Mating behaviour of the ‘cosmopolitan’ species Phyllognathopus viguieri (Copepoda: Harpacticoida) and its systematical significance
Das Paarungsverhalten des ‘kosmopolitischen’ Harpacticoiden Phyllognathopus viguieri (Copepoda) und seine systematische Bedeutung
Abstract
enThe mating behaviour was studied and recorded on video with individuals of four cultures of Phyllognathopus viguieri from different populations obtained from the interstitial water of a slow sand filter near the river Ruhr (Germany) (Ruhr population), from a compost heap in Bethesda (Maryland, USA) (Maryland population), from a rain gauge in Windsor Campbell farm (Jamaica) (Jamaica population), and a tree trunk with moss in a forest in the municipality of Rio de Janeiro (Brazil) (Brazil population). The mating behaviour was divided into the well-known initial phase, copula phase and postcopulatory mate guarding phase. An additional phase prior to the initial phase serves to recognize the female, the recognition phase. The mating behaviour is identical in the males of the Jamaica and Brazil populations of P. viguieri. A postcopulatory mate guarding phase is not found in these two groups. Here, we refute the hypothesis, that a postcopulatory mate guarding phase is found in taxa in which only adult males grasp adult females. The males of the Ruhr and Maryland populations differ from each other in their mating behaviour. Generally, the males of all four populations do not mate with fertilized females which are equally unattractive to the males, i.e., females mate only once in their lifetime to produce offspring. These results corroborate the view that the different populations of P. viguieri do not belong to a single cosmopolitan species.
Zusammenfassung
kaDas Kopulationsverhalten wurde an Vertretern aus vier Populationen von Phyllognathopus viguieri mit unterschiedlicher geographischer Herkunft mit Videoaufzeichnung untersucht. Die Tiere stammen aus dem Grundwasser der Ruhr (Langsamsandfilter) Deutschland (Ruhr-Population), aus Moospolstern im städtischen Wald von Rio de Janeiro, Brasilien (Brazil-Population), aus einem Komposthaufen in Bethesda, Maryland, USA (Maryland-Population) und aus einer Zisterne der Windsor Campbell farm, Jamaika (Jamaica-Population). Das Kopulationsverhalten kann in wie bereits bekannt Initialphase, Kopulaphase und Postkopulaphase eingeteilt werden. Während der Initialphase findet keine Balz statt. Als zusätzliche Phase findet vor der Initialphase eine Prüfphase statt, die dem Erkennen des Weibchens dient. Es konnte festgestellt werden, daß die Vertreter der vier Populationen sich in Bezug auf ihr Kopulationsverhalten voneinander unterscheiden. Das Kopulationsverhalten der Tiere aus Jamaika und Brasilien war identisch, sowohl die Dauer der einzelnen Phasen, als auch das Verhalten der einzelnen Versuchsindividuen. Bei ihnen trat keine Postkopulaphase auf. Bei den Tieren der Ruhr- und der Marylandpopulation trat eine Postkopulaphase von unterschiedlicher Dauer auf. Auch das Fortpflanzungsverhalten der Männchen war unterschiedlich. Die in der Literatur vertretene Hypothese, dass bei Taxa, in denen die Männchen nur adulte Weibchen greifen, eine Postkopulaphase vorkommt, wird in dieser Studie widerlegt. Die Weibchen kopulieren nur einmal in ihrem Leben, was für ihre gesamte Reproduktion ausreichend ist. Männchen kopulieren in der Regel nicht mit bereits begatteten Weibchen. Bereits begattete Weibchen zeigen ein Abwehrverhalten, um die Männchen an der Anheftung der Spermatophore zu hindern. Die Spermatophore wird ohne Hilfe der Schwimmbeine übertragen. Die Befestigung der Spermatophore am Genitalfeld des Weibchens geschieht mit einer Kittsubstanz, die vom Männchen abgegeben wird. Die Individuen der Ruhr- und der Marylandpopulation zeigen trotz einer bei beiden vorkommenden Postkopulaphase unterschiedliches Fortpflanzungsverhalten. Wir schließen daraus, dass sie unterschiedlichen biologischen Arten angehören. Die Individuen der Jamaika- und Brazil-Populationen sind einer Art zuzuordnen, die sich von diesen beiden Arten unterscheidet. Die in dieser Arbeit gemachten Beobachtungen sind eine Bestätigung dafür, dass P. viguieri keine kosmopolitische Art ist, sondern dass es sich tatsächlich um eine Gruppe kryptischer valider Arten handelt.
Introduction
The harpacticoid copepod Phyllognathopus viguieri (Maupas, 1892) has been reported from most continents and has been collected from a vast variety of freshwater habitats (Reid 2001; Glatzel and Königshoff 2005; Bozkurt 2007). Behavioural observations as well as morphological and genetic studies may help in addressing the question whether the different populations can be considered belonging to a single species (see Ganz and Burton 1995; Lee 2000). Cross-breeding experiments within the genus Phyllognathopus Mrázek, 1893 (Copepoda, Harpacticoida) were already performed by Božić (1966) and Glatzel and Königshoff (2005), assuming that a larger number of species exist within the genus and that P. viguieri, as defined at the moment, represents more than one species.
This paper describes the mating behaviour observed in four different populations of P. viguieri of different geographical origin. Relevant observations in P. viguieri were already described by Chappuis (1916), who referred to a manuscript by Maupas, carried out his investigations as early as 1892 (Chappuis 1916). The mating behaviour of Copepoda can be divided into different phases (Boxshall 1990; Dürbaum 1995; Glatzel and Schminke 1996). In the precopulatory mate guarding phase, the male grasps a female copepodid, over the course of one or more successive moults until it reaches sexual maturity following the final moult. This strategy serves to secure paternity (Boxshall 1990). The next phase is the initial phase, during which the male grasps the adult female and sometimes shows a courtship phase. In some species, the actual copula phase is followed by a postcopulatory mate guarding phase during which the male escorts the female following the copula, until the attached spermatophore is evacuated/discharged (Dürbaum 1995; see Glatzel and Schminke 1996).
In addition to the results of the cross-breeding experiments, the question of whether these mating phases also occur in P. viguieri could be clarified.
Materials and Methods
Abbreviations
♀♀: females
♂♂: males
A1: antennula (first antenna)
A2: antenna (second antenna)
P1–P5: first to fifth leg
C1–C5: copepodid stages 1–5
Breeding cultures
Four different populations of P. viguieri were established from existing laboratory cultures of different origin. From these populations, new cultures were prepared in glass Petri dishes (80 × 15 mm) filled with filtrated pond water. Dried ash tree leaves (Fraxinus excelsior L.) served as food (see Glatzel and Königshoff 2005).
The culture vessels were stored at room temperature (21–28°C). Already after 1 week, nauplii and copepodids were observed in all culture plates. Two weeks later, the cultures had developed such that they could be used as initial populations for observations of mating behaviour.
Origin of the four populations
The animals from Germany were found in a slow sand filter on the Ruhr near Echthausen (Ruhr population). The Jamaican animals were taken from a rain gauge in Windsor Campbell farm. In Brazil, samples were collected from damp terrestrial moss, from stones and from pieces of a tree trunk both covered with moss, along the stream Rio Grande in Pedra Grande Massif (Brazil population). The animals from USA, came from a compost heap in Bethesda (Maryland population).
Single individual culture
For observations of the mating behaviour, it is crucial to keep the adult ♂♂ and ♀♀ separately to ensure that no copulations occur prior to the observations. Animals were taken from the culture vessels when at copepodid stage C2–C4 and first pipetted in small glass Petri dishes. Single animals were then transferred into glass block vessels avoiding transporting detritus possibly containing eggs into the vessels. Forty-five copepodids per population were isolated and matured to adult animals within 1–2 weeks at room temperature under daily control. The different development of antennulae, filled oviducts in ♀♀ and visible spermatophores in ♂♂ enabled the sex of the animals to be determined in vivo.
Experiments
The mating behaviour was observed using a microscope with photo tube, to which a video camera was connected with a monitor and an S-VHS time-lapse video recorder (tape system). Filming was done with various objectives from fourfold to 40-fold magnification.
Only sexually mature animals were selected for the experiments. In mature ♀♀, newly developed, unfertilized eggs were visible in the oviducts. In ♂♂, the spermatophore divided into two phases was discernible. An adult ♀ and an adult ♂ were pipetted onto a slide with concave depression within an open drop of water, and then put under the microscope immediately.
