The larval head morphology of Xyela sp. (Xyelidae, Hymenoptera) and its phylogenetic implications
Abstract
Larval head structures of Xyela sp. are described in detail. The characters are compared to conditions found in larvae of other groups of Hymenoptera and Endopterygota. Like other symphytan larvae the immature stages of Xyelidae are mainly characterized by presumably plesiomorphic features of the head. The head sutures are well developed and all parts of the tentorium are present. The labrum is free and a complete set of labral muscles is present. The maxillae are in a retracted position. In contrast to other hymenopteran larvae Xyela possesses a clypeofrontal suture, a comparatively long antenna and three well-developed antennal muscles. Apomorphic features of Xyela are the absence of muscles associated with the salivarium and the complete absence of Musculus craniocardinalis. A clade comprising Orussidae and Apocrita is supported by the unsegmented maxillary and labial palps and the absence of the lacinia. Six potential autapomorphies for the Hymenoptera were revealed: (1) the caudal tentorial apodeme, (2) the bifurcated tendon of Musculus craniomandibularus internus, (3) the lateral lobe of the cardo, (4) the origin of M. tentoriohypopharyngalis from the posterior head capsule, (5) the exceptionally strong prepharyngo-pharyngeal longitudinal muscle and (6) the longitudinal muscle of the silk press. The maxillolabial complex, the vestigial M. craniocardinalis and a distinctly developed labio-hypopharyngeal lobe bearing the opening of the salivary duct are potential synapomorphies of Hymenoptera and Mecopterida. The globular, orthognathous head capsule, the modified compound eyes, the occipital furrow and the X-shaped tentorium are features with unclear polarity shared by Hymenoptera and Mecoptera.
Introduction
Xyelidae is very likely the sistergroup of all other hymenopteran taxa (Vilhelmsen 2001; Schulmeister 2003), and therefore crucial for the reconstruction of the groundplan of the order. A number of detailed studies dealing with the anatomy of different regions of the body of adult basal Hymenoptera, including Xyelidae, have recently been conducted (see Vilhelmsen 2001; Schulmeister 2003 and references therein). In contrast, studies dealing with larval anatomy of basal Hymenoptera are scarce and mostly not done in a phylogenetic context. Yuasa (1922) provided an overview of the external anatomy of basal Hymenoptera. Maxwell (1955) studied selected aspects of internal structures in some detail for a substantial number of taxa. The only study to deal with the skeleto-musculature of the larval head so far is Parker (1935), who only included very few taxa, and no Xyelidae. The present study is thus the first to deal with the anatomy of the larval head of Xyelidae, and the most detailed study of this subject for any hymenopteran. It has primarily been undertaken to elucidate the larval groundplan of Hymenoptera with the aim to provide phylogenetically relevant information at the interordinal level.
Characters with potential phylogenetic relevance are listed and presented in a data matrix. A parsimony analysis was not carried out at this stage. The characters will be combined with an extensive data set comprising features of different stages and body parts and analysed cladistically in a future study.
Materials and Methods
List of taxa examined
Hymenoptera, Xyelidae: Xyela sp. (presumably ultimate and penultimate instars; fixed in 70% ethanol; collected in Austria by Dr. E. Altenhofer)
Tenthredinidae: Dolerus sp. (fixed in FAE = formaldehyde–ethanol–acetic acid; collected in Austria by Dr. E. Altenhofer)
Diprionidae: Neodiprion sertifer (Geoffroy, 1785) (fixed in FAE; collected in Austria by Dr. E. Altenhofer)
Cephidae: Janus compressus (Fabricius, 1793) (fixed in FAE; collected in Austria by Dr. E. Altenhofer)
Xiphydriidae: Xiphydria camelus (Linnaeus, 1758) (fixed in FAE; collected in Austria by Dr. E. Altenhofer)
Mecoptera, Nannochoristidae: Nannochorista philpotti (Tillyard, 1917) (Pampel's fluid, Bouin, 70% ethanol)
Boreidae: Boreus westwoodi Hagen, 1866 (fixed in FAE)
Panorpidae: Panorpa sp.
Zoroaptera: Zorotypus hubbardi Caudell, 1918 (late nymphal stage; fixed in ethanol)
Methods
Specimens were embedded in Araldit® (Plano GmbH, Wetzlar, Germany), cut at 1 μm with a Microm microtome (HM 360; Microm, Walldorf, Germany) and stained with Azan. Drawings were made using an ocular grid or a camera lucida (cross-sections). For SEM micrographs (JEOL JSM-6335F; JEOl GmbH, Eching, Germany) specimens were cleaned in 0.1% Triton, dehydrated in an ascending series of ethanol, fixed in iso-amylacetate, dried (critical point) and sputter-coated with gold-platinum.
Results
External features of the head capsule (1)
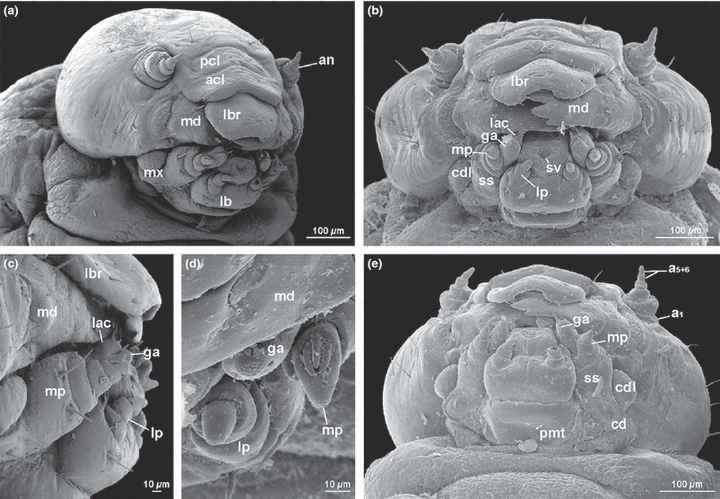
Xyela sp., larval head. (a) frontolateral view; (b) ventral view; (c) mouthparts, lateral view; (d) mouthparts, frontal view; (e) posterior view. acl, anteclypeus; an, antenna; cd, cardo; cdl, lateral lobe of cardo; ga, galea; lac, lacinia; lbr, labrum; lb, labium; lp, labial palp; md, mandible; mp, maxillary palp; mx, maxilla; pcl, postclypeus; ss, stipes; sv, opening of salivary duct
The head is globose and orthognathous and only very slightly retracted into the prothorax. The head is nearly round in frontal view. The foramen occipitale is very wide and approximately trapezoid, with a rounded upper margin. It is divided by the well-developed posterior tentorial arms and the very strongly developed tentorial bridge. It is enclosed by a moderately broad postoccipital ridge dorsally and laterally.
The lateral part of the postoccipital ridge is continuous with a distinct paired ridge, which is visible externally as a longitudinal furrow (occipital ridge). The cuticle of the head capsule is largely smooth, slightly wrinkled in some areas and very lightly pigmented. The setation is sparse. The distribution of setae is shown in Fig. 1a,b,e. The single lateral eye (ocularium) is vestigial; it is visible as a subcuticular pigment spot below the articulating area of the antenna (antacorium); ommatidia are not developed and a convex cuticular lense is also absent. The U-shaped frons is enclosed by distinct frontal sutures. The coronal suture (=epicranial stem) is moderately long. The clypeus is transverse and separated from the frons by an evenly curved, frontoclypeal (epistomal) suture, which is distinct medially but obliterated towards the lateral clypeal base. The clypeus is subdivided into a dorsal postclypeus and a ventral anteclypeus by a transverse suture. The postclypeus is semicircular and bears a row of four setae along its ventral margin. The anteclypeus is distinctly concave at its anterior margin and adjacent to the free labrum. A median endocarina, hypostomal rods or ventral epicranial ridges are absent. Anterior or posterior tentorial pits are not recognizable externally. The pleurostoma is covered with wart-like structures. A gula or a hypostomal bridge is absent. The dorsal margin of the postmentum is adjacent to the ventral cervical membrane.
