetica (Walker, 1856) | NHM_MYC_ER_094 | EU219599 | DQ787920 | DQ787945 | DQ787895 |
| Pseudorymosia fovea (Dziedzicki, 1910) | NHM_MYC_ER_102 | EU219578 | DQ787910 | DQ787935 | DQ787885 |
| Rymosia sp. | NHM_MYC_ER_003 | EU219574 | DQ787905 | DQ787930 | DQ787880 |
| Stigmatomeria crassicornis (Stannius, 1831) | NHM_MYC_ER_082 | EU219594 | DQ787916 | DQ787941 | DQ787891 |
| Synplasta gracilis (Winnertz, 1863) | NHM_MYC_ER_083 | EU219595 | DQ787917 | DQ787942 | DQ787892 |
| Tarnania dziedzickii (Edwards, 1941) | NHM_MYC_ER_098 | EU219600 | DQ787923 | DQ787948 | DQ787898 |
| Tribe Mycetophilini | |||||
| Dynatosoma reciprocum (Walker, 1848) | NHM_MYC_ER_092 | EU219597 | DQ787903 | DQ787928 | DQ787878 |
| Epicypta aterrima (Zetterstedt, 1852) | NHM_MYC_ER_108 | EU219579 | EU219568 | EU219603 | EU219562 |
| Macrobrachius sp. | NHM_MYC_ER_122 | EU219581 | EU219570 | EU219605 | EU219564 |
| Mycetophila fungorum (De Geer, 1776) | NHM_MYC_ER_017 | EU219583 | DQ787902 | DQ787927 | DQ787877 |
| Phronia strenua Winnertz, 1863 | NHM_MYC_ER_019 | EU219585 | EU219571 | EU219606 | EU219565 |
| Platurocypta testata (Edwards 1925) | NHM_MYC_ER_049 | EU219590 | EU219567 | EU219601 | EU219560 |
| Sceptonia sp. | NHM_MYC_ER_005 | EU910592 | EU910591 | EU910589 | EU910590 |
| Trichonta sp. | NHM_MYC_ER_029 | EU219588 | EU219572 | EU219607 | EU219566 |
| Zygomyia angusta Plassmann, 1977 | NHM_MYC_ER_113 | EU219580 | EU219569 | EU219604 | EU219563 |
| Outgroup taxa | |||||
| Boletina sp. | NHM_MYC_ER_047 | EU219589 | DQ787901 | DQ787925 | DQ787876 |
| Docosia sp. | NHM_MYC_ER_072 | EU219592 | DQ787900 | DQ787926 | DQ787875 |
| Leia sp. | NHM_MYC_ER_066 | EU219591 | DQ787899 | DQ787924 | DQ787874 |
- 1Some samples are represented only by female individuals, and therefore, cannot be determined to species.
We attempted to include representatives of all currently recognized Mycetophilinae genera in the study, but this could not be achieved. Unfortunately, available collection material turned out not suitable for molecular analyses, i.e. the DNA extractions did not yield genetic material of reasonable quality to serve as appropriate PCR template. The outgroup taxa were the same genera as used by Rindal and Søli (2006), i.e. Boletina, Leia and Docosia belonging to Gnoristinae and Leiinae (Mycetophilidae).
Morphological data
The morphology-based taxonomy and nomenclature of Mycetophilinae species follows Søli (1997), and the morphological characters and the respective data matrix have been published by Rindal and Søli (2006). The present study is based on these morphological data. The data set of Rindal and Søli (2006) was trimmed to match the taxa included in the molecular analyses presented here, and consist of 65 characters for 24 genera.
Molecular data
DNA was extracted from the abdomen of the specimens following the instructions of the Puregene kit (Gentra Systems, Minneapolis, MN, USA). The genitalia were stored in glycerol in micro vials as vouchers and deposited in the entomological collection of the Natural History Museum, Oslo.
Details on the molecular methods for amplification and sequencing of the nuclear 18S rDNA, and the mitochondrial 16S rDNA and coxI genes are described in Rindal et al. (2007). The amplification programme for the 28S gene was 94°C for 3 min; 35 cycles of 94°C for 30 s, 50°C for 30 s and 72°C 1 min 50 s; and a final extension step at 72°C for 7 min. All PCR amplifications were performed using the recombinant Taq polymerase of Roche (Basel, Switzerland). The primers used for PCR amplifications and sequencing for 28S are Forward_C1: ACC CGC TGA ATT TAA GCA T and Reverse_C1: TGA ACT CTC TCT TCA AAG TTC TTT TC. All sequences have been deposited in Genbank and their accession numbers are listed in Table 1.