In case of contact, the entire course of mating as well as the subsequent separation was filmed. If no contact occurred within 10–15 min, the animals were returned to the glass block vessels. Even following a copula, the animals continued to be kept separately in glass block vessels. During the following days and weeks, respectively, it was checked, if and when the ♀♀ deposited eggs and whether nauplii actually hatched from them. Thereby, it was possible to observe, how many eggs the ♀♀ produced and how long their development took. For this purpose, single eggs were additionally isolated and their development observed in detail. In addition to the pairs that were filmed, pairs in glass block vessels filled with pond water were observed to determine whether the mating behaviour deviated under different conditions. Mating pairs were also observed in the culture vessels.
Some of the already fertilized ♀♀, were put together with new ♂♂ to find out, whether they mated again. Males that had mated before were put together with unfertilized ♀♀, to determine, how quickly a new spermatophore can be produced and how often ♂♂ can mate.
To find out, whether the ♂♂ grasp copepodids, too, single ♂♂ from two populations (Germany and Jamaica) were pipetted into glass block vessels together with 20 copepodids (C3–C5), the sex of which could not be determined at this stage, and were observed for several minutes.
Results
General description of mating behaviour
Although a copula phase was observed in most pairs of the four investigated populations, there were also some cases that failed to result in a copula. When the ♀♀ were still very young and the ovaries not yet completely developed, it happened that the ♂ examined the ♀, but did not try to grasp the inner terminal setae of caudal rami. Some ♂♂ did grasp ♀♀, but could not attach a spermatophore successfully in spite of several attempts. In some couples, the ♀♀ had already mated and rejected the attempt of a new ♂ by bending strongly and swimming away quickly so that the ♂ could not attach a spermatophore.
In those cases where a copula occurred, the ♂ was observed to pursue the ♀ for some seconds or minutes after pipetting in many additional pairs. Subsequently, the ♂ palpated the ♀ with its antennulae in most cases, which mostly happened laterally, if the ♀ was positioned on its ventral side, or ventrally at the thorax or abdomen of the ♀, or rarely at its A1 or caudal rami. While being examined by the ♂, the ♀ remained motionless. Thereafter, the ♂ tried to grasp the ♀ around the inner terminal setae (V) of the caudal rami with its antennulae. The time period during which the ♂ pursues the ♀ and examines it, is called recognition phase. It enables the ♂ to recognize the ♀ and its maturity. However, the ♀ was not examined by the ♂ in all couples. Some ♂♂ directly grasped the inner terminal setae of caudal rami of the ♀♀ without prior examination, and hence there was no recognition phase in these pairs. Nevertheless, the term recognition phase is also used in these couples for the period of time between pipetting and grasping the caudal setae.
In some cases, the ♂ initially grasped only one inner terminal seta of the caudal ramus, either the right or the left one, and then the inner terminal setae of both caudal rami a few seconds later. Occasionally, the ♂ grasped the A1 or legs (P2 or P3) of the ♀ with its A1. At that moment, the ♀ became very restless and tried to shake off the ♂. The ♂ then loosened its grip, ran its A1 along the body of the ♀ down to the caudal rami and then grasped the inner terminal setae. In about half of the cases, the ♂ grasped the setae of the ♀ so that its ventral side faced the dorsal side of the ♀. Then, the ♂ loosened one antennula and turned around so that it could reach the ventral side of the ♀ with its ventral side. These movements occurred so rapidly that their exact course was impossible to observe.
As soon as the ♂ had grasped the tip of the inner terminal setae of the caudal rami, it proceeded to the base of these setae, to hold the ♀ close to the caudal rami.
The time period from grasping the inner terminal setae of the caudal rami with both geniculate antennulae to attaching the spermatophore is called initial phase. The ♂ bent dorsally more or less strongly during this phase and moved under the ♀ so that the animals faced each other with their ventral sides. Grasping the A1 or legs of the ♀ prior to grasping the inner terminal setae was not taken into account for time measurements. Sometimes there were pauses during which the ♂ lost contact with the inner terminal seta of the ♀ and then grasped it again within a few seconds or minutes. In such case time was measured after the pause (Table 1).
| Ruhr population P. viguieri | Jamaica population P. viguieri | Brazil population P. viguieri | Maryland population P. viguieri | |
|---|---|---|---|---|
| Number of couples/pairs | 58 | 45 | 45 | 43 |
| Number of copulae | 23 | 31 | 32 | 32 |
| Copulae with offspring | 17 | 26 | 30 | 31 |
| Copulae without offspring | 6 | 5 | 2 | 1 |
| No copula | 35 | 14 | 13 | 11 |
| Re-copula | 1 | 2 | 2 | 4 |
| Recognition phase | ± | ± | ± | ± |
| Average | 2 : 41 | 1 : 30 | 2 : 43 | 0 : 53 |
| Minimum | 0 : 10 | 0 : 00 | 0 : 20 | 0 : 10 |
| Maximum | 5 : 54 | 8 : 31 | 9 : 55 | 4 : 00 |
| Initial phase | + | + | + | + |
| Average | 1 : 08 | 0 : 27 | 0 : 18 | 0 : 20 |
| Minimum | 0 : 12 | 0 : 02 | 0 : 02 | 0 : 03 |
| Maximum | 6 : 24 | 2 : 07 | 1 : 23 | 1 : 47 |
| Postcopula phase | + | − | − | + |
| Average | 7 : 29 | 0 : 21 | 0 : 17 | 1 : 39 |
| Minimum | 2 : 40 | 0.03 | 0 : 05 | 1 : 05 |
| Maximum | 20 : 00 | 2 : 51 | 0 : 36 | 2 : 39 |
| Discharching of spermatophore | 10 : 00 | 35 : 00 | 20 : 00 | 3 : 00 |
The actual copula phase is an extremely short phase during which contact of both genital fields is established and the spermatophore is transferred. The attachment of the spermatophore does not take longer than 1 s. The spermatophore emerged first from bottom of the ♂ gonopore, which is located on the ventral side of the genital somite. It was then placed with its thin neck onto the female genital field by the pressing male abdomen and was attached instantly by means of an adhesive material that is released from the gonopore of the ♂ following the emergence of the spermatophore (Fig. 1). Subsequently the ♂ either remained in the copula position or immediately moved away from the ♀.
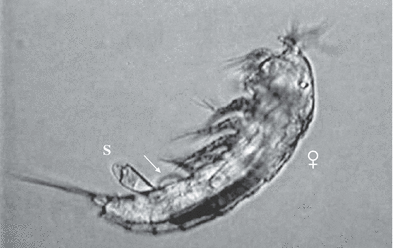
Attached spermatophore (S) just after copulation, showing the adhesive material (arrow) on the female genital field and the bipartite structure of the spermatophore, Phyllognathopus viguieri– Brazil population
As contact after the copula phase, the time period was measured during which the ♂ holds onto the ♀ after attaching the spermatophore. In some cases this contact lasted until the spermatophore was discharged. This phase is called postcopulatory mate guarding phase. It is the time of male’s grasping the female’s inner terminal setae of caudal rami between the copula and the complete discharge of the spermatophore into the female’s seminal receptacles.
Sometimes the ♂♂ grasped the ♀ once again some seconds or minutes after breaking off the contact. Some ♂♂ bent their abdomen dorsally, while others held the ♀ tight without moving. This re-grasping, which lasted only a few seconds, was not taken into account for the time measurements.
Mating behaviour of the Ruhr population
Of the 58 couples observed under identical conditions, only 23 couples mated. No copula phase occurred in 35 couples. In 15 of those 35 couples, the ♀ had mated before. Seven ♀♀ repulsed the ♂ actively, the other eight ♀♀ were not grasped by the ♂♂. In the other 20 cases without copula phase, there was no contact at all. In some cases the ♂ did examine the ♀, however, made no attempt to grasp the inner terminal setae of caudal rami.
When a copula occurred, the pairs stayed together for 2 min and 41 s on average, before the ♂ grasped the inner terminal setae of the ♀. This recognition phase took 15 min at most, but in one couple it lasted only 10 s. Only seven ♂♂ examined the ♀ before grasping it. The others immediately grasped the inner terminal setae with their A1 at the moment of contact. In those cases, there was no recognition phase. Only one ♂ grasped the A1 of the ♀ first and had to change its grip. In three couples, the ♂ lost contact and had to grasp once again. The initial phase lasted 1 min and 8 s on average (Table 1).