Internal skeletal structures (2-5)
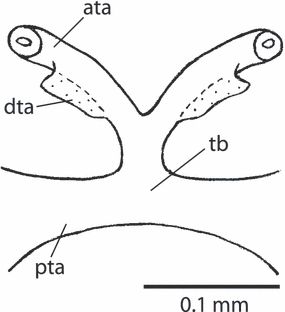
Xyela sp., tentorium, anterior (dorsal) view. ata, anterior tentorial arm; dta, base of dorsal tentorial arm; pta, posterior tentorial arm; tb, tentorial bridge
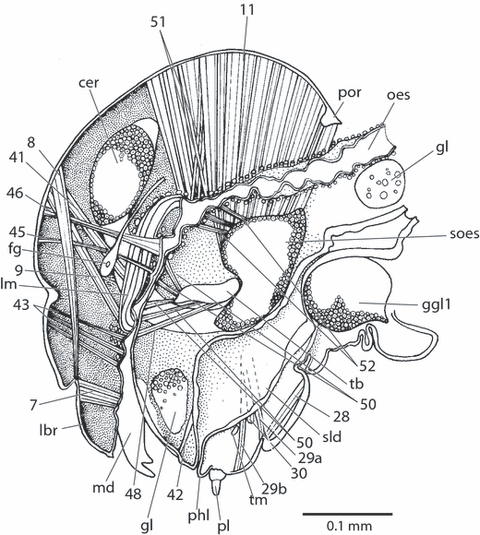
Xyela sp., larval head, sagittal section. cer, brain; fg, ganglion frontale; ggl1, prothoracic ganglion; gl, gland; lbr, labrum; lm, longitudinal muscle; md, mandible; oes, oesophagus; phl, prelabiohypopharyngeal lobe; pl, labial palp; por, postoccipital ridge; sld, salivary duct; soes, suboesophageal ganglion; tb, tentorial bridge; tm, transverse muscle; 7, M. labroepipharyngalis; 8, M. frontolabralis; 9, M. frontoepipharyngalis; 11, M. craniomandibularis internus; 28, M. submentopraementalis; 29a, b, subcomponents of M. tentoriopraementalis inf.; 30, M. tentoriopraementalis sup.; 41, M. frontohypopharyngalis; 42, M. tentoriohypopharyngalis; 43, M. clypeopalatalis; 45, M. frontobuccalis ant.; 46, M. frontobuccalis post.; 48, M. tentoriobuccalis ant.; 50, M. tentoriobuccalis post.; 51, M. verticopharyngalis; 52, M. tentoriopharyngalis
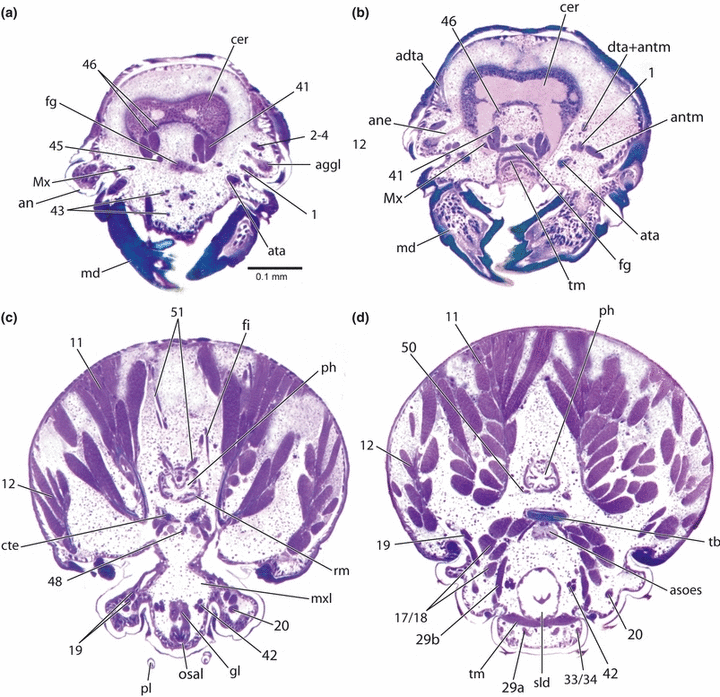
Xyela sp., larval head, cross-sections. (a) clypeal region; (b) anatomical mouth region; (c) anterior pharyngeal region; (d) posterior pharyngeal region. adta, attachment of dorsal tentorial arms; aggl, antennal ganglion; an, antenna; ane, antennal nerve; antm, antennal muscle; asoes, anterior part of suboesophageal ganglion; ata, anterior tentorial arm; cer, brain; cte, corpora tentorii; dta + antm, dorsal tentorial arm and antennal muscle; fg, frontal ganglion; md, mandible; Mx, lateral component of M. frontohypopharyngalis; mxl, maxillolabial complex; osal, opening of salivary duct; ph, pharynx; pl, palpus labialis; rm, ring muscle; sld, salivary duct; tb, tentorial bridge; tm, transverse muscle; 1, M. tentorioscapalis anterior; 2–4, extrinsic antennal muscles; 11, M. craniomandibularis int.; 12, M. craniomandibularis ext.; 17/18, Mm. tentoriocardinalis/stipitalis; 19, M. craniolacinialis; 20, M. stipitolacinialis; 29a,b, M. tentoriopraementalis inferior; 33/34, Mm. praementopalpales ext. /int.; 41, M. frontohypopharyngalis; 42, M. tentoriohypopharyngalis; 43, M. clypeopalatalis; 45, M. frontobuccalis ant.; 46, M. frontobuccalis post.; 50, M. tentoriobuccalis post.; 51, M. verticopharyngalis
The tentorium is well developed and the main parts form an X-shaped structure. The short and strong posterior arms are connected by a very massive tentorial bridge. The anterior margin of each posterior arm is inflected, thus forming a posteriorly directed apodeme-like structure (caudal tentorial apodeme). The corpora tentorii (posterior common stems of anterior and dorsal arms) are contiguous at their origin from the tentorial bridge. The dorsal arms are connected with the corpora tentorii by their broad and ligamentous basal part. Their major part is thin and the apices are attached to the anterolateral head capsule by numerous fibrillae. The broad and distinctly sclerotized anterior arms diverge towards the anterior tentorial pits.
A very thin fibre connecting the lateral edges of the tentorial bridge with the dorsal wall of the head capsule was observed in the largest larva sectioned (frontal section; Fig. 5c: fi), but could not be identified in the other specimens (cross sections and longitudinal sections).
Labrum (1, 4)
The labrum is separated from the anterior clypeal margin by a deep fold and is interiorly connected with it by a membrane. It is well developed, rounded laterally and slightly concave anteromedially. It covers the distal parts of the mandibles in their resting position. A transverse row of four setae is present in the middle region of the labrum and one seta close to the ventrolateral edge. Short setae (partly reduced to warts) are inserted along the ventral margin.
Musculature (Fig. 4): M. 7: M. labroepipharyngalis, present, two well-developed bundles, O (=origin): posterior part of the anterior labral wall, close to the median line, I (=insertion): ventral part of epipharynx, close to median line; M. 8: M. frontolabralis, long and strongly developed, O: central area of the frons, above the brain, I: medially on the dorsal margin of the anterior labral wall; M. 9: M. frontoepipharyngalis, strongly developed, O: laterally on the frons, I: strongly developed lateral apodemes (=tormae) arising from the ventral section of the epipharynx, close to the secondary mandibular joint.
Antenna (1, 5)
The antenna is short (<25% of the maximum width of the head capsule), tapering apically and consists of five distinctly recognizable segments that are situated concentrically. The apical segment represents the largely fused antennomeres 5 and 6. In contrast to the other antennomeres, it is equipped with two instead of one row of reduced sensilla. The insertion point of the antenna is abutting the frontal suture. The basal antennomere is short, anelliform and dorsally covered by a globose cuticular bulge. Antennomere 2 is globular, about half as long as wide and apically bearing a row of reduced (wart-like) setae. Antennomeres 3 and 4 are distinctly smaller than 2, cylindrical, short and characterized by an apical row of short setae. The apical segment (antennomeres 5 + 6) is slender, slightly narrowing distally and apically rounded. The apex is devoid of any sensilla.
Musculature (Fig. 5a): well developed, M. tentorioscapalis anterior (M. 1), two parallel bundles, O: anterior tentorial arm and basal part of dorsal arm; I: ventrally on the base of the scapus; M. tentorioscapalis posterior/lateralis/medialis (Mm. 2–4), two well-developed bundles, the homologization with v. Kéler's (1963) designations is problematic, O: anterior arm and base of dorsal arm, and upper part of dorsal arm respectively, I: dorsally and posteriorly on the base of the scapus, respectively. Mm. scapopedicellaris lateralis/medialis (Mm. 5/6), absent.
A slender muscle, which originates immediately close to or at the anterior margin of the antennal base (Fig. 5a,b: Mx) and merges with the two large bundles of M. 41 is tentatively interpreted as an aberrant subcomponent of M. frontohypopharyngalis here. It cannot be fully excluded that this muscle belongs to the antenna.
Mandible (1, 3, 4, 5)
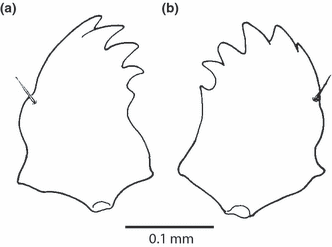
Xyela sp., mandibles, anterior (dorsal) view: (a) left mandible; (b) right mandible
The slightly asymmetric mandibles articulate with the head capsule through an anterior socket (3, 5) and a posterior condyle. They are stout, moderately broad at their base and rounded laterally. A strong seta is inserted on the dorsolateral edge. Six roughly triangular teeth are present on the right mandible and five on the left mandible. The proximal tooth is distinctly smaller than the others. Mola and prostheca are absent. The adductor tendon is bifurcated from its base (Fig. 5b,c).
Musculature (4, 5): M. 11: M. craniomandibularis internus, large muscle, divided into two subcomponents, O: dorsally and posterodorsally from the head capsule, I: both branches of the adductor tendon; M. 12: M. craniomandibularis externus, O: laterally from the head capsule, I: abductor tendon; M. 13: M. tentoriomandibularis, thin but distinctly developed, O: anterior tentorial arm, I: dorsomesally on the mandibular base.