Proofreading of the obtained nucleotide sequences and subsequent alignment was straightforward and initially performed using genetools 2.0 (Wishart and Fortin 2001) and the alignment was subsequently optimized by eye. Variable regions in the 18S and 28S sequence alignment that were considered arbitrary because of the occurrence of indels (up to 51 bp per sequence), were omitted from the subsequent analyses.
Phylogenetic reconstruction
The phylogenetic analyses were performed on two different data sets. First the nucleotide sequence data were analysed, using both a maximum parsimony (MP) and a Bayesian approach. Subsequently, a combined data set consisting of both morphological and molecular data was subjected to a MP analysis.
Bayesian analyses of the molecular data set were conducted with an online version of MrBayes (Huelsenbeck and Ronquist 2001) implemented at the Bioportal at the University of Oslo (http://www.bioportal.uio.no). Modeltest 3.06 (Posada and Crandall 1998) was used to estimate the best-fitting substitution model for the analyses. Using the Akaike information criterion (AIC), the best model of nucleotide substitution for the 18S, 16S and coxI was the general time reversible model with gamma distributed rate heterogeneity and a significant proportion of invariable sites (GTR + I + G), for the 28S data set it was the GTR + I model. Bayesian inference analyses were performed under 4 000 000 generations and four Metropolis-coupled Markow chains, taking samples every 100 generations, with the first 4000 samples discarded as burn-in. From the resulting trees a posteriori probabilities for individual clades were assessed based on their observed frequencies.
Due to the substitution saturation of cox1 (see Results), additional runs were conducted: (1) without coxI, (2) with 3rd position of coxI excluded and (3) using only the 2nd position of coxI.
The settings for the parsimony analyses were the same for both the molecular and the combined approach. paup* 4 beta 10 win (Swofford 2003) as implemented at the Bioportal at the University of Oslo (http://www.bioportal.uio.no) was used to construct the most parsimonious (MP) cladograms. The parsimony analysis utilized a heuristic search with 1 million replicates and treating gaps as a fifth character state. Gaps can be treated in different ways. If coded as missing data they will not be informative, and thus not contribute to the phylogenetic reconstruction. Alternatively, treating gaps as a fifth state allows for retaining the evolutionary information associated to an assumed indel. Bootstrap analyses were performed with 1000 replicates and 100 searches within each bootstrap replicate.
Pair-wise partition homogeneity tests as implemented in paup* 4 beta 10 win (Swofford 2003) with 200 replicates and 10 searches within each replicate were conducted for the 18S, 28S, 16S and coxI data sets.
Saturation plot
Saturation plots (Fig. 1a–g) were made using p-distances plotted against the distances based on the model chosen by the Modeltest analyses, i.e. GTR + I + G distances for 18S, 16S and coxI and GTR + I distances for 28S, in accordance with Sullivan and Joyce (2005). Individual plots were made for the 18S, 28S, 16S and coxI respectively. The different codon positions of the coxI gene were also plotted separately.
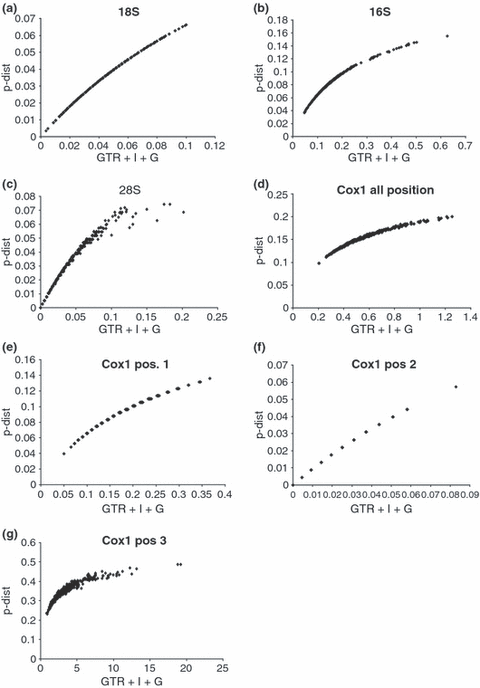
Saturation plots for four molecular markers for 25 Mycetophilinae species, GTR + I + G distances were plotted against p-distances for: (a) 18S rDNA, (b) 16S rDNA, (c) 28S rDNA, (d) coxI, all positions, (e) coxI – first codon positions only, (f) coxI – second positions only, (g) coxI – third positions only