Copula phase
The inner terminal setae of the caudal rami, which are grasped by the ♂♂, are modified (swollen) in the ♀♀ of the Ruhr population. Having grasped both inner terminal setae the ♂ stayed behind the ♀ first, repeatedly bending its abdomen dorsally to the extent that the thorax and abdominal somites were curled up and the inner terminal setae touched its A1. Subsequently the ♂ moved under the ♀ and bent the abdomen dorsally again in this position. Additionally, it beat rhythmically with its legs (P1–P4), touching the ♀ only occasionally. Shortly before the spermatophore was attached, the caudal rami of the ♀ were bent dorsally and the P5 of the ♀, which normally clings to the body, was spread out. Then the spermatophore emerged from the gonopore of the ♂ and by bending its body dorsally the ♂ pressed it onto the genital field of the ♀ within the area of the copulatory pore (Fig. 2). The ♂ remained in this position for 30 s up to 1 min and continued beating with its legs. Both A2 of the ♂ positioned laterally relative to the abdomen of the ♀ also moved backwards and forwards rhythmically. According to our observations, the mouthparts did not move. After the ♂ had removed itself from the genital field of the ♀, it stayed under the ♀ with its body stretched out (see Fig. 3) and continued to grasp both inner terminal setae of caudal rami. The P6 of the ♂, which did not move during the copula phase, was repeatedly raised during this phase. Some time lapsed before the ♂ resumed its position behind the ♀. The ♀, which remained completely motionless during the copula phase, swam ahead and either pulled the ♂ with it or the ♂ pushed the ♀ ahead. After some minutes, the ♀ became restless and started bending dorsally and ventrally or rotated around its own antero-posterior axis. Some seconds to minutes later, the ♂ released contact. The contact following the copula, i.e. the postcopulatory mate guarding phase, took 7 min and 29 s on average; none of the ♂♂ grasped the same ♀ again. In some ♀♀ discharge of the spermatophore took up to 10 min.
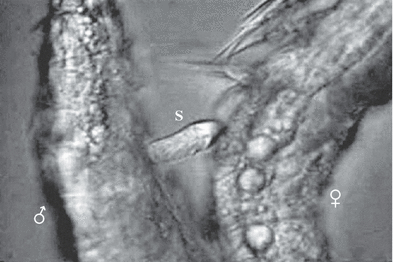
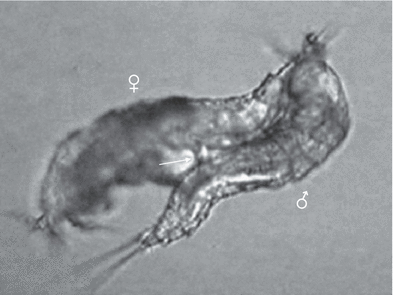
Male on left side, female on right side just after the copula. The extruded spermathophore (S) is between the couple, Phyllognathopus viguieri– Ruhr population
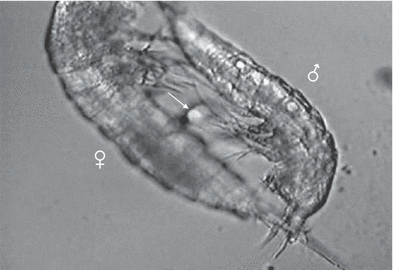
Male (above) and female just after copulation with spermatophore (arrow). Male grasps with its antennulae the inner terminal setae of caudal rami of the female, Phyllognathopus viguieri– Jamaica population
Production of offspring
Following the copula phase only 17 of the 23 ♀♀ produced offspring. The first eggs were observed in five ♀♀ only. They were produced after 3.2 days on average. Two eggs were produced per clutch. The first nauplii were clearly visible in all ♀♀ and hatched after 5.4 days on average. In the course of their different life spans, the ♀♀ produced up to 129 offspring (65 broods). The maximum life span observed for an adult ♀ was 116 days (without nauplius and copepodid stages). The duration of development could be observed in isolated eggs in the glass block vessels at a room temperature of 21°C. It took 9 days to develop the egg into the first copepodid (including the development of the egg before the nauplius hatches). The five copepodid stages were passed within 6–7 days, so that the entire development from egg deposition until adult animal took 15 days (Table 2).
| Ruhr population | Jamaica population | Brazil population | Maryland population | |
|---|---|---|---|---|
| Temperature | 21°C | 23°C | 25°C | 25°C |
| Development in the produced eggs1 | 2 | 1 | 2 | 2 |
| Nauplius stages2 | 7 | 7 | 2 | 3–4 |
| Copepodid stages3 | 6–7 | 5 | 4 | 5–6 |
| Complete development4 | 15 | 13 | 8 | 10–12 |
- 1Egg production to hatching of nauplius.
- 2First nauplius stage to first copepodid stage.
- 3First copepodid stage to adult.
- 4Total time of development from egg production to the adult.
Re-copula experiment
Sixteen ♀♀ that had already mated were again brought together with a ♂, the interval between successive copulae varying between 1 h and 62 days. Some of these ♀♀ had produced offspring after the first copula, while others had not. A single ♀ mated again with a ♂ following the first copula. This ♀ had not produced offspring until that time. In all other ♀♀, independent of whether or not they had produced offspring, there was no second copula. Seven ♂♂ attempted to copulate after grasping the ♀, however, the ♀♀ did not remain motionless, but prevented spermatophore transfer by continuous swimming and bending their body backwards and forwards. After several minutes, the ♂♂ loosened the contact. In the other, eight of the 15 ♀♀ that did not mate again, the ♂♂ did not attempt to grasp the ♀.
The ♂♂ of the Ruhr population brought together with a ♀ again after their first successful copula did not mate a second time except for one ♂. The interval between copulae was between 18 and 48 h. The only ♂ that mated again attached a spermatophore to another unfertilized ♀ 26.5 h after the first copula. This ♀ produced viable offspring.
Mating behaviour of the Jamaica population
Of the 45 couples observed under identical conditions, 14 did not copulate. Eight of the respective 14 ♀♀ had already mated before and repulsed the ♂ or the ♂ did not attempt to grasp the ♀ at all. In another four couples, the ♂♂ did grasp the respective ♀, however, were not able to attach a spermatophore in spite of several attempts. In two couples there was no contact at all.
A copula occurred in 31 couples. The recognition phase lasted 1.5 min on average. Two thirds of the ♂♂ examined the ♀♀ before grasping them laterally, at the A1 or at the caudal rami with their A1. No recognition phase was observed in the other couples. In only two cases the contact broke after grasping the inner terminal setae of caudal rami so that the ♂♂ had to grasp once more. Only one ♂ first grasped the ♀ at the A1 and had to loosen the contact. The other ♂♂ grasped the inner terminal setae at once. The initial phase prior to the attachment of the spermatophore lasted 27 s on average (Table 1).
Copula phase
The ♂♂ of the Jamaica population moved under the ♀♀ immediately after grasping the inner terminal setae of the caudal rami such that the ventral sides of the animals faced each other (Fig. 4). The inner terminal setae are long, not modified and not swollen in the ♀♀ of the Jamaica population. The ♂ then repeatedly bent its abdomen dorsally, but did not curl up as strongly as the ♂♂ of the Ruhr population from Germany. The ♂ only beat with its legs (P1–P4) for a moment without touching the ♀. The A2 and mouthparts did not move. Only some seconds later, the spermatophore emerged from the gonopore of the ♂ and was attached to the ♀ by pressing the abdomen against it. Before that, the caudal rami of the ♀ were also bent upwards and the P5 of the ♀ turned away from the body. Then, the ♂ immediately detached its genital field from the ♀. The grasp around the inner terminal setae of the caudal rami was maintained for an average of 21 s following the actual copula, the ♂ lying under or behind the ♀ and bending its abdomen dorsally several times. Thereafter it loosened the grasp. The ♀ lay still throughout the contact. After breaking the contact, several ♂♂ again examined the ♀ in lateral aspect some seconds to minutes later, however, none of the ♂♂ attempted to grasp the ♀ once more. The complete discharge of the attached spermatophore (Fig. 5) took 35 min in the ♀♀ of the Jamaica population (Table 1).
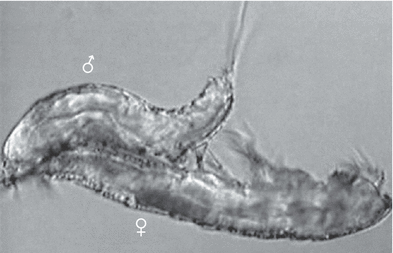
Male (above) grasping the female’s inner terminal setae of the caudal rami with its antennulae and pressing its genital field against the genital field of the female during copulation, Phyllognathopus viguieri– Jamaica population
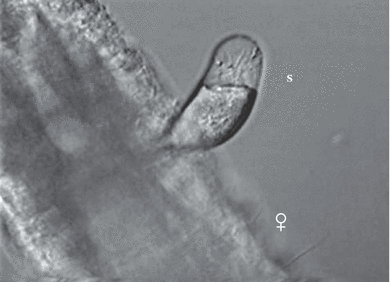
Attached spermatophore (S) just after copulation, showing the bipartite structure; the proximal part contains the spermatozoa, Phyllognathopus viguieri– Jamaica population
Production of offspring
Of the 31 ♀♀ that had mated, 26 produced offspring. In 10 ♀♀, the first eggs (two per clutch) were observed after 5.1 days on average while the first nauplii appeared after 6.3 days on average. The maximum life span of a ♀ was 121 days. This ♀ produced 270 offspring (135 clutches) within this period. The duration of development totalled 13 days at 23°C. The nauplii emerged from the isolated eggs after 2 days. The moult from sixth nauplius stage to first copepodid stage was reached 6 days later. The five copepodid stages were passed within 5 days (Table 2).