Maxilla (1, 5)
The maxilla is closely connected with the labium and hypopharynx. The entire maxillolabial complex closes the head capsule ventrally. The cardo is represented by a narrow part proximad of the stipes and a large, semicircular lateral lobe (Fig. 1e: cd, cdl). The stipes is composed of a transverse basal part and a more elongate distal part. The subunits are separated by an indistinct transverse line. One long seta is inserted on the basal part and two setae on the distal portion, close to its anterolateral margin. The maxillary palp is three-segmented. The basal segment is very broad and short. The second palpomere is about as long as broad. Palpomere 3 is comparatively long and slender; three large elongate sensilla are inserted on its dorsal side, close to the base; few very small sensilla are present on the apex. The galea is a fairly large, mesally directed lobe with a rounded apical margin, which bears a group of small sensilla. The lacinia is flat and largely covered by the galea. Three strong setae are inserted on its apex.
Musculature (Fig. 5b–d): M. 15: M. craniocardinalis, absent; M. 17: M. tentoriocardinalis, two strongly developed parallel bundles, O: medially fused posterior part of the corpora tentorii, I: ventral surface of the cardo; M. 18: M. tentoriostipitalis, composed of two intercrossing subcomponents, M. 18a, O, anterior part of the corpora tentorii, I: mesal surface of the posterior stipes; M. 18b, O: tentorial bridge, I: mesal edge of the anterior stipes; M. 19: M. craniolacinialis, two well-developed parallel bundles, O: posterior head capsule, between the attachment areas of M. 11 and M. 12, I: dorsomesally on the base of the lacinia; M. craniodististipitalis, absent; M. stipitolacinialis, O: ventrally on the basal part of the stipes, I: together with M. 19; M. stipitogalealis, absent; Mm. 22/23: M. stipitopalpalis externus, absent; Mm. 24–27: Mm. palpopalpalis primus – tertius, absent.
Labium and hypopharynx (1, 4, 5)
The moderately sized undivided postmentum is inserted between the maxillae. It is distinctly broader than long and the lateral margin is rounded. Its upper margin is the ventral border of the foramen occipitale. The well-developed prementum is of similar shape and size. Glossae and paraglossae are absent and a ligula is also lacking. The labial palp is three-segmented. Palpomeres 1 and 2 are short and fairly broad. The distal palpomere is more slender, slightly longer and cone-shaped. The anterior part of the labium forms a functional unit with the anterior hypopharynx. The large ovoid ligular sclerome above the palp bears the Y-shaped opening (meatus) of the salivary duct (Fig. 1b). The dorsal surface of the hypopharynx forms a ramp leading towards the anatomical mouth. The posteriormost part is laterally fused with the posterior epipharyx, thus forming a short prepharyngeal tube. The sclerotized lateral edge represents the hypopharyngeal suspensorium (suspensorial apodeme). However, it is not distinctly separated from the floor of the prepharyngeal tube.
Labial musculature (4, 5): M. 28: M. postmentopraementalis, O: medially from the posterior margin of the postmentum, I: ventrolaterally on the premental base; M. 29: M. tentoriopraementalis inferior, composed of two subcomponents, M. 29a, O: caudal tentorial apodeme, I: ventral surface of prementum between palps; M. 29b: well developed almost vertical bundle, O: base of caudal tentorial apodeme, I: ventrolaterally on premental base, together with M. 28; M. 30, M. tentoriopraementalis superior, well developed, O: caudal tentorial apodeme, posterad of M. 29a, I: membranous fold connecting prementum and mesal stipital margin; M. 31: M. praementoparaglossalis, absent; M. 32: M. praementoglossalis, absent; M. 33/34: M. praementopalpalis internus/externus, very small, O: lateral wall of prementum, I: laterally on the base of the palp; M. 35/36: M. palpopalpalis labii primus/secundus, absent.
Hypopharyngeal musculature (4, 5): M. 41: M. frontohypopharyngalis, well developed, O: central area of frons, laterad of M. 8, I: posterolateral edge of the prepharyngeal tube at apex of suspensorial apodeme, laterad of anatomical mouth; M. 42: M. tentoriohypopharyngalis, a well developed, long muscle; O: laterally on the postoccipital ridge, I: laterally on the base of the prelabiohypopharyngeal lobe.
The muscle referred to as M. tentoriohyopharyngalis (M. 42) in studies on the head morphology of beetles and other insects (e.g. Beutel 1986; Beutel and Weide 2005; Beutel and Vilhelmsen in press) functions as a tentorial retractor of the hypopharnyx, but is homologous to v. Kéler's M. tentoriobuccalis anterior (M. 48). M. tentoriobuccalis anterior lies within the circumoesophageal connectives, M. tentoriohyopharyngalis laterad of them.
A well-developed transverse muscle (M. transversalis labialis) connects the lateral walls of the labiohypopharyngeal unit.
Epipharynx (4)
The ventral part of the epipharynx is symmetrical, medially concave and devoid of trichia. A thin outer layer of the cuticle is sclerotized. The dorsal epipharynx is separated from the anterior part by the origin of strong lateral apodemes (=tormae) of M. frontoepipharyngalis. It is laterally connected with the mandibular base, asymmetrical, with a paramedian concavity and a slightly irregular surface structure. The dorsalmost part is laterally fused with the posterior hypopharynx (see above). A thin longitudinal sclerotized bar connects the apodeme of M. frontopepipharyngalis with the hypopharyngeal suspensorium.
Musculature (4, 5): M. 43: M. clypeopalatalis, cibarial dilator composed of six thin bundles, O: successively on the postclypeus, I: successively on the epipharynx, close to the median line.
Well-developed transverse muscles separate the bundles of M. 43. They are attached to the wall of the cibarium mesad of the longitudinal sclerotized bar of the epipharynx. An extremely strong and compact mass of longitudinal muscles reach from the upper surface of the posterior epipharynx to the upper surface of the anterior postcerebral pharynx.
Pharynx (4, 5)
The pharynx is narrower than the salivary duct (4, 5). Dorsolateral and ventrolateral longitudinal folds serve as attachment areas for the dilator muscles.
Musculature (4, 5): M. 45: M. frontobuccalis anterior, a well-developed bundle, O: central region of frons; I: dorsally of the anatomical mouth, adjacent to the insertion of M. 41; M. 46: M. frontobuccalis posterior, three thin transversely arranged parallel bundles, O: frons, between the origins of M. 8 and M. 45, I: dorsally on the precerebral pharynx, close to the anatomical mouth; M. 48: M. tentoriobuccalis anterior, a well developed muscle; O: ventromedially on the tentorial bridge, I: posteromedially on the hypopharynx; M. 50: M. tentoriobuccalis posterior, several bundles, O: anteriorly on the tentorial bridge, adjacent to the origin of M. 48, I: ventrally on the precerebral pharynx, posterad of the insertion of M. 48 and opposite the attachment of M. 45 and M. 46; M. 51: M. verticopharyngalis, composed of several very thin bundles, O: dorsally on the posterior vertex, I: dorsal folds of the postcerebral pharynx, M. 52: M. tentoriopharyngalis, composed of two subcomponents, M. 52a, several very thin bundles, O: ventrolaterally on the postoccipital ridge, the posterior tentorial arms and the tentorial bridge; I: ventrolateral and lateral folds of the postcerebral pharynx; M. 52b, very thin, O: ventrolaterally on the cervical membrane; I: ventral and ventrolateral folds of the postcerebral pharynx.
A well-developed ring muscle layer is present over the entire length of the pharynx (Fig. 4). The dorsal longitudinal muscles of the pharynx (and posterior epipharynx, see above) are very strongly developed.
Salivarium (4, 5)
The salivarium is represented by the anterior salivary duct, which is V-shaped in cross section and moderately narrow close to its Y-shaped opening (meatus) on the prelabio-hypopharyngeal lobe (Fig. 1a,b), but widens strongly after a short distance (4, 5). The wall of this part is unusually thick in one of the specimens examined (Fig. 4). Posteriorly the salivary duct is connected to extremely long gland reservoirs, which extend to the posterior abdominal region.
Musculature (4, 5): M. 37: M. hypopharyngosalivarialis, absent; M. 38: M. praementosalivaris anterior, absent; M. 39: M. prementosalivaris posterior, absent; M. 40: M. annularis salivarii, absent.
Cerebrum suboesophageal complex (4, 5)
The dumbbell-shaped brain is moderately sized relative to the head capsule. It is located in the central part of the head. Optic neuropils are not developed. The optic nerve connecting the protocerebrum with the vestigial lateral eye is extremely thin. The tritocerebral commissure is thin. It separates M. tentoriohypopharyngalis (M. 42) and M. tentoriobuccalis posterior (M. 50). The circumoesophageal connectives are elongate. The anterior end of the suboesophageal compound ganglion lies below the tentorial bridge. The larger part is located in the cervical region. Small ganglia are present at the base of the labrum, the antennal base and the base of the mandibles. They are connected to the frontal connective, the deutocerebrum and the suboesophageal complex, respectively.
Glands
Fairly large more or less globular glands are present in the lateral clypeal region, above the anterior mandibular articulation and in the anterior hypopharyngeal region. The latter gland lies immediately above the anterior part of the salivary duct and is connected to it (4, 5).
Fat body
Fat body tissue is present in the neck region between the pharynx and the gland reservoir but absent in other parts of the head.
Phylogenetically relevant characters
The characters are listed in a morphology-based sequence (see results). The coding as (0) does not necessarily imply that this is the plesiomorphic state.