Results
Total alignment of the four molecular markers includes 2374 bp; of which 842 bp correspond to the 18S RNA, 320 bp to the 28S RNA, 529 bp to the 16S RNA, and 683 bp to the coxI genes. A total of 464 sites were parsimony informative, 35 of these sites involved sequences with gaps. The base frequencies are for coxI are A = 30.3%, C = 14.8%, G = 14.3% and T = 40.5%; for 16S A = 39.6%, C = 15.7%, G = 9.9% and T = 34.7%; for 28S A = 29.3%, C = 21.0%, G = 27.2% and T = 22.3%; and for 18S A = 28.6%, C = 18.9%, G = 23.9% and T = 28.5.5%. The 18S, 28S, 16S and CoxI alignments were deposited in the EMBL database and can be retrieved electronically from ftp://ftp.ebi.ac.uk/pub/databases/embl/align/, accession numbers ALIGN_001228–ALIGN_001231. Little or no saturation was detected by means of saturations plots for the nuclear 18S and 28S genes (Fig. 1a,c). In contrast, there is substantial saturation in the mitochondrial 16S and coxI genes, respectively (Fig. 1b,d). As can be concluded from Fig. 1e–g, the saturation of coxI is largely attributed to the third and, to some extent first, codon positions. There is little indication of saturation at the second codon position of coxI.
The partition homogeneity tests reveal significant differences between the coxI and the rest of the genes (p = 0.005), but when the genes are analysed separately against each other coxI is not significantly in conflict with any of the other genes. Though in this analysis 18S is in conflict with 28S (p = 0.005) and 16S (p = 0.005).
Phylogenetic analyses using only the nucleotide sequence data recovered the monophyly of Mycetophilinae and, within the subfamily, the monophyly of the tribe Exechiini with high bootstrap support or posterior probability in all trees (Fig. 3). However, within the tribe Exechiini little support was found for groups of closely related genera, which is congruent with earlier results presented by Rindal et al. (2007) based on 18S, 16S and coxI. The additional 320 bp of the 28S gene appear to lack sufficient phylogenetic signal for obtaining a better resolution of the Exechiini genera.
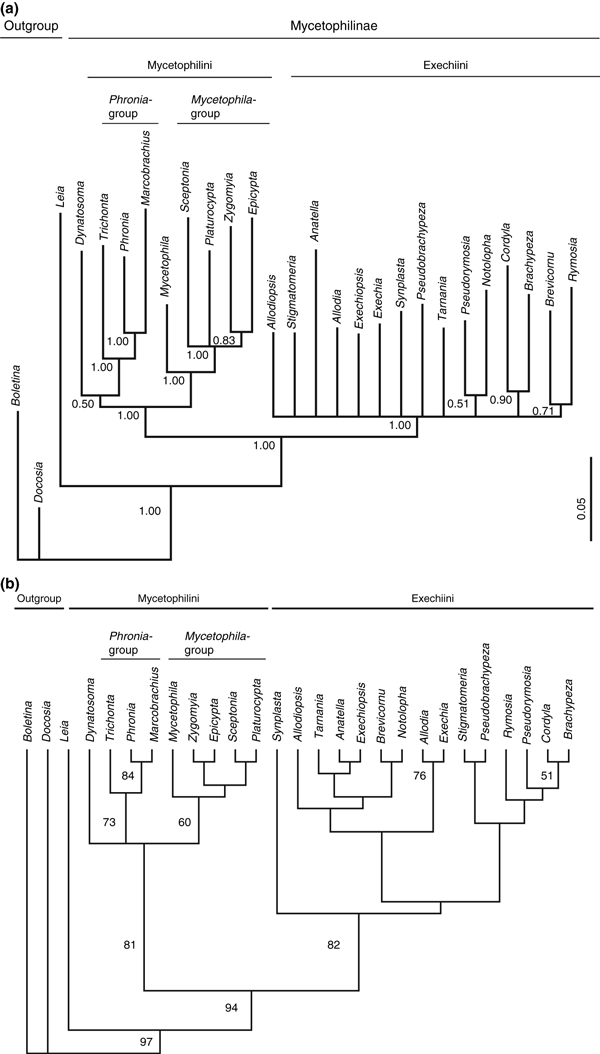
(a) Phylogenetic hypothesis of the fungus gnat subfamily Mycetophilinae as obtained with MrBayes using the GTR + I + G model for the nuclear 18S and the mitochondrial 16S rDNA and the coxI genes, and the GTR + I model for the 28S rDNA. Posterior probabilities exceeding 0.5 are indicated. (b) Consensus of two most parsimonious tree of 2478 steps (RI = 0.318; CI = 0.351), complete molecular data set
The tribe Mycetophilini was found monophyletic, except in the parsimony analyses with the coxI data entirely excluded (data not shown). When using the complete molecular data set, there was very high statistical support for the monophyly of the tribe both in the MP (81%) and the Bayesian analyses (94%). Within Mycetophilini there is also statistical support for the Phronia-group (73% bootstrap; 100% posterior probability) consisting of the genera Phronia, Trichonta, Macrobrachius, and the Mycetophila-group (60% bootstrap; 100% posterior probability) consisting of the genera Mycetophila, Zygomyia, Sceptonia, Platurocypta and Epicypta. The phylogenetic position of Dynatosoma, however, remains ambiguous; while it was found in a trichotomy with the Mycetophila- and Phronia-groups in the MP analysis (Fig. 3b), Dynatosoma was recovered as a sister group of the Phronia-group in the Bayesian analysis (Fig. 3a).