Re-copula experiment
The question of whether a repeated copula occurred was investigated in an experiment with 10 ♀♀. The interval between the successive copulae was between 15 min and 55 days. Two ♀♀ mated again. One of them had produced offspring before, the other one had not. Of the eight ♀♀ that did not mate again, three repulsed the grasping of the ♂. Thus, the ♂♂ were not able to attach a spermatophore because of the movements of the ♀. In the other five couples, the ♂♂ did not attempt to grasp the respective ♀.
Nine ♂♂ of P. viguieri mated several times. Two ♂♂ mated four times at intervals of 1–5 days. In five ♂♂, another copula occurred 24 h following the previous one. With the exception of three ♀♀, all of the 17 ♀♀ mating with a ♂ that had mated before produced offspring.
Mating behaviour of the Brazil population
Of the Brazil population, 45 couples were observed under identical conditions, 32 of them having mated. Of the 13 couples without copula, nine ♀♀ had already mated before and repulsed the respective ♂. In the other four couples that did not mate, the ♂♂ grasped the inner terminal setae of caudal rami of the ♀♀, but failed to attach a spermatophore.
In the couples displaying copula, 24 of the 32 ♂♂ examined the ♀♀ laterally or ventrally before grasping them. On average, the recognition phase lasted 2 min and 43 s, before the ♂ grasped the inner terminal setae of caudal rami of the ♀. There was no recognition phase in the other eight couples. In six couples, contact was interrupted temporarily so that the ♂♂ had to grasp once again. The initial phase prior to the copula phase took 18 s on average (Table 1).
Copula phase
The inner terminal seta of the caudal ramus is long and not swollen in the ♀♀ of the Brazil population. Hence, it corresponds to that in the ♀♀ of the Jamaica population. After grasping the two inner terminal setae of the caudal rami the ♂♂ of the Brazil population moved under the ♀♀ very quickly. The abdomen was repeatedly bent dorsally. Then both caudal rami of the ♀ were bent dorsally, the P5 of the ♀ being turned away from the somite and by folding its body dorsally the ♂ attached the spermatophore emerging from its gonopore to the genital field of the ♀. The female and male genital fields then separated immediately. The ♂♂ maintained their position below the ♀, moving their abdomen dorsally very often and quickly. On average, 17 s after the attachment of the spermatophore the ♂♂ released contact. Prior to and following the copula, the ♂♂ also temporarily moved the legs (P1–P4) away from the body without touching the abdomen of the ♀. Mouthparts and A2 of the ♂ did not move. The ♀♀ lay still throughout the contact. Several ♂♂ examined the ♀ again directly following the contact or after some minutes without grasping the inner terminal setae or swam beside the ♀.
The spermatophore attached to the genital field of the ♀♀ was completely discharged within 20 min (Table 1).
Production of offspring
In the ♀♀ of the Brazil population, only two of the 32 ♀♀ did not produce offspring following the copula. One of them mated again 10 days after the first copula and then produced offspring after that second copula. First eggs, two per clutch, were produced, if they were observed, after 2.6 days on average. The nauplii hatched 4.2 days after the copula on average. The maximum life span of a ♀ after the copula was 52 days. The maximum life span observed in a ♀ that had not mated was 156 days (excluding development time of nauplii and copepodid stages). The ♀♀ produced up to 294 offspring (147 clutches). Their development took 8 days at 25°C. Two days after egg production, the nauplii hatched, which then reached the first copepodid stage after 4 days. Two to three days later they already reached their adult stage (Table 2).
Re-copula experiment
The experiment was performed in 11 ♀♀ 1–24 days after a copula. Two ♀♀ mated again. One of them had not produced offspring within the 10 days following the first copula. The second ♀ had mated 24 days before and had produced offspring for 11 days and then no longer for 13 days. No second copula occurred in the other nine ♀♀. Most ♀♀ prevented the attachment of a spermatophore by swimming or bending their bodies ventrally. Only two ♂♂ did not attempt to grasp the ♀.
Contrary to the other populations, the experiment to find out whether the ♂♂ mated several times could not be performed, because the majority of ♂♂ employed in the experiments died 1 or 2 days following a copula. Also the ♂♂ that had not mated died within few days, because they had crawled out of the glass block vessels and had dried out. Two days after their first copula, two ♂♂ (with visible spermatophores) were brought together with a new ♀ that had not mated before, but neither mated again with it.
Mating behaviour of the Maryland population
Forty-three couples were observed under identical conditions. No copula occurred in 11 couples. Eight of those 11 ♀♀ had already mated before and repulsed the ♂♂. In the three couples without successful copula, the ♂♂ did grasp the inner terminal setae of the caudal rami of the ♀♀, however, did not attach a spermatophore.
A copula occurred in 32 couples. Only five ♂♂ examined the ♀, while the others immediately grasped the inner terminal setae of the caudal rami of the ♀♀ without prior recognition phase. This recognition phase lasted 53 s on average. In only one pair, it happened that the ♂ initially grasped the A1 of the ♀ and had to loosen its grasp to clasp the inner terminal setae of the caudal rami. The initial phase took an average of 20 s.
Copula phase
The inner terminal seta of the caudal rami of the ♀♀ from the Maryland population is short and modified (swollen). Having grasped both inner terminal setae, some ♂♂ stayed behind the ♀♀ in the beginning. Others immediately took a position below the ♀ or grasped the inner terminal setae from the ventral side. The legs of the ♂ were shortly moved away from its body without touching the ♀. The ♂♂ bent their abdomen repeatedly dorsally and then bent the caudal rami of the ♀ upwards. The P5 of the ♀ subsequently turned away from the ventral surface of the somite. The spermatophore emerging from the gonopore of the ♂ was attached by bending the male abdomen dorsally and pressing it to the genital somite of the ♀ (Fig. 6). Subsequently, the ♂♂ remained in the copula position for 20–30 s. Then the ♂ stretched out its abdomen such that the direct contact of both genital fields ceased, however, the cephalothorax of the ♂ remained closely below the abdomen of the ♀. The grasp of the A1 at the inner terminal setae of the caudal rami shifted from the base of the setae to their median part and further up to their distal tips, bending them still further upwards. At the same time the ♂ positioned its cephalothorax around the caudal rami of the ♀ such that the antennulae of the ♂ lay dorsally above the caudal rami of the ♀. The distance between the abdomen of the ♀ and the abdomen of the ♂ became larger and the ♂ grasped ventrally around the abdomen of the ♀. During the contact following the attachment of the spermatophore, the ♂♂ frequently and rapidly moved their abdomen up and down whilst beating their legs (P1–P4) and A2. Temporarily an A1 of the ♂ lost contact with the inner terminal seta of the caudal ramus, which was then grasped anew in most cases. The ♂ released contact after an average of 1 min and 39 s and withdrew from the ♀. The ♀♀, which remained motionless up till then, started swimming again. Many ♂♂ examined the ♀♀ once again after some seconds to minutes and some grasped the inner terminal setae again. The ♀♀ remained still, the ♂♂ moving their abdomen fast up and down and then breaking the contact again.
Copula position with spermatophore (arrow), male below, of Phyllognathopus viguieri– Maryland population
The spermatophore attached to the ♀ was fully discharged after maximally 3 min (Table 1).
Production of offspring
Only one of 32 ♀♀ did not produce offspring after the copula. This ♀ mated again 7 days after the first copula and then produced offspring. The eggs were deposited, as far as observed, already after 1.6 days. The nauplii hatched 3.3 days after the copula. The highest number of nauplii was 232 (116 clutches) and was produced by one ♀ in 65 days. The maximum life span was 85 days. The duration of development observed in isolated eggs was 10–12 days at a room temperature of 25°C. Development from deposited egg to first copepodid stage took 5–6 days. The five copepodid stages were passed also within 5–6 days (Table 2).
Re-copula experiment
Of 12 ♀♀ brought together with a ♂ for a second time, four ♀♀ mated again. The interval between the copulae was between 1 day and 20 days in the 12 experiments. One of the four ♀♀ that mated again had not produced offspring within 7 days after the first copula. The other three ♀♀ that had produced offspring following the first copula mated again 9–20 days after the first copula. In the eight experiments, in which no re-copula occurred, the ♀♀ repulsed the ♂♂. Typically, the ♂♂ grasped the inner terminal setae of caudal rami of the ♀♀ and attempted to mate, however, failed to attach a spermatophore due to the movements of the ♀♀ which incessantly bent their bodies dorsally and ventrally.