1. (omp) Orientation of mouthparts: (0) orthognathous; (1) prognathous or slightly inclined. The head is distinctly orthognathous in larvae of Xyela (1, 4) and other genera of Xyelidae (Smith and Middlekauff 1987). This is also the case in other symphytan lineages (Yuasa 1922; Parker 1935; Smith and Middlekauff 1987), with very few exceptions (Heterarthrinae [Tenthredinidae] partim; Smith and Middlekauff 1987). An orthognathous condition is also found in Mecoptera excluding Nannochorista (Pilgrim 1972; Byers 1987), in Trichoptera (partim; Wiggins 1987), in some groups of Coleoptera (e.g. Chrysomelidae, Curculionidae partim; Anderson 1991; Lawson 1991) and in many groups of Lepidoptera-Ditrysia (Hasenfuss and Kristensen 2003). The head is slightly inclined in Sialidae (Röber 1942) and in many groups of beetles (e.g. Myxophaga, Hydraenidae, Leiodidae; Beutel and Molenda 1997; Beutel et al. 1998) (coded as 1). It is distinctly prognathous in Corydalidae, Neuroptera, Raphidioptera (e.g. Crampton 1921; Tauber 1991), Coleoptera (partim, e.g. Archostemata, Adephaga; Beutel 1993; Beutel and Hörnschemeyer 2002a,b), Strepsiptera (first instar, maxillae and labium posteriorly directed; Pohl 2000), Trichoptera (partim, ‘spicipalpian grade’; Wiggins 1987), Siphonaptera (Sharif 1937), basal lineages of Diptera (e.g. Bibionidae; Cook 1944a,b, 1949; Hennig 1973; Oosterbrook and Theowald 1991) and several groups of Lepidoptera (e.g. Micropterigidae, Eriocraniidae; Stehr 1987; Hasenfuss and Kristensen 2003). A prognathous head is also characteristic for the first instar larvae of some apocritan Hymenoptera with a derived cleptoparasitic (e.g. Antophoridae partim; Rozen 1991) or parasitoid lifestyle (e.g. Chalcidoidea partim; Parker 1924).
2. (hsh) Head shape: (0) globular; (1) flattened dorsoventrally. The head capsule is globular in larvae of Xyela (Fig. 1a,b) and in larvae of other symphytan groups, with the exception of Heterarthrinae (partim; Smith and Middlekauff 1987; see above). A similar condition is found in Mecoptera excluding Nannochorista (Pilgrim 1972; Byers 1987), in some groups of Coleoptera (e.g. Chrysomelidae, Curculionidae; Anderson 1991; Lawson 1991) and in many groups of Lepidoptera (Stehr 1987; Hasenfuss and Kristensen 2003). The head is more or less distinctly flattened in larvae of the other groups of Endopterygota (e.g. Crampton 1921; Sharif 1937; Röber 1942; Cook 1949; Hennig 1973; Widhalm-Finke 1974; Beutel 1993, 1995; Hasenfuss and Kristensen 2003). Prognathism and flattening of the head often coincide, but are not necessarily correlated (e.g. Agathiphagidae; Kristensen 1984).
3. (lso) Light sense organs: (0) multifaceted compound eyes; (1) unicorneal composed eyes; (2) several stemmata; (3) lateral eyes absent or vestigial. The lateral eyes are vestigial or absent in Xyela (Fig. 1a) and in larvae of several other groups with endophytic, woodboring or parasitic habits (e.g. Heterarthrinae, Xiphydriidae, Siricidae, Orussidae; Smith and Middlekauff 1987; Vilhelmsen 2003), and also in larvae of Apocrita (Evans et al. 1987). More or less well-developed eyes are present in free-living symphytan larvae (e.g. Macroxyela; Pamphilidae, Argidae, Cimbicidae, Pergidae, Diprionidae, Tenthredinidae [major part]; Smith and Middlekauff 1987). They comprise several rhabdoms (e.g. Neodiprion), but lack individual corneal lenses and crystalline cones (‘unicorneal composed eyes’; N.P. Kristensen, personal communication). The optic neuropils are well developed. Multifaceted compound eyes are present in Mecoptera (distinctly simplified in Nannochoristidae; Melzer et al. 1994). Several stemmata are found in most groups of endopterygote insects (e.g. Röber 1942; Wiggins 1987; Beutel 1993, 1999). Larvae of Siphonaptera and Cupedidae (Beutel and Hörnschemeyer 2002b) and larvae of many nematoceran families (e.g. Bibionidae, Tipulidae) are eyeless (e.g. Foote 1991). The compound eyes occurring in some Culicoidea are precociously developed adult compound eyes that remain almost unchanged during metamorphosis [N.P. Kristensen, personal communication; see also Paulus (1979, 1986, 2000)].
4. (fcl) Frontoclypeal suture: (0) distinct; (1) absent or only vaguely indicated. The frontoclypeal suture is present in Xyela (Fig. 1), but is apparently reduced in other symphytan larvae (e.g. Tenthredinidae; Parker 1935; Smith and Middlekauff 1987). It is also preserved in Mecoptera (Applegarth 1939; Bierbrodt 1942; Pilgrim 1972; Russell 1982), Sialidae (Röber 1942) and in different groups of Coleoptera (e.g. Cupedidae, Staphylinoidea partim; Beutel and Molenda 1997; Beutel and Hörnschemeyer 2002b). It is absent in Trichoptera (R. Beutel, personal observation; Winkler 1959), Lepidoptera (Hasenfuss and Kristensen 2003), Siphonaptera (Sharif 1937; Widhalm-Finke 1974), Diptera (Cook 1949; Hennig 1973), Raphidioptera (Beutel and Ge in press), Corydalidae and Neuroptera (Crampton 1921; Grebennikov 2004).
5. (acl) Division of clypeal area into anteclypeus and postclypeus: (0) absent; (1) present. The clypeal area is divided into an anterior anteclypeus without muscle attachment, and a posterior postclypeus in Xyela and in other symphytan larvae (e.g. Tenthredinidae; Parker 1935). The two areas are also separated by a suture in Apterobittacus (Mecoptera; Applegarth 1939: Fig. 53A), Lepidoptera (groundplan, e.g. Micropterix; Hasenfuss and Kristensen 2003), Trichoptera (Winkler 1959: Fig. 3; Rhyacophila; R. Beutel, personal observation) and also in Raphidia (Beutel and Ge in press).
6. (occ) Occipital furrow: (0) absent; (1) present. A longitudinal furrow on the posterior head capsule is present in Xyela and usually also in other symphytan larvae (e.g. Megaxyela, Pamphilidae, Pergidae, Argidae, Diprionidae, Tenthredinidae; Smith and Middlekauff 1987). The absence in Xiphydriidae is likely an autapomorphy of this family and the partial reduction a potential synapomorphy of the siricoid families (see Smith and Middlekauff 1987). The homology of this structure with a true occipital suture (e.g. Bierbrodt 1942) is uncertain. The furrows are absent in all other groups of Endopterygota (e.g. Crampton 1921; Sharif 1937; Cook 1949; Wiggins 1987; Foote 1991; Beutel 1993, 1999; Beutel and Hörnschemeyer, 2002a,b) with the exception of Mecoptera (major part; Bierbrodt 1942; Byers 1987).
7. (gul) Gula: (0) absent; (1) undivided sclerotized gula; (2) medially divided sclerotized gula-submentum. A gula or any other type of ventral closure of the head is absent in Xyela and in other symphytan larvae (Parker 1935; Smith and Middlekauff 1987). The posterior tentorial pits are adjacent to the foramen occipitale. This is likely a plesiomorphic condition. A sclerotized gula is present in Raphidioptera, Corydalidae (Crampton 1921), Coleoptera (partim; e.g. Adephaga, Hydrophiloidea; Beutel 1993, 1999), Trichoptera (partim; Malicky 1973: Figs 26 and 30) and Siphonaptera (Widhalm-Finke 1974; R. Beutel, personal observation). The triangular sclerite (=ventral apotome; Wiggins 1987) of larvae of some groups of Trichoptera (e.g. Limnephilidae, Rhyacophilidae; Crampton 1921: Figs 20 and 21; Winkler 1959: Fig. 4; Malicky 1973: Figs 29 and 30) is not a gula as it lies far anterad of the origin of the posterior tentorial arms (coded as 0). A medially divided gula-submentum is present in Nannochoristidae. The elongate, fissure-shaped posterior tentorial pits are in contact with the foramen occipitale.
8. (dta) Dorsal tentorial arm: (0) present; (1) absent. Dorsal tentorial arms are present in Xyela, Neodiprion (Diprionidae), Tenthredinidae (e.g. Dolerus; see also Parker 1935), Janus (Cephidae), Xiphydria (Xiphydriidae) and probably also in other symphytan lineages. This plesiomorphic condition is also found in Osmylidae (Wundt 1961), Agathiphaga (Lepidoptera; Kristensen 1984), Trichoptera (Fotius-Jaboulet 1961: Fig. 23) and in most groups of Coleoptera (e.g. Beutel 1993, 1999). They are absent in Mecoptera (Bierbrodt 1942; R. Beutel, personal observation), Sialidae (Röber 1942), Strepsiptera (Pohl 2000), Lepidoptera-Glossata (Hasenfuss and Kristensen 2003), Siphonaptera (Sharif 1937; Widhalm-Finke 1974) and usually also in Diptera (Cook 1949; Hennig 1973).