Within the Phronia-group, a sister-group relationship was found between Macrobrachius and Phronia, a result that was well supported in all trees. Within the Mycetophila-group, Mycetophila is found as the sister-group to the remaining taxa.
The phylogenetic analysis of the combined data set (Fig. 2), including the nucleotide sequences of the four molecular markers and 65 morphological characters (Rindal and Søli 2006), yielded two most parsimonious tree of 2582 steps (RI = 0.371; CI = 0.360), with a topology in some respects similar to that obtained with the molecular data alone. However, the monophyly of the tribes Exechiini and Mycetophilini had higher statistical support compared with the analyses based on the nucleotide sequences. The combined data set also supports the genus Dynatosoma as a sister to the Mycetophila- and Phronia-group. Within the tribe Exechiini, few phylogenetic relationships among the genera could be resolved with substantial statistical support.
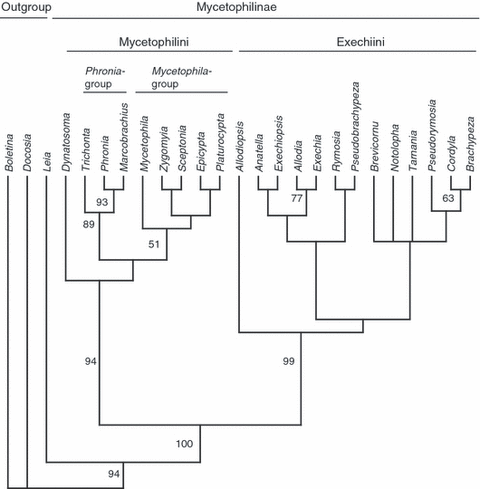
The most parsimonious tree of 2837 steps (RI = 0.3769; CI = 0.3754) recovered for the fungus gnat subfamily Mycetophilinae based on the combined morphological and molecular data set. Bootstrap values based on 1000 replicates that exceed 50 are indicated
Discussion
The recovery of robust phylogenetic relationships depends heavily on the choice of included ingroup and outgroup taxa; it is always recommended to include a large representation of both in phylogenetic analyses. In the present study, outgroup taxa were chosen that allow to use the morphological data from Rindal and Søli (2006). Although our data set may be biased toward a sampling of Palaearctic taxa, this reflects the difficulty in obtaining suitable material from the southern hemisphere either with respect to morphological or molecular analyses, or both.
The subfamily Mycetophilinae
The present study, based on a combined data set including nucleotide sequences of nuclear (18S and 28S rDNA), mitochondrial (16S rDNA and coxI) and morphological characters, confirms the monophyly of the subfamily Mycetophilinae with high statistical support. The current results are congruent to those obtained by Rindal and Søli (2006) and Rindal et al. (2007). Rindal and Søli (2006) analysed exclusively morphological characters, whereas Rindal et al. (2007) based their conclusions exclusively on nucleotide sequence data with the tribe Mycetophilini only represented by two genera.
The tribe Mycetophilini
The combined analyses of the morphological and molecular data also provided strong support for the monophyly of the tribe Mycetophilini including the genus Dynatosoma. As with the subfamily Mycetophilinae, this is in agreement with previous results by Rindal and Søli (2006) and Rindal et al. (2007). Hence, the notion of Tuomikoski (1966), who suggested that Mycetophilini might be paraphyletic and instead considered the Mycetophila- and the Phronia-groups tribes in their own right, is not supported, but cannot formally be rejected on the basis of the taxa included in this study.