Several ♂♂ of the Maryland population mated frequently. The shortest interval between two copulae was 22 h. One ♂ mated once a day on five successive days. Another ♂ mated 11 times within 2 weeks and the 12th time 14 days afterwards. All of the ♀♀ that had mated produced offspring.
Mating between males and copepodids in all populations
Adult ♂♂ that were put together with copepodids of the second to fifth stage C2–C5 (sex of copepodids not determined) did not attempt to grasp them. Four single ♂♂ of different age of the Ruhr population were put together with 20 copepodids in a glass block vessel each. The ♂♂ did not pursue them and in case of accidental contact the copepodids were not examined either. Two ♂♂ of the Jamaica population were tested and did not attempt to establish contact with the copepodids, either. One ♂ of the Brazil population put together with a C5 ♀ checked the copepodid repeatedly. After several minutes, the ♂ grasped the inner terminal setae of the caudal rami of the juvenile ♀, but released it after few seconds. Subsequently, the ♀ was examined repeatedly around the caudal rami, however, the ♂ did not attempt to grasp the ♀ again.
Mating between males in all populations
In several isolated ♂♂ put together into a glass block vessel, it was observed that they grasped each other and attempted to mate. Two ♂♂ had grasped each other and tried to attach a spermatophore onto one another. Although the ♂♂ clasped by other ♂♂ were very restless and tried to repel their partner ♂, spermatophores were nevertheless attached in such cases. In one glass block vessel, a ♂ was observed with three spermatophores attached to its abdomen, however, such observations were made only if there were no ♀♀ in the vessel and the ♂♂ had been kept isolated before.
Discussion
Mating behaviour
Although mating behaviour in Harpacticoida is very variable, the entire process can be dissected into comparable phases in different taxa, which are also applicable to Calanoida and Cyclopoida.
These phases are:
-
recognition phase – described for the first time
-
precopulatory mate guarding (Boxshall 1990),
-
initial phase and stimulation/courtship phase (Glatzel and Schminke 1996)
-
copula phase and
-
postcopulatory mate guarding (Dürbaum 1995).
Precopulatory mate guarding
Precopulatory mate guarding phase, in which an adult ♂ grasps a juvenile ♀, to mate with it after its terminal moult, occurs within the Cyclopoida in some parasitic taxa, but only exceptionally in free-living species [e.g. Orthocyclops modestus (Herrik, 1883)]. In Calanoida, a precopulatory mate guarding phase has thus far not been documented (Boxshall 1990).
The precopulatory mate guarding phase, which frequently occurs in Harpacticoida, was not found in the populations of P. viguieri observed in this study. The ♂♂ brought together with copepodids did not examine them as appropriate ♀♀.
A precopulatory mate guarding phase has been described in various species of Tigriopus Norman, 1869 (Harpacticidae). Males of T. fulvus (Fischer, 1860) (Lazzaretto et al. 1994), T. californicus (Baker, 1912) (Burton 1985), and T. japonicus Mori, 1938 (Kelly and Snell 1998) grasp very young ♀♀ of the second and even first copepodid stages. Males usually dominate and must therefore secure an appropriate ♀ for future mating as early as possible. Hence, they spend up to 15 days in the precopulatory mate guarding phase (Lazzaretto et al. 1994), but release contact with an early copepodid stage when a more advanced/mature ♀ comes into their reach. The ♂♂ can determine sex and age of the copepodid, even at an early stage of development (Burton 1985). The ♂♂ of Zausodes arenicolus Wilson, 1932 (Harpacticidae) (Kern et al. 1984) and Coullana canadensis (Willey, 1923) (Canuellidae) (Lonsdale et al. 1988) grasp very early copepodid stages as well. In other taxa of Harpacticoida, the ♂♂ clasp female copepodids of the fourth and fifth stages (Walker 1981; Hicks and Coull 1983; Dürbaum 1997). The Harpacticoida stated so far are exclusively marine taxa, however, a precopulatory mate guarding phase also occurs in some freshwater taxa. In Harpacticella inopinata Sars, 1908 (Evstigneeva 1993), juvenile ♀♀ are clasped from the third copepodid stage C3 on, but ♂♂ mainly clasp ♀♀ of the fifth stage C5. During the terminal moult of the ♀ the ♂ releases its grasp and waits besides the ♀ for the moult to be completed before the copula takes place.
The copula takes place following the terminal moult of the ♀ in all taxa with precopulatory mate guarding phase. Thus, it is ensured that the ♀ has no spermatozoa stored in its seminal receptacle (Ridley 1983). The ♂♂ can mate the moment the ♀ becomes mature without investing time in locating an adult, virgin ♀. Therefore, the precopulatory mate guarding phase means an increase in reproductive success and thus a selective advantage to the ♂. Precopulatory mate guarding phase frequently occurs in taxa in which the ♂♂ mate only once so that each unfertilized ♀ is a potential partner even if it is still juvenile. In these taxa, there are often only few ♀♀ in relation to the ♂♂ so that the ♂♂ compete with each other (Boxshall 1990). According to Ridley (1983), the occurrence of a precopulatory mate guarding phase is determined by the female’s capability of conceiving which is mostly connected with the moult cycle.
The position of the animals during the precopulatory mate guarding phase differs from that during the actual copula phase. For example, the ♂♂ of the Laophontidae grasp the ♀ at the exopod of the fourth leg pair P4 (Lang 1948), others, as e.g. Tigriopus spp., at the posterior edge of the cephalothorax. A transfer of spermatophores cannot take place in this position, because the copulatory pore of the ♀ lies ventrally in the middle of the genital segment. The ♂♂ have to grasp anew prior to the copula. According to Boxshall (1990), species in which the ♂♂ produce spermatophores several times and mate with more than one ♀, do not show precopulatory mate guarding. Frequently, these are species with few ♂♂. Since Phyllognathopus populations do not show a precopulatory mate guarding phase, it can be assumed that there are enough ♀♀ so that it is not necessary to secure paternity in this way.
Pheromones and mating behaviour
Pheromones serve to recognize female copepodids and adult ♀♀ in Copepoda without precopulatory mate guarding phase. They have been documented in Calanoida [Eurytemora sp. (Katona 1973)], Cyclopoida [Oithona davisae (Uchima and Murano 1988)] and Harpacticoida. Snell and Carmona (1994) studied various species of all three taxa and showed glycoproteins occurring on the body surface serve as pheromones. They are concurrently found at the abdomen, the setae of caudal rami, the copulatory pores, at the edge of the genital somite and at the edges of somites in the different species. Hence, the latter regions play an important part in grasping the ♀♀, during the copula phase and the transfer of spermatophores. The body regions of the ♀ where pheromones were found correspond with the regions where the ♂♂ of the four P. viguieri populations examine the ♀♀ prior to grasping. This indicates pheromone production in the ♀♀ of the four P. viguieri populations agrees with the observations reported by Snell and Carmona (1994). In this context, it is relevant to note the important distinction between contact pheromones on the body surface of the ♀♀ and diffusible pheromones that are released into the ambient water (Lonsdale et al. 1998).
The ♂♂ of some species show special search behaviour, in particular when ♀♀ were in the water before (Griffiths and Frost 1976). Some can recognize ♀♀ at a distance of up to 20 mm (Katona 1973) and change their normal swimming behaviour in response to that. According to experimental observations of Temora longicornis Müller, 1792 (Doall et al. 1998), ♀♀ are detected by ♂♂ even at a distance of more than 34 mm. Males of Cyclops scutifer Sars, 1863 can follow traces left in the water by ♀♀, increasing their swimming speed and synchronizing their swimming behaviour with the ♀ (Strickler 1998). In the four populations of P. viguieri observed, no special mate tracking behaviour was found during the experiments or in the culture vessels. Often, a ♂ clasped the ♀ when accidentally swimming into it. Furthermore, the population density in the culture vessels was so high that special search behaviour was not required, since the ♂♂ met a fertilizable ♀ at adequate intervals.
In addition to recognizing ♀♀, fertile ♀♀ can be distinguished from infertile ♀♀ in Diaptomus sp. (Chow-Fraser and Maly 1988) and Oithona davisae Ferrari and Orsi, 1984 (Uchima and Murano 1988). In the four P. viguieri populations, ♂♂ also recognized immature ♀♀. Often, the ♂♂ did not attempt to grasp a ♀, when its oviducts were not completely filled with mature eggs. One ♀, in which an oviduct on one side was completely filled, was grasped and fertilized. Thus, recognition and release of pheromones, respectively seem to be connected with the degree of egg maturity. It is not quite clear, whether ♂♂ can distinguish between fertilized and unfertilized ♀♀. A few ♂♂ attempted to grasp fertilized ♀♀ to mate with them, however, other ♂♂ did not show a reaction.