9. (ten) Shape of tentorium in cranial view: (0) H-shaped; (1) X-shaped, anterior arms contiguous posteriorly. The posterior and anterior tentorial arms and the tentorial bridge form an X-shaped structure in Xyela and in other symphytan larvae (e.g. Parker 1935), and a similar condition is found in Mecoptera excluding Nannochorista (Bierbrodt 1942; R. Beutel, personal observation). The tentorium is H-shaped in other groups of Endopterygota (e.g. Sharif 1937; Fotius-Jaboulet 1961: Figs 23 and 28; Wundt 1961; Kristensen 1984: Fig. 4; Beutel 1993; Hasenfuss and Kristensen 2003). The character is not applicable for Strepsiptera as the tentorium is largely reduced (Pohl 2000).
10. (wtb) Width of tentorial bridge: (0) not distinctly broadened; (1) approximately 4 times as wide as corpora tentorii. The tentorial bridge is very massive and broad in larvae of Xyela (2-5 ) and this is also the case in the other symphytan larvae examined (see also Parker 1935: Fig. 9). Yet, it is highly reduced in larvae of Orussus (‘short stubs’; Parker 1935: Fig. 53). A broad tentorial bridge is also present in Osmylidae (Wundt 1961) and Sialidae (Röber 1942; Wundt 1961), and in Mecoptera (Bierbrodt 1942; R. Beutel, personal observation) and Micropterigidae. It is narrow or reduced in the other mecopterid lineages (Hasenfuss and Kristensen 2003) and also in many groups of Coleoptera (Beutel 1993, 1999). It is completely absent in Archostemata (Beutel and Hörnschemeyer 2002a,b) and in Strepsiptera (Pohl 2000).
11. (cta) Process formed by inflection of anterior margin of posterior tentorial arm (caudal tentorial apodeme): (0) absent; (1) present. The process is present in Xyela, Neodiprion and Janus (not identified in Dolerus, but see Parker 1935: Fig. 9: hstap). It serves as attachment area of thoracic muscles and as area of origin of extrinsic labial muscles. A somewhat similar structure occurs in Sialis and Osmylus (Röber 1942; Wundt 1961) and in some groups of Adephaga (Beutel 1993). However, in these taxa the process arises from the hind margin of the posterior arms (Röber 1942: Fig. 5; Beutel 1993: Fig. 13) (coded as 0). It mainly serves as attachment area of ventral postpharyngeal dilators in Sialis and Adephaga. The process is absent in other groups of Endopterygota.
12. (lbr) Articulation of labrum: (0) labrum free; (1) labrum fused with clypeus. The labrum is articulated with the clypeus in Xyela (1, 4) and in other symphytan larvae (Smith and Middlekauff 1987). This is presumably plesiomorphic condition is also found in Mecoptera (Pilgrim 1972; Russell 1982; Byers 1987), Raphidioptera and Megaloptera (Crampton 1921), in larvae of many groups of Coleoptera (e.g. Archostemata, Staphyliniformia partim; Beutel and Molenda 1997; Beutel and Hörnschemeyer 2002a,b), and in larvae of Trichoptera (Wiggins 1987), Lepidoptera (Hasenfuss and Kristensen 2003), Diptera (groundplan; e.g. Cook 1944a,b, 1949; Kramer 1954) and Siphonaptera (Sharif 1937; Widhalm-Finke 1974). The labrum is fused with the head capsule in larvae of several groups of Coleoptera (e.g. Adephaga, Hydrophiloidea; Beutel 1993, 1999), in Neuroptera (Wundt 1961; New 1991) and in first instar larvae of Strepsiptera (Pohl 2000).
13. (8) M. frontolabralis (M. 8): (0) present; (1) absent (Table 1). M. frontolabralis is present in Xyela (Fig. 4) and in other symphytan larvae (Das 1937; Parker 1935). It does also occur in Mecoptera (Bierbrodt 1942), Raphidioptera (Das 1937; Beutel and Ge in press), Megaloptera (Röber 1942), Trichoptera (Das 1937; Winkler 1959), Micropterigidae (Hasenfuss and Kristensen 2003), Siphonaptera (Sharif 1937; Widhalm-Finke 1973) and in nematoceran larvae (Cook 1944a,b, 1949; Kramer 1954, Hennig 1973). It is absent in Lepidoptera (excluding Micropterigidae; Hasenfuss and Kristensen 2003), Neuroptera (Myrmeleontidae, Osmylidae; Korn 1943; Wundt 1961), Coleoptera (Das 1937; Beutel 1993, 1995, 1999; Beutel and Molenda 1997; Beutel and Hörnschemeyer 2002a,b) and Strepsiptera (Pohl 2000).
| Taxa/characters | 1 omp | 2 hsh | 3 lso | 4 fcl | 5 acl | 6 occ | 7 gul | 8 dta | 9 ten | 10 wtb | 11 cta | 12 lbr | 13 8 | 14 9 | 15 ans |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Xyelidae | 0 | 0 | 1&3 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 |
| Diprionidae | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 3 |
| Tenthredinidae | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1&2 |
| Cephidae | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1&2 |
| Siricidae | 0 | 0 | 3 | 1 | 1 | 1 | 0 | ? | ? | ? | ? | 0 | ? | ? | 3&4 |
| Xiphydriidae | 0 | 0 | 3 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 2&3 |
| Orussidae | 0 | 0 | 3 | 1 | 1 | 0? | 0 | ? | ? | – | ? | 0 | ? | ? | 5 |
| Apocrita | 0 | 0 | 3 | 1 | 1 | 0? | 0 | ? | ? | ? | ? | 0 | ? | ? | 5 |
| Nannochoristidae | 1 | 1 | 1 | 0 | 0 | 0 | 2 | 1 | 0 | 1 | 0 | 0 | 0 | 0? | 3 |
| Boreidae | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 4 |
| Panorpidae | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 3 |
| Diptera (‘Nematocera’) | 1 | 1 | 3 | 1 | 0 | 0 | 0 | 1 | 0 | – | 0 | 0 | 0 | 0 | 4&5 |
| Siphonaptera | 1 | 1 | 3 | 1 | 0 | 0 | 1 | 1 | 0 | – | 0 | 0 | 0 | 0? | 4 |
| Lepidoptera (‘Aglossata ’) | 1 | 0&1 | 2&3 | 1 | 0&1 | 0 | 0 | 0 | 0 | 0&1 | 0 | 0 | 0&1 | 0 | 3&5 |
| Trichoptera | 0&1 | 1 | 2 | 1 | 1 | 0 | 0&1 | 0 | 0 | – | 0 | 0 | 0 | 0 | 5 |
| Archostemata | 1 | 1 | 3 | 0 | 0 | 0 | 0 | 1 | 0 | – | 0 | 0 | 1 | 0 | 2 |
| Polyphaga | 0&1 | 0&1 | 2 | 0&1 | 0 | 0 | 0&1 | 0&1 | 0&1 | 0&1 | 0 | 0&1 | 1 | 0&1 | 3 |
| Megaloptera | 1 | 1 | 2 | 0&1 | 0&1 | 0 | 0&1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1&2 |
| Raphidioptera | 1 | 1 | 2 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Neuroptera | 1 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0&1 |
| Strepsiptera | 1 | 1 | 2 | 1 | 0 | 0 | 0 | 1 | – | – | 0 | 1 | 1 | 1 | 5 |
| Zoraptera | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | ? | 0 | 0 | 0 | 0 | 0 |
| Taxa/characters | 16 1–4 | 17 o1– | 18 iam | 19 mol | 20 13 | 21 t11 | 22 pmx | 23 mxl | 24 car | 25 lcl | 26 lac | 27 gal | 28 smp | 29 15 | |
| Xyelidae | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 2 | |
| Diprionidae | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | |
| Tenthredinidae | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | |
| Cephidae | 2 | – | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | |
| Siricidae | ? | ? | ? | 1 | ? | ? | 0 | 1 | 1 | 1 | 0 | 0 | 0 | ? | |
| Xiphydriidae | 2 | – | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | |
| Orussidae | ? | ? | ? | 1 | ? | ? | 0 | 1 | 1 | 1 | 1 | 1 | 1 | ? | |
| Apocrita | ? | ? | 1 | 1 | ? | ? | 0 | 1 | ? | ? | 1 | 1 | 1 | ? | |
| Nannochoristidae | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | |
| Boreidae | 0 | 1 | 1 | 1 | ? | ? | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | |
| Panorpidae | 0 | 3 | 1 | 0 | 1? | 0 | 1 | 1 | 1 | – | 0 | 0 | 0 | 2 | |
| Diptera (‘Nematocera’) | 0&1&2 | 2&– | 1&– | 1 | 1? | 0 | 1 | 0 | 0&1 | 0 | 0&1 | 0&1 | 0&1 | 2 | |
| Siphonaptera | 0 | 1 | 1 | 1 | 1? | 0 | 1 | 0 | 0 | 0 | 1? | 0? | 0 | ? | |
| Lepidoptera (‘Aglossata ’) | 0&2 | 4&–? | 1 | 0&1 | 0 | 0 | 0 | 1 | 0 | 0 | 0&1 | 0&1 | 0 | 2 | |
| Trichoptera | 2 | – | – | 1 | 1? | 0&1 | 1 | 1 | 0 | 0 | 1 | 2 | 0 | 2 | |
| Archostemata | 0 | 4 | 1 | 0 | 1? | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | |
| Polyphaga | 0 | 1&4 | 1 | 0&1 | 0&1 | 0 | 0&1 | 0&1 | 0 | 0 | 0&1 | 0&1&2 | 0 | 0&2 | |