The systematic position of Dynatosoma is particularly interesting. Dynatosoma was considered by Tuomikoski (1966) to be a member of the Phronia-group, without specifying synapomorphic characters in more detail. Later, Rindal and Søli (2006) found Dynatosoma in a trichotomy with Epicypta and Plarurocypta, thus considering the genus as part of the Mycetophila-group. In the parsimony analysis of the complete molecular data set, Dynatosoma is found in a trichotomy with the Mycetophila- and Phronia-group, whereas in the combined data set it is the sister-taxa to all other Mycetophilini taxa. On the other hand, the Bayesian analysis places the genus basal to the Phronia-group, though with low posterior probability. Nevertheless, the clade consisting of Phronia, Trichonta and Macrobrachius is recovered with high posterior probability in the Bayesian analysis.
The Mycetophila- and the Phronia-groups are also well resolved within the Mycetophilini. A diagnostic morphological character for separating the Phronia-group from the Mycetophila-group is the presence of a distinct, small, ovate plate above the antennal socket (for further details see Fig. 4 published in Rindal and Søli 2006). In Dynatosoma this area is well sclerotized and furnished with setae, but connected to the frons. At first glance, this condition is more similar to that found in the species of the Phronia-group than it is to the outline of a bare, weakly sclerotized membrane that is characteristic for the species in the Mycetophila-group. This may be taken as support for a basal position of Dynatosoma in the Phronia-group as recovered by the Bayesian analyses presented here. However, it needs to be stressed that the affinity of Dynatosoma and the Phronia-group in the Bayesian hypothesis is not supported by high posterior probability. Moreover, Dynatosoma does not share similarities with members of the Phronia-group in general appearance or in genital structures.
Within the Mycetophila-group, Sceptonia and Zygomyia are recovered as sister-groups in the combined analysis. This may indicates that the loss of M4 in wing venation, following the interpretation of Amorim and Rindal (2007) is a suitable diagnostic synapomorphy for this group. However, the Bayesian and parsimony analysis based on molecular data contradicts this and places Sceptonia together with Epicypta. The phylogenetic position of Mycetophila as the sister-group to the other genera within the Mycetophila-group is found in all trees. The monophyly of the highly diversified genus Mycetophila has never been properly demonstrated, and, currently, it cannot be excluded that the genus is paraphyletic. However, an adequate test of the monophyly of Mycetophila is beyond the scope of this study.
The tribe Exechiini
The combined analysis of morphological and molecular data did not recover any intergeneric relationships within the tribe Exechiini with high statistical support. In the parsimony analyses (2, 3) Exechiini is divided into three clades, though the composition differ between the trees and little statistical support is found for any of the arrangements. The only noteworthy grouping relates to the genera Cordyla and Brachypeza that form a common clade in all trees, though with low statistical support. It is interesting to note that these two genera also share some morphological traits, in particular between Cordyla and Paracordyla, a subgenus of Brachypeza. Their resemblance was also mentioned by Tuomikoski (1966) in his description of the genus. Tuomikoski, however, suggested that this might be a result of convergence.
Concluding remarks
The present study provides support for the monophyly of the subfamily Mycetophilinae and its two tribes, Mycetophilini and Exechiini, as well as the monophyly of the Phronia-group within Mycetophilini. The internal phylogeny of Exechiini, however, remains largely unresolved. Combining data set might give an increased resolution as compared to data sets including only morphological or molecular characters (reviewed in Wortley and Scotland 2006), and sometimes also a better statistical support (Wahlberg et al. 2005). Our study yielded a better resolution, but did not show a significantly better support than found in Rindal and Søli (2006) and Rindal et al. (2007). Thus, future studies need to address the phylogenetic relationships within Exechiini through new approaches. Better phylogenetic resolution may be achieved by including more species for each genus. Geographical variation and biogeography may offer additional useful criteria for the selection of taxa.
Acknowledgements
We thank Magnus Popp for help with the combined analysis, and J. Kjaerandsen, L. O. Hansen, K. Sundt, O. Lønnve, Ø. Gammelmo, T. Darup and P. Tripotin for support with the sampling. Matt Bertone provided valuable comments on the manuscript. The project was supported by the ‘National Centre for Biosystematics’ (Project no. 146515/420), co-funded by the Norwegian Research Council and the Natural History Museum, University of Oslo, Norway.