According to Lazzaretto et al. (1990)♀♀ of Tigriopus fulvus can recognize their own offspring, because they prey on unrelated nauplii, but do not show cannibalistic behaviour. Pheromones are species-specific, because ♂♂ can distinguish between ♀♀ of their own species and ♀♀ of other species (Jacoby and Youngbluth 1983; Chow-Fraser and Maly 1988; Lazzaretto et al. 1990; Ting et al. 2000). Lazzaretto et al. (1994) demonstrated that slightly modified pheromones occur already in different geographical populations of T. fulvus, as ♂♂ show a less strong reaction to ♀♀ of other populations than to ♀♀ of their own population.
Recognition phase
Already in 1976, Griffith and Frost assumed that the aesthetascs on the antennulae of male Calanus pacificus Brodsky, 1948 are the main receptors for detecting pheromones, because aesthetascs are better developed in ♂♂ than in ♀♀. It is conceivable that contact pheromones are detected with the antennulae, as in various species ♂♂ examine ♀♀ with their A1 before grasping them. Males of Tigriopus japonicus examine the copepodids around the ramal setae and the abdomen (Ting et al. 2000). Male Tachidius discipes Giesbrecht, 1881 examines the juvenile ♀ at its ventral side (Dürbaum 1997). Also in the ♂♂ of the four P. viguieri populations, the receptors for pheromones must be located on the antennulae, because ♀♀ are examined by ♂♂ using their A1, exclusively. As this checking of ♀♀ occurs in different taxa, and also in the observed populations of P. viguieri, this behaviour prior to grasping the inner terminal setae of the caudal rami is considered a separate phase of the mating behaviour and is called recognition phase. It enables the ♂ to recognize the ♀. In all four populations investigated, some couples did not show a recognition phase. Instead, the ♂♂ immediately grasped the inner terminal setae of the caudal rami of the ♀♀. Obviously, the ♀♀ were instantly recognized as appropriate mating partners when first encountered, rendering additional examination unnecessary. Although it has been suggested for other species (Coullana canadensis and Coullana sp.) that structures of the antennae (A2) instead of those of the antennulae (A1) presumably serve as receptors to examine the ♀ (Lonsdale et al. 1996), this seems improbable, as there are no aesthetascs on the A2.
Male examination may also be a signal for the ♀♀. In the four P. viguieri populations, the ♀♀ remained motionless while being examined and also kept still afterwards. The examination by ♂♂ may be interpreted as a sign of the copula becoming imminent. The motionless ♀ thus enables the ♂ to clasp it. Females that had already mated often swam away when they were examined by a ♂, to prevent the ♂ from grasping. Some ♂♂ did not examine the respective ♀ and did not attempt to grasp the inner terminal setae of the caudal rami either, even though the ♀ had completely filled oviducts. In those cases, the ♂♂ were probably too young. Although clearly visible, their spermatophore was not sufficiently developed to be released.
Initial phase
Prior to the copula, a ♂ has to clasp a ♀. The adult ♀ is either clasped directly, or the ♂ must grasp it anew following her terminal moult, as exemplified by the species displaying precopulatory mate guarding. The appendages used for grasping are usually sexually dimorphic (Ridley 1983). Typically, the antennulae are transformed into a geniculate appendages in the ♂♂ (unilaterally in Calanoida, ambilaterally in Harpacticoida and Cyclopoida), however, maxillipeds and legs can also exhibit sexual dimorphism.
In the Harpacticoida, the ♂♂ grasp with both geniculate antennulae. Lang (1948) claimed that the differences in grasping, i.e. the places on the body of the ♀♀ where the ♂♂ grasp them, are family-specific. The former general view had been that the ♂♂ always clasp the ramal setae of the ♀♀. However, sometimes ♂♂ grasp around the posterior edge of the cephalothorax (for example: Tachidiidae, Harpacticidae, Peltidiidae), at the base of the caudal rami (Canuellidae), between the two terminal body somites (Porcellidiidae) or around the distal exopod segment of the fourth leg 4 (Laophontidae). In the Laophontidae, the ♂♂ are reported to frequently grasp immature ♀♀ (Fiers 1998). For the other taxa stated in Lang (1948), precopulatory mate guarding phase and copula phase are not differentiated.
The ♂♂ of the four P. viguieri populations also grasp around the inner terminal setae of the caudal rami of the ♀♀ in the usual way. The inner terminal setae of caudal rami are short and modified (swollen) in the ♀♀ of the Ruhr and Maryland populations, while they are long and thin in the ♀♀ of the Jamaica and Brazil populations. These different forms do not influence the grasping of the ♂♂. It rarely happened that the ♂♂ grasped around the A1 or legs of the female. A successful copula is not possible when assuming the latter postures.
Kern et al. (1984) use the term initial phase to describe the mating behaviour of Euterpina acutifrons (Dana, 1847), which was studied by Haq (1972), although the author himself did not use this term. Accordingly, the ♂ grasps the ♀ during the initial phase with its antennulae around the terminal setae of the caudal rami. Courtship is not described. The subsequent phase is the copula phase, i.e. the transfer of the spermatophore (Kern et al. 1984).
The initial phase in the four populations of P. viguieri starts with the grasping of the inner terminal setae of the caudal rami. The ♂ then proceeds to the base of the inner terminal setae and pulls itself underneath the ♀ so that the ventral sides face each other. Rhythmic dorsal movements of the abdomen by the ♂ precede the release of the spermatophore, and the beating legs are probably connected with that the latter process. The behaviour in the four P. viguieri populations cannot be called courtship, as the ♀ is hardly touched and thus not stimulated as such. The length of the initial phase is different in the four populations observed (see Table 1).
In some copepods, the initial phase includes a courtship phase. In Parastenocaris phyllura Kiefer, 1938, the initial phase was described by Glatzel and Schminke (1996) as follows: the ♂ clasps the ramal setae of the ♀ with its A1. Both animals then move together and the ♀ tries to loosen the grasp of the ♂ with its mouthparts. In this phase, the ♀ has the possibility to repulse the ♂. Subsequently, the courtship phase commences during which the ventral side of the ♂ faces that of the ♀. With its modified third legs, the ♂ then grasps the genital double segment of the ♀ and additionally holds it tightly with the caudal rami and the A2, after having loosened the clasp of the A1 and grasping the A2. The fourth legs of the ♂ then beat against the ventral side of the ♀. Dürbaum (1995) observed a similar behaviour in Tisbe sp., in which the ♂♂ hold the ♀♀ tightly with A1, A2, maxillipeds, maxillulae and endopods of P2. The legs P2 to P4 beat against the ventral side of the abdomen of the ♀. The ♀ then bends its abdomen upwards and exposes its genital field, which is then no longer covered by the P5.
Although a courtship phase as described above does not occur in the four P. viguieri populations, the abdomen of the ♀ is bent upwards and the P5 spread out so that the genital field is exposed. Whether the ♀ actively bends the abdomen upwards itself or the ♂ causes this to happen by its movements, cannot be clarified on the basis of the observations made in the experiments.
In T. discipes the legs beat rhythmically against the P5 of the ♀, when the ♂ has controlled the genital field of the ♀ with its second, third, and fourth legs, which takes place following the terminal moult of the ♀. Males of T. discipes also mate with ♀♀, which they have not previously accompanied during the precopulatory mate guarding phase, however, only with immature ♀♀. By means of an inspection of the genital field they can determine, whether the ♀ has already mated (Dürbaum 1997).
Blades and Youngbluth (1979) observed a similar behaviour in Labidocera aestiva Wheeler, 1900 (Calanoida). The ♂ firmly strokes the abdomen of the ♀ with the modified endopod of the left P5 before transferring the spermatophore. This inspection serves the ♂ to examine, whether it has grasped correctly, whether it is lying properly relative to the abdomen of the ♀ and whether a spermatophore has been attached before. The preceding phase, during which the ♂ grasps the setae of the caudal rami of the ♀ with the right antennula (A1) (as in many Calanoida), is called ‘initial physical contact’ by Blades and Youngbluth (1980) corresponding to the above-mentioned initial phase.
Copula phase
For Calanoida, a basic course of mating behaviour can be described as follows: the ♂♂ grasp the setae of caudal rami of the ♀♀ with the geniculated right antennula A1. The abdomen of the ♀♀ is then clasped with the right P5, whereupon the grasp of the A1 is loosened in some species (Blades and Youngbluth 1980). The extruded spermatophore is then grasped around its neck with the left P5 of the ♂ and attached to the genital field of the ♀ (Dussart and Defaye 2001). Special setae on the exopodite of P5 are available for this purpose in Centropages typicus (Krøyer, 1849) (Blades 1977). Female calanoids often bear several spermatophores deposited by various ♂♂, of which only one will discharge its content. In Calanoida, a second mating seems to be necessary to produce new egg clutches (Dussart and Defaye 2001). This was confirmed for Eudiaptomus gracilis (Sars, 1863), in which the ♀♀ have to mate again prior to each brood (Berger and Maier 2001).