| Megaloptera | 0 | 4 | 1 | 1 | 1? | 1 | 0 | 0 | 0 | 0 | 0&1 | 0 | 0 | 0 | |
| Raphidioptera | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Neuroptera | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0&2 | |
| Strepsiptera | 2 | – | – | 1 | ? | 0 | 0 | 0 | 1 | – | 1 | 2 | 0 | ? | |
| Zoraptera | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Taxa/characters | 30 men | 31 nlp | 32 glp | 33 28 | 34 34 | 35 sal | 36 phl | 37 o42 | 38 37 | 39 38 | 40 tml | 41 msp | 42 dlm | ||
| Xyelidae | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | ||
| Diprionidae | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | ||
| Tenthredinidae | 1 | 0 | 1 | 1? | 0 | 0 | 1 | 1 | 1 | 0? | 1 | 1 | 1 | ||
| Cephidae | 0&1? | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | ||
| Siricidae | 1 | 1&2 | 1 | ? | ? | ? | 1 | ? | ? | ? | ? | ? | ? | ||
| Xiphydriidae | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | ||
| Orussidae | 1 | 3 | 1 | ? | ? | ? | 0 | ? | ? | ? | ? | ? | ? | ||
| Apocrita | 1 | 2&3 | 1 | ? | ? | ? | 0&1 | ? | ? | ? | ? | ? | ? | ||
| Nannochoristidae | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0? | 0 | 1 | 1 | 0 | 0 | ||
| Boreidae | 0 | 1&2 | 1 | 1 | 1 | 0 | 0 | 2 | 0 | 1 | 1 | 0 | 0 | ||
| Panorpidae | 4 | 1 | 1 | 1 | 1 | 0 | 0 | 2 | 0 | 1 | 1 | 0 | 0 | ||
| Diptera (‘Nematocera’) | 2 | 1&2 | 1 | 1 | 1 | 0 | 0 | 2 | 0 | 1 | 1 | 0 | 0 | ||
| Siphonaptera | 1 | 2 | 1 | 0 | 1 | 0 | 0 | 2 | 0 | 1 | 1 | 0 | 0 | ||
| Lepidoptera (‘Aglossata ’) | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 2 | 0 | 0&1 | 1 | 0 | 0 | ||
| Trichoptera | 0 | 1&2 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | ||
| Archostemata | 1 | 1&2 | 1 | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 0&1 | – | 0 | ||
| Polyphaga | 0 | 1 | 1 | 0 | 0&1 | 1 | 0 | 2 | 1 | 1 | 0&1 | – | 0 | ||
| Megaloptera | 0 | 0 | 1 | 0 | 0 | 1? | 0 | 0 | ? | ? | 1 | – | 0 | ||
| Raphidioptera | 3 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 1 | 0 | 0 | ||
| Neuroptera | 0&1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0&1 | 1 | 0 | 0 | ||
| Strepsiptera | 4 | 3 | 1 | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 1 | – | 0 | ||
| Zoraptera | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 |
- ?, unknown character state; – , inapplicable.
14. (9) M. frontoepipharyngalis (M. 9): (0) present (1) absent. M. frontoepipharyngalis is present with the typical insertion on the tormae in Xyela (Fig. 4) and in other symphytan taxa (e.g. Neodiprion, Dolerus, Janus, Xiphydria; Parker 1935; Das 1937). This presumably plesiomorphic condition is also found in some groups of Coleoptera (e.g. Archostemata; Das 1937; Beutel and Hörnschemeyer 2002a,b), in Neuropterida (Das 1937; Röber 1942; Wundt 1961; Beutel and Ge in press), Trichoptera (Das 1937; Winkler 1959) and Lepidoptera (Hasenfuss and Kristensen 2003), and in larvae of several nematoceran families (Cook 1944a,b, 1949 [tormal muscle/messorial muscles]; Kramer 1954). The muscle has an unusual insertion close to the median line in Siphonaptera (Sharif 1937; Widhalm-Finke 1971) and probably also in Nannochorista. It is absent in Panorpa (Bierbrodt 1942) and Boreus, in larvae of many groups of Coleoptera (Beutel 1993, 1995, 1999) and in first instar larvae of Strepsiptera (Pohl 2000).
15. (ans) Number of antennal segments: (0) multisegmented; (1) 5–7; (2) 4; (3) 3; (4) less than 3; (5) vestigial, no segmentation recognizable. Contrary to Smith (1967) we observe five, not six antennomeres in Xyela; the discrepancy is apparently caused by us not counting the basal membranous socket as an antennomere; the extrinsic antennal muscles attach to the antennomere distal to the socket, we consider the former to be the first antennomere. The antennae are generally short in larvae of Hymenoptera and show a distinct tendency towards reduction within the order. Comparatively long antennae with five to six antennomeres are present in Xyelidae (Fig. 1a,b,d; Smith 1967), and seven antennomeres are recorded for Pamphiliidae (Smith and Middlekauff 1987). A relatively high number is likely a plesiomorphic condition for the Hymenoptera. Four or five segments are usually present in Tenthredinidae and Cephidae, three in Diprionidae (strongly shortened), Anaxyelidae and Xiphydriidae, and only one or two in Argidae, Pergidae, Cimbicidae and Siricidae (Smith and Middlekauff 1987). The antennae are vestigial or absent in larvae of Orussus (Parker 1935: Fig. 53; Vilhelmsen 2003). The larvae of higher (apocritan) Hymenoptera are characterized by reduced sensory organs and their antennae are usually one-segmented or absent. Three antennomeres are present in larvae of Mecoptera (excl. Boreus; Byers 1987), Lepidoptera (groundplan, e.g. Micropterigidae, Heterobathmioidea; Kristensen 1999, 2003), Chrysopidae (New 1991) and in most polyphagan larvae (e.g. Beutel 1995, 1999; Beutel and Molenda 1997). The antennae are multisegmented in some groups of Neuroptera (e.g. Nevrorthidae, Psychopsidae, Osmylidae; New 1991), five antennomeres are present in Corydalidae (Crampton 1921) and Ithonidae (New 1991), four in larvae of Sialidae (Röber 1042), Cupedidae and Adephaga (Beutel 1993; Beutel and Hörnschemeyer 2002b), two in Boreidae (Russell 1982; Byers 1987) and Siphonaptera (Sharif 1937) and only one in larvae of Trichoptera (Hasenfuss and Kristensen 2003), Agathiphagidae (Lepidoptera; Kristensen 1984), and in several basal groups of Diptera (e.g. Bittacomorpha, Tipulidae, Culicidae, Bibionidae and other Nematocera; Cook 1949; Kramer 1954; Foote 1991), and also in larvae of Strepsiptera (Pohl 2000).
16. (1–4) Extrinsic antennal muscles: (0) well developed; (1) vestigial; (2) absent. Three well-developed extrinsic antennal muscles are present in Xyela (Fig. 5a). Only one thin muscle is present in Pseudoclavellaria (Parker 1935: Fig. 20) and a group of extremely thin fibres in Neodiprion and Dolerus (coded as 1). Antennal muscles are entirely lacking in Janus and Xiphydria. Extrinsic muscles are usually well developed in endopterygote larvae (e.g. Bierbrodt 1942; Neuroptera, Korn 1943; Culicidae, Bittacomorpha, Cook 1944a, 1949; Kramer 1954; Wundt 1961; Lepidoptera, Kristensen 1984; Mecoptera; R. Beutel, personal observation; Coleoptera, Beutel 1993, 1999; Beutel and Hörnschemeyer 2002b). Antennal muscles are often lacking in nematoceran larvae (e.g. Bibionidae, Tipulidae; Cook 1949) and are also absent in Trichoptera (e.g. Rhyacophila, Limnephilus; Winkler 1959), Agathiphagidae (Lepidoptera; Kristensen 1984) and Strepsiptera (Pohl 2000).
17. (o1-) Origin of antennal muscles: (0) dorsal tentorial arms; (1) at least partly from anterior tentorial arms; (2) posterior arms; (3) ligament between anterior arm and gena; (4) head capsule. The antennal muscles originate from the dorsal tentorial arms in Xyela and Tenthredinidae (Parker 1935), and this is also the case in larvae of Adephaga (Beutel 1993). They arise from the anterior arms in Nannochorista, Boreus (one of two muscles), Ctenocephalides (Widhalm-Finke 1974) and in polyphagan larvae (major part; e.g. Beutel and Molenda 1997), from a ligament in Panorpa (Bierbrodt 1942), from the head capsule in Sialis (Röber 1942) and in larvae of Archostemata and Elateriformia (Beutel and Hörnschemeyer 2002a,b; Beutel 1995), and from the posterior tentorial base in some nematoceran larvae (Schremmer 1950). Two of four muscles arise from the anterior arm in Osmylus and two from the dorsal arms (Wundt 1961).