In Cyclopoida, the ♂ grasps either the fourth swimming leg pair or the abdomen of the ♀ with both geniculate A1. Then the ♂ pulls itself forward, until the genital somites of ♂ and ♀ face each other. Two spermatophores are attached close to the copulatory pore, a process that involves the setae of P2 or P3 in some species (Dussart and Defaye 2001). In Oithona davisae, the legs of the ♂ are not used for the transfer and the spermatophores are attached between the right and left genital apertures (Uchima and Murano 1988). The ♀♀ mate only once in their lifetime like, e.g., Cyclops vicinus Ulyanin, 1875 (Maier 1992).
The basic course of the copula phase in Harpacticoida was described by Lang (1948): having grasped the setae of caudal rami, the ♂ proceeds to their bases using its A1, turns underneath the ♀ and bends its abdomen dorsally, to face its ventral side to that of the ♀. The spermatophore is extruded by bending the male abdomen and attached to the genital field of the female genital double-somite. The behaviour of the animals of the four P. viguieri populations observed here corresponds with this description of the copula phase. The ♂♂ grasp with their A1 exclusively, without additionally employing the A2 or the mouth parts. The A2 of the ♂ lying laterally relative to the abdomen of the ♀ are not used to hold the ♀, as they move freely during the mating behaviour. This behaviour also corresponds with the description by Chappuis (1916) concerning the mating behaviour von P. viguieri.
Some Harpacticoida, such as e.g. Tisbe species also use the maxillules, maxillipeds and endopods of P1 for holding on tight to the ♀ (Dürbaum 1995). The ♂♂ of P. phyllura fix the ♀ with A1, A2, P3 and the caudal rami (Glatzel and Schminke 1996). Following the courtship phase mentioned above for some species, the spermatophore protrudes from the gonopore of the ♂ with its bottom first. The narrow neck of the spermatophore is attached to the genital field of the ♀ with a glue-like substance. This adhesive material originates from the gonopore of the ♂ immediately after extrusion of the spermatophore and flows across the narrow neck of the spermatophore onto the genital field of the ♀. In some copulae of the four P. viguieri populations, this process was observed in detail in a frame-by-frame video analysis. In all animals observed, the ♂ attaches the spermatophore without using its legs. In the copulae of P. viguieri observed by Chappuis (1916), the animals were connected by a short thick string following the copula. This string observed by Chappuis may be the glue-like substance or part of it. Such a ‘string’ was not observed in this study. The ♂ observed by Chappuis may have pulled off part of the adhesive material while separating from the ♀, although the spermatophore was still attached to the ♀.
In Tisbe species, the endopodites of P3 and P4 are used to attach the spermatophore. In Paramphiascella fulvofasciata Rosenfield and Coull, 1974, the spermatophore is attached without using the legs (Dürbaum 1995).
In Harpacticoida, the ♀♀ usually mate only once in their lifetime (Boxshall 1990). This is also true for the ♀♀ of the four P. viguieri populations, as the ♀♀ can produce up to 147 broods (=294 offspring) after one copula and do not mate a second time as a rule. Of the nine ♀♀ in the four P. viguieri populations, which mated a second time, four had not yet produced offspring. The other five ♀♀ did produce offspring in the first few days following the copula, but then did not produce further eggs for several days. From that it can be concluded that the ♀♀ are fertilized only once in their lifetime. If the eggs are not fertilized after the first copula, however, the ♀♀ mate a second time in order to produce offspring.
The question of why single ♀♀ mated again while they were still producing offspring cannot be answered at present. The defensiveness frequently observed in ♀♀ that had already mated was too weak or not observed in them at all. The majority of ♀♀ observed repulsed any attempts of a second copula by moving strongly and thus preventing ♂♂ from clasping them and attaching a spermatophore (see Chappuis 1916: 533).
The fact that ♀♀ mated only once was also described for various taxa of Tigriopus (Burton 1985; Lazzaretto et al. 1994), for Tisbe sp. (Vilela 1969), for E. acutifrons (Haq 1972) and for Diarthrodes cystoecus Fahrenbach, 1954 (Hicks and Coull 1983). The ♀♀ are able (in these cases) to mate any time after their terminal moult. This was not observed in the ♀♀ of the four P. viguieri populations. In general, the ♂♂ do not attempt to mate with very young ♀♀ (adult for <1 day). Only 1 or 2 days afterwards, the eggs in the oviducts were ready for fertilization. In rare cases, ♀♀ produced mature eggs, although they had not mated. These unfertilized eggs did not develop further. Shortly before the nauplii hatched, fertilized eggs, in which embryogenesis had taken place, appeared brighter under the binocular, whereas unfertilized eggs looked diffuse and did not change in appearance or showed any development.
The ♀♀ of Scottolana canadensis (Willey, 1923) have also been reported to produce egg sacs, even if they had never established any contact with a ♂ during their adult stage. Similarly, nauplii do not hatch from these eggs (Lonsdale et al. 1988).
Spermatophore
Within the Harpacticoida the spermatophore of E. acutifrons (Haq 1972) and Tisbe sp. (Dürbaum 1995) is subdivided into a distal and a proximal part. In both species, the proximal part is extruded by the swollen distal part and is discharged through the fertilization tube of the spermatophore via the copulatory pore and duct into the seminal receptacle of the ♀. In the four representatives of the Phyllognathopus populations, the spermatophore also consists of two portions. In one case, the spermatophore was torn off the ♀ immediately after the copula, thus enabling us to observe its discharge. The content of the proximal part (spermatozoa) discharged via a fertilization tube and dispersed in the ambient water. Following the spermatozoa, another secretion extruded and accumulated as a bubble-like substance directly at the end of the tube. Swelling of the content in the distal part of the spermatophore extrudes the spermatozoa through the fertilization tube.
In Heterolaophonte minuta (Boeck, 1873), the spermatophore has a tripartite structure and contains three secretions. The inner secretion becomes firm in contact with marine water, thus forming a fertilization tube elongating the neck of the attached spematophore. The middle secretion swells to extrude the content of the spermatophore. The outer secretion contains the spermatozoa which form a border layer below the wall of the spermatophore. When the spermatophore extrudes from the gonopore of the ♂, a glue-like substance is added attaching the spermatophore to the genital field of the ♀ (Hosfeld 1994). The extruding glue substance was also observed in the ♂♂ of the four P. viguieri populations when attaching their spermatophores.
Postcopulatory mate guarding phase
According to Dürbaum (1995) the postcopulatory mate guarding phase is defined as the period when the ♂♂ accompany the ♀♀ between the copula and the complete discharge of the spermatophore into the seminal receptacle. For Tisbe bulbisetosa Volkmann-Rocco, 1972 (Vilela 1969) and E. acutifrons (Haq 1972), such a phase was already described without giving it a specific name. For Tisbe species, Dürbaum (1995) stated an average duration of 39–67 min (max. 1012) for the postcopulatory mate guarding phase, while the actual discharge of the spermatophore takes only up to 45 min. After the transfer of the spermatophore, the ♂♂ hold on to the terminal setae of caudal rami of the ♀ with their maxillules. A postcopulatory mate guarding phase has also been reported for P. phyllura, during which the ♂♂ cling to the ♀♀ again for 3 min following the copula phase, however, the time for discharge of the spermatophore was not observed (Glatzel and Schminke 1996). In E. acutifrons, the postcopulatory mate guarding phase can take between 30 min and 10 h (Haq 1972).
Among the Calanoida, a postcopula phase is reported in Centropages typicus, however, not in the sense of the definition given in this study. Blades (1977) only described the discharge of the spermatophore after the ♂ had released the ♀. The ♂ stayed with the ♀ for some minutes following the copula phase, however, the time of discharge of the spermatophore is not known. According to Dürbaum (1995) a postcopulatory mate guarding phase can be expected in those species, in which the ♂♂ grasp adult ♀♀ only. Males either grasp only adult ♀♀ (in that case, presumably postcopulatory mate guarding phase) or only juvenile ♀♀ (precopulatory mate guarding phase) (Boxshall 1990). Thus, one of the two strategies should occur in any case. Consequently, a postcopulatory mate guarding phase was to be expected in the four populations of P. viguieri, in which the ♂♂ always clasp adult ♀♀. This hypothesis, however, is inconsistent with our observation, because a postcopulatory mate guarding phase did not occur in two of the four populations investigated, although only adult ♀♀ were clasped. Another hypothesis proposed that the clasping of caudal setae is only found in species with postcopulatory mate guarding phase (Dürbaum 1995). Our results equally disagree with this opinion, as the ♀♀ were clasped at their inner terminal setae of the caudal ramus in the latter two populations without displaying any postcopulatory mate guarding. The ♂♂ of the four observed populations of P. viguieri show different mating behaviour after completion of the copula phase. The ♂♂ of the Jamaica and the Brazil populations loosen their grasp around the inner terminal setae of the caudal rami relatively soon and release the ♀, which effectively ends the copula phase. The ♂♂ of the Ruhr and the Maryland populations continue clasping the inner terminal setae of caudal rami following the attachment of the spermatophore and accompany the ♀ for an average of 7 min and 29 s and 1 min and 39 s respectively. These durations approximately correspond with the discharge of the spermatophore, which takes maximally 10 min in the Ruhr population and 3 min in the Maryland population. Therefore, a postcopulatory mate guarding phase occurs in both two populations (see Table 1).
In the Jamaica and the Brazil populations, contact after the copula was maintained for only 21 and 17 s respectively, while the discharge of the spermatophore took 35 min in the ♀♀ of the Jamaica population and 20 min in the ♀♀ of the Brazil population. According to its definition, there is no postcopulatory mate guarding phase in these two populations.
The postcopulatory mate guarding phase serves to secure paternity and is useful in case there is a risk of other ♂♂ tearing off the spermatophores already deposited or of ♀♀ being able to mate several times (Dürbaum 1995). A repeated copula is prevented by the ♀♀ themselves in the populations of P. viguieri. As the ♀♀ do not mate several times, it is consequently not necessary to secure paternity in this species, however, it is possible, that the spermatophore attached to the ♀ is torn off. Since P. viguieri occurs in small water puddles as found in the leaf axils of bromeliads and moss, or also in compost heaps, moist soils, pieces of tree trunks and leaf litter, the animals often move within a very thin film of water on a surface and not within the free water column. While the ♀ crawls on the soil, the spermatophore may be damaged or break off. During the postcopulatory mate guarding phase, the spermatophore is protected by the ♂. Moreover, the movements of the ♀♀ are still restricted in this phase so that they crawl about very little or not at all. An attached spermatophore being torn off by another ♂ was never observed.
In case of an excess of potential ♀♀, a postcopulatory mate guarding phase is not necessary, because the ♂♂ can mate with several ♀♀. If there are only a few ♀♀, the pressure of competition among ♂♂ increases so that this measure to secure paternity becomes advantageous, since other ♂♂ are prevented from approaching the ♀ (Dürbaum 1995). With a postcopulatory mate guarding phase, the ♂♂ can be sure that their spermatophores are effectively discharged into the seminal receptacle of the ♀. However, the question arises why the ♂♂ do not approach juvenile ♀♀ when adult ♀♀ are scarce. This question cannot be answered on the basis of our observations.
The four observed populations of P. viguieri differ with respect to the postcopulatory mate guarding phase. There are so many differences in the conditions for life and/or the gender ratio in nature that a strategy to secure paternity has been developed in two populations which seems to be unnecessary in the other two populations.
In the Jamaica and the Brazil populations, it is improbable that the spermatophore is torn off or even removed by another ♂, as the spermatophore is not protected by the ♂. In addition, the sex ratio is more balanced towards ♂♂. There are enough potentially receptive ♀♀ in the culture vessels to avoid significant competition among the ♂♂.
In the Ruhr and Maryland populations, there seems to be a higher risk for the spermatophore to be torn off. Furthermore, the number of ♀♀ in relation to the ♂♂ may be so small that the postcopulatory mate guarding phase is useful to secure paternity with regard to the pressure of competition among the ♂♂.
The postcopulatory mate guarding phase is also disadvantageous to the ♂♂, because they cannot feed or search for other ♀♀ during that phase, and there is an increased predator risk. In Cyclops vicinus, couples in mating and egg-bearing females are the preferred prey item of fish as they are bigger than single animals. Their larger size makes them more conspicuous to visual predators and potentially slows down their predator escape response (Maier et al. 2000).
The different duration of the postcopulatory mate guarding phase in the Ruhr and the Maryland populations is connected with the different timing at which spermatophores discharge. Although the spermatophores of the ♂♂ in the Maryland population require considerably less time (2–3 min) to be discharged, it should be noted that they are much smaller (in relation to the body size of the ♀♀) than those of the ♂♂ in the Ruhr population.
Males among themselves
Males attaching spermatophores to other ♂♂ have also been reported for E. acutifrons (Haq 1972), Eurytemora affinis (Poppe, 1880) (Katona 1975) and Oithona davisae (Uchima and Murano 1988), however, none of the authors offered an explanation for this behaviour. Assuming that only the ♀♀ and not the ♂♂ produce sexual pheromones, the ♂♂ seem to be able to mate without this stimulus. All observed ♂♂ of the four populations of P. viguieri had been kept isolated prior to mating. Not being able to attach the mature spermatophore to a receptive ♀ for some time, the ♂♂ mate with other ♂♂ to dispose of the spermatophore. This observation is probably due to artificial conditions, since the ♂♂ are not isolated from the ♀♀ in nature. Hence, these results cannot be applied to a complex natural situation.
Systematical significance
If a reunion of the spatially isolated populations was assumed to be a certainty, the populations could be considered members of one biological species. However, if the populations diverge finally, they would be classified with different species. Cross-breeding experiments with organisms of recent populations enable us to experimentally examine, whether or not the division of species is already irreversible (Wägele 2005). According to Wägele, laboratory studies are admissible to prove the species membership.
Lang (1948) considered P. viguieri a cosmopolitan species. He classified all species described at that time as geographic variations of P. viguieri. According to Bruno and Cottarelli (1999) it is more likely that they represent a group of morphologically similar species. Their redescription of P. bassoti Rouch, 1972 from the Philippines is based on morphological features. Karanovic and Reddy (2004) redescribed P. bassoti from India once again, but did not recognize the populations from Papua New Guinea, the Philippines and India as different species. The authors synonymized P. insularis Chappuis, 1940 and P. camptoides, Božić 1965 with P. chappuisi, Delachaux, 1924 and P. volcanicus Barclay, 1969 with P. viguieri and concider four valid species (P. paracamptoides Božić, 1968; P. bassoti; P. viguieri and P. chappuisi) in the genus Phyllognathopus. Karanovic and Reddy accept only species being valid within the family of Phyllognathopodidae, which are distinguished by significant morphological differences.
The morphological differences within the four populations of P. viguieri were not significant to delimit species (Glatzel and Königshoff 2005).
While observing the mating behaviour of the four populations of P. viguieri investigated in this study, we noticed the differently shaped posterolateral and inner terminal setae of caudal rami of the ♀♀ (swollen or not modified). They have been described as being variable in P. viguieri (Karanovic and Reddy 2004). This variable dimorphism among the females of the four populations is not confirmed by this study nor observed in other cultures that have been kept in the laboratory for several years. This different shape has no influence on the clasping of the ♀♀ by the ♂♂. In addition, these characters are not appropriate to describe species, because the ♀♀ of both the Maryland and Ruhr populations have swollen setae of caudal rami, however, specimens cannot be cross-bred (Glatzel and Königshoff 2005) and show different mating behaviour.
Based on the observations of the different mating behaviour and the cross-breeding experiments (Glatzel and Königshoff 2005) the four investigated populations are classified among three different species (for other taxa see: Ritchie and Gleason 1995; Töpfer-Hofmann et al. 2000; Savitsky 2007).
Phyllognathopus viguieri is regarded as the sole species of the genus Phyllognathopus occurring in Europe (Kiefer 1960; Janetzky et al. 1996). If the Ruhr population from Germany as the only European population investigated in this study is considered to be P. viguieri on the basis of morphological characters, then, contrary to our initial assumption, the populations from Brazil and Maryland are not P. viguieri but independent species. On the basis of their similar mating behaviour, the individuals of the Jamaica and Brazil populations can be classified as one independent species.
We are convinced that genetic experiments on the species complex will support our results, improve our knowledge of the biological species concept and finally will require a revision of the genus Phyllognathopus.
Acknowledgements
We are very grateful to Wolfgang Janetzky and Peter Rumm, Germany, Paul Lehman and Janet W. Reid, USA, and Rodrigo Johnsson Tavares da Silva, Brazil, for providing us with living material of Phyllognathopus viguieri; Janet Reid’s field collections in Rio de Janeiro, Brazil, were supported by a grant from CAPES to the Museu Nacional, Universidade Federal do Rio de Janeiro. Sincere thanks are also due to Angelika Sievers for helping with the English text.