18. (iam) Intrinsic antennal muscles (Mm. scapopedicellaris): (0) present; (1) absent. Intrinsic muscles of the scapus are absent in Xyela and the other symphytan larvae examined (Parker 1935; R. Beutel, personal observation). They are also lacking in all other endopterygote lineages (e.g. Bierbrodt 1942; Röber 1942; Korn 1943; Kramer 1954; Wundt 1961; Kristensen 1984; Beutel 1993, 1995, 1999; Beutel and Ge in press).
19. (mol) Mola: (0) present; (1) absent or strongly reduced. A mandibular mola with a grinding surface is absent in Xyela (Fig. 3) and other symphytan larvae (Parker 1935; Smith and Middlekauff 1987), and also in larvae of Mecoptera (excl. Panorpa; Applegarth 1939; Pilgrim 1972; Byers 1987), Neuropterida (e.g. Röber 1942; Wundt 1961), Adephaga, Hydrophiloidea (Beutel 1993, 1999), Trichoptera (Wiggins 1987), Siphonaptera (Sharif 1937), Diptera (Cook 1949; Foote 1991) and Strepsiptera (Pohl 2000). It is present in larvae of Panorpa (Bierbrodt 1942), in many groups of Coleoptera (Archostemata, Myxophaga, Polyphaga partim; Beutel and Molenda 1997; Beutel and Haas 1998; Beutel and Hörnschemeyer 2002a) and in most groups of Lepidoptera (absent in Micropterix but present in Neomicropterix; Hasenfuss and Kristensen 2003).
20. (13) M. tentoriomandibularis: (0) present; (1) absent. M. tentoriomandibularis is present in Xyela, and also present but extremely thin in the other symphytan larvae examined. M. tentoriomandibularis is also present and extremely thin in Nannochorista, and is recorded for Osmylus (three subcomponents; Wundt 1961), Raphidia (Beutel and Ge in press), Oryzaephilus (Coleoptera) (composed of one muscle fibre and eight multipolar sensory cells; Honomichl 1978) and for larvae of basal groups of Lepidoptera (e.g. Agathiphaga; few muscle fibres and a cluster of sensory neurons; Kristensen 1984, 2003; Vegliante 2005). It is absent (or has been overlooked due to its very small size) in larvae of other endopterygote taxa (e.g. Das 1937; Sharif 1937; Bierbrodt 1942; Röber 1942; Winkler 1959; Widhalm-Finke 1974; Beutel and Hörnschemeyer 2002a,b).
21. (t11) Tendon of M. craniomandibularis internus: (0) not bifurcated to its base; (1) bifurcated to its base. A deeply cleft tendon of M. craniomandibularis is present in Xyela (Fig. 5b,c) and other symphytan larvae (e.g. Neodiprion, Janus, Xiphydria; Parker 1935: principal apodeme and auxillary apodeme). This is also the case in Sialis (Röber 1942: Fig. 5), in Limnephilus (Winkler 1959) and in larvae of some aquatic groups of Adephaga (Beutel 1993). An undivided or only slightly bifurcated tendon is present in most beetle larvae (e.g. Beutel 1993, 1999).
22. (pmx) Position of maxilla: (0) retracted; (1) protracted. The maxilla is in a retracted position in Xyela (Fig. 1b,e) and other symphytan larvae (Parker 1935; Smith and Middlekauff 1987), and also in larvae of non-pistilliferan Mecoptera (Pilgrim 1972; Russell 1982), in Megaloptera (Crampton 1921; Röber 1942), Neuroptera (e.g. Crampton 1921; Wundt 1961), Coleoptera (with some exceptions; Beutel and Hörnschemeyer 2002a,b; Beutel and Haas 1998) and Lepidoptera (Hasenfuss and Kristensen 2003). This is likely a plesiomorphic condition, which is also found in almost all nymphs of hemimetabolous insects (e.g. Zoraptera). The maxillary bases are protracted in Pistillifera (Applegarth 1939; Bierbrodt 1942), Raphidioptera, Siphonaptera (Widhalm-Finke 1974), Adephaga (Beutel 1993), Histeridae (Beutel 1999), Trichoptera (Winkler 1959; Wiggins 1987) and in nematoceran larvae (Cook 1944a,b, 1949; Kramer 1954).
23. (mxl) Maxillolabial complex: (0) absent; (1) present. The ventral mouthparts form a maxillolabial complex in larvae of Xyelidae (Fig. 1) and in other symphytan lineages. An articulatory membrane is absent and the entire structure is mainly moved in vertical direction as one unit. This derived condition is also found in Mecoptera (Bierbrodt 1942; Pilgrim 1972; Russell 1982), Trichoptera (Winkler 1959; Fotius-Jaboulet 1961), Lepidoptera (Kristensen 1984; Hasenfuss and Kristensen 2003) and in some groups of Coleoptera (e.g. Elateriformia [major part], Cleroidea; Beutel 1995; Beutel and Pollock 2000), but not in Diptera and Siphonaptera (e.g. Sharif 1937; Cook 1944a,b, 1949; Kramer 1954; Widhalm-Finke 1974). The maxillary bases are also distinctly separated from the anterior labium in Neuropterida (Crampton 1921; Das 1937; Röber 1942; Wundt 1961; New 1991; Beutel and Ge in press), in most groups of Coleoptera (e.g. Beutel 1993, 1999; Beutel and Molenda 1997; Beutel and Hörnschemeyer 2002a,b) and in Strepsiptera (Pohl 2000).
24. (car) Cardo: (0) present as a separate sclerite; (1) not present as a separate sclerite. The cardo is present in larvae of Xyela (Fig. 1a,e) and in most other symphytan larvae, but largely or completely reduced in Siricidae and Orussidae, respectively (Parker 1935). The cardo is also not differentiated in larvae of Pistillifera (Applegarth 1939; Bierbrodt 1942), Culicidae (Cook 1944a) and Strepsiptera (Pohl 2000). It is well developed in other groups of Endopterygota with very few exceptions in subordinate groups (e.g. Crampton 1921; Das 1937; Röber 1942; Chiswell 1955; Wundt 1961; Beutel 1999; Beutel and Hörnschemeyer 2002a,b).
25. (lcl) Lateral cardinal lobe: (0) absent; (1) present. The presence of a lateral cardinal lobe in Xyela (Fig. 1e: cdl) and other symphytan larvae (Parker 1935) is perhaps a derived groundplan feature of Hymenoptera. The lobe is large and conspicuous in Xyela, whereas it is small, but still distinctly recognizable in tenthredinid larvae (Parker 1935: Figs 2 and 14, cdlo). It is usually absent in other groups of Endopterygota (e.g. Crampton 1921; Röber 1942; Beutel and Ge in press; Beutel 1993, 1995, 1999; Hasenfuss and Kristensen 2003; Cook 1944a,b, 1949), but occurs in larvae of Archostemata (Beutel and Hörnschemeyer 2002a,b).
26. (lac) Lacinia: (0) present as a separate structure; (1) not present as a separate structure or completely reduced. The lacinia is distinctly developed and equipped with mesally directed spines (‘rake’; Parker 1935) in Xyela (Fig. 1c) and other symphytan larvae (incl. Xiphydria). It is absent in larvae of Orussus (Parker 1935) and Apocrita (e.g. Finlayson 1987; Evans 1987). The lacinia is still recognizable but distinctly reduced in larvae of Nannochoristidae (Fig. 5a; Pilgrim 1972), Boreidae (Russell 1982: Fig. 4), Apterobittacus (Applegarth 1939), Panorpa (Bierbrodt 1942), Raphidioptera (Das 1937) and Tenthredinidae (Parker 1935: rectangular, sclerotized piece bearing about fourteen spine-like teeth; Fig. 28). A distinct peg-like lacinia is articulated close to the palp in larvae of Trichoptera (partim), but the lacinia is vestigial in Limnephiloidea (Wiggins 1987). It is present as a small, pointed structure in Micropterigidae (partim), but fused with the galea in larvae of other lepidopteran groups (Hasenfuss and Kristensen 2003). The lacinia is modified in many different ways in Coleoptera or completely reduced (Beutel 1993, 1995, 1999; Beutel and Hörnschemeyer 2002a,b), completely fused with the galea (see char. 33) in Neuroptera (e.g. Wundt 1961), and is not recognizable as a separate element of the maxilla in Corydalidae (Crampton 1921) and Strepsiptera (Pohl 2000: Fig. 3). A strongly developed hook-shaped structure (without mesally directed spines) is present in Sialis. However, this structure was interpreted as the galea by Röber (1942). A strongly modified lacinia is probably present in the groundplan of Diptera (Anthon 1943; Hennig 1973), but only one endite lobe is present in most taxa (e.g. Kramer 1954). It is probably represented by a mesal area densely covered with adhesive microtrichia in Siphonaptera.
It has to be noted that character state 1 of this and the following character may be the result of the same evolutionary transition, i.e. the fusion of both endite lobes.
27. (gal) Galea: (0) present as a separate structure; (1) fused with the lacinia or absent. The galea is well developed as a separate element in Xyela and usually also in other symphytan and endopterygote lineages (e.g. Röber 1942; Beutel and Ge in press; Beutel 1993; Beutel and Hörnschemeyer 2002a,b). It is small and palp-like in Xiphydria and Siricidae (Parker 1935: Fig. 46) and absent in Orussus (Parker 1935) and Apocrita (e.g. Evans 1987; Finlayson 1987). A fusion of the galea with the lacinia occurs in Coleoptera (e.g. Beutel et al. 1998; Beutel and Friedrich 2005), Trichoptera (Kristensen 1984) and in Lepidoptera excl. Micropterigidae (Hasenfuss and Kristensen 2003).
28. (smp) Segmentation of maxillary palp: (0) present; (1) absent. The maxillary palp is segmented in Xyela (Fig. 1a,e) and this is usually also the case in other symphytan larvae (Parker 1935, Smith and Middlekauff 1987) and in other endopterygote lineages (e.g. Crampton 1921; Röber 1942, Beutel 1993, 1995, 1999; Hasenfuss and Kristensen 2003; Beutel and Ge in press). It is unsegmented and often vestigial in larvae of Orussus (Parker 1935) and Apocrita (Evans et al. 1987).
29. (15) M. craniocardinalis (M. 15): (0) well developed; (1) very small, attached with a long and very thin tendon; (2) absent. The muscle is absent in Xyela. It is present in the other symphytan larvae examined, but extremely small and attached by means of a long, very thin tendon. It is absent in larvae of Mecoptera (Bierbrodt 1942; R. Beutel, personal observation), Osmylus (Korn 1943: M. rem. max.; Wundt 1961; present in Myrmeleon), Lepidoptera (Das 1937; Hasenfuss and Kristensen 2003), Trichoptera (Das 1937) and Diptera (Das 1937; Cook 1949; Kramer 1954: Fig. 7; Hennig 1973). It is not clear if M. craniocardinalis is present or absent in Siphonaptera. The homologization of the extrinsic maxillary muscles is problematic.
30. (men) Mentum: (0) separated from submentum; (1) fused with submentum (=postmentum); (2) fused with hypostomal plate; (3) membranous and strongly reduced; (4) absent. The postmentum (=submentum + mentum) is undivided in larvae of Xyela. This condition is also usually found in tenthredinid larvae, but a separating fold is recorded for the cephid Trachelus tabidus (Fabricius, 1775) by Parker (1935). An undivided postmentum is also present in Osmylidae, Ithonidae and Polystoechotidae (Wundt 1961; Grebennikov 2004), in Lepidoptera (Hasenfuss and Kristensen 2003), in Siphonaptera (Sharif 1937) and in Cupedidae (Beutel and Hörnschemeyer 2002b). The mentum is strongly reduced and membranous in different groups of beetles (e.g. Adephaga, Histeridae; Beutel 1993, 1999) and in Raphidioptera (Beutel and Ge in press), and both posterior labial parts are probably absent in Strepsiptera (Pohl 2000) and also reduced in Pistillifera (e.g. Applegarth 1939; Bierbrodt 1942). Submentum and mentum are separated in Nannochoristidae, Boreidae, Nevrorthidae (New 1991; Fig. 34.5S), Chrysopidae (Crampton 1921: Fig. 51), Megaloptera (Crampton 1921), Trichoptera (Wiggins 1987) and in larvae of many groups of Coleoptera (e.g. Beutel and Molenda 1997). The labium is more or less simplified in nematoceran larvae and the posterior parts are often fused to the hypostomal plate (e.g. Kramer 1954; Foote 1991; Teskey 1991).
31. (nlp) Number of segments of labial palp: (0) three; (1) two; (2) one; (3) vestigial. The labial palp is composed of three palpomeres in Xyelidae (Fig. 1C; Smith 1967; Smith and Middlekauff 1987) and Tenthredinidae (Parker 1935), and in most other symphytan larvae (one- to two-segmented in Siricidae; Smith and Middlekauf 1987). The palp is vestigial in Orussus (Parker 1935; Vilhelmsen 2003) and disc-like or papilliform and without distinct segmentation in Apocrita (Yuasa 1922; Evans et al. 1987; see also Vilhelmsen 2003). The presumptive plesiomorphic number of three is also found in Raphidioptera and Megaloptera (Crampton 1921; Röber 1942), and more than three segments are usually present in Neuroptera (New 1991). Two palpomeres are usually present in Mecoptera (Pilgrim 1972; Applegarth 1939; Bierbrodt 1942), in Coleoptera (with few exceptions; Beutel 1993, 1995, 1999; Beutel and Hörnschemeyer 2002b; Beutel 1993, 1995, 1999), Trichoptera (partim, e.g. Rhyacophila; Hinton 1958; Wiggins 1987) and Lepidoptera (Hasenfuss and Kristensen 2003). Only one palpomere is present in Caurinus (Russell 1982), in few groups of Coleoptera (e.g. Micromalthidae; Beutel and Hörnschemeyer 2002a), in some groups of Trichoptera (Hinton 1958), in Siphonaptera (Sharif 1937) and in many nematoceran larvae (e.g. Bibio, Kramer 1954; Foote 1991).
32. (glp) Glossa and paraglossae (0) present; (1) absent. The glossa and paraglossae and associated muscles are absent in Xyela and in all other endopterygote larvae (e.g. Crampton 1921; Parker 1935; Röber 1942; Cook 1944a,b, 1949; Beutel 1993, 1995, 1999).
33. (28) M. postmentopraementalis: (0) present; (1) absent (Table 1). M. postmentopraementalis is present in Xyela (Fig. 4), Neodiprion, Janus and Xiphydria, but is apparently absent in tenthredinid larvae (e.g. Dolerus; Parker 1935). It is also absent in Mecoptera (Bierbrodt 1942; R. Beutel, personal observation) and in larvae of most other groups of Mecopterida with the exception of Siphonaptera (Sharif 1937). It is generally present in other groups of endopterygote insects (e.g. Das 1937) but reduced in larvae of some groups of Coleoptera (Beutel 1993; Beutel and Hörnschemeyer 2002a,b) and in first instar larvae of Strepsiptera (Pohl 2000).
34. (34) M. praementopalpalis: (0) present; (1) absent (Table 1). M. praementopalpalis is present in Xyela and is also preserved as a very thin bundle in most other symphytan larvae (e.g. Neodiprion, Janus; Parker 1935). It is also present in Neuropterida (Röber 1942; Wundt 1961; Beutel and Ge in press), and in the groundplan of Coleoptera (e.g. Das 1937; Beutel 1993, 1995). It is absent in Xiphydria, Mecoptera (misinterpreted by Bierbrodt 1942 [origin from the tentorium]), Trichoptera (Rhyacophila; R. Beutel, personal observation), Lepidoptera (Hasenfuss and Kristensen 2003), Diptera (Das 1937; Cook 1949; Kramer 1954; Hennig 1973), Siphonaptera (Sharif 1937; Widhalm-Finke 1974) and in Strepsiptera (Pohl 2000).
35. (sal) Salivarium: (0) present; (1) absent. A salivarium transformed into a tube-like duct is present in larvae of Xyela (4, 5) and other symphytan larvae. It is usually also present in other groups (e.g. Sharif 1937; Bierbrodt 1942; Kramer 1954; Winkler 1959; Fotius-Jaboulet 1961; Wundt 1961; Kristensen 1984, 2003), but is absent in Sialidae (Röber 1942), Coleoptera (Beutel 1993, 1995, 1999; Beutel and Molenda 1997; Beutel and Hörnschemeyer 2002a,b) and Strepsiptera (Pohl 2000).
36. (phl) Prelabio-hypopharnygeal lobe: (0) absent or strongly modified; (1) present. As the boundary between the labium and hypopharynx is defined by the salivarium, the prelabio-hypopharyngeal lobe is apparently formed by both elements as already pointed out in Hasenfuss and Kristensen (2003). This structure is usually present in symphytan larvae (1, 4; Parker 1935) but is extremely reduced in Orussus (Parker 1935: Fig. 53). It is usually well developed in Lepidoptera and in Trichoptera (Hasenfuss and Kristensen 2003), but strongly modified (e.g. Nannochorista) or reduced in Antliophora (Sharif 1937; Applegarth 1939: Fig. 55A,D; Bierbrodt 1942: Figs. 12, 14; Anthon 1943: Fig. 4; Kramer 1954: Fig. 8).
37. (o42) Origin of M. tentoriohypopharyngalis: (0) tentorium; (1) occipital ridge; (2) absent. M. tentoriohypopharyngalis originates from the lateral postoccipital ridge in Xyela (4, 5) and also in other symphytan larvae (e.g. Neodiprion, Janus, Xiphydria; Parker 1935: muscle 27). It originates on the posterior tentorium in larvae of Osmylus (Wundt 1961) and in Limnophilus (Winkler 1959). The muscle is absent in most groups of Endopterygota (e.g. Bierbrodt 1942; Beutel and Hörnschemeyer 2002a,b; Hasenfuss and Kristensen 2003).
38. (37) M. hypopharyngosalivaris: (0) present; (1) absent. M. hypopharyngosalivaris is present in Janus but absent in Xyela and the other symphytan larvae examined (see also Parker 1935). The muscle is usually present in larvae with a well developed salivary duct (e.g. Neuroptera, Mecoptera, Diptera, Siphonaptera; Bierbrodt 1942; Kramer 1954; Wundt 1961; Hasenfuss and Kristensen 2003), but is absent in Raphidia, Coleoptera and Strepsiptera (Beutel and Ge in press).
39. (38) M. prementosalivaris: (0) present; (1) absent. M. prementosalivaris is absent in Xyela, Janus and Xiphydria, but present in Dolerus and Neodiprion, and probably also in Pseudoclavellaria (Parker 1935: 31). The muscle arises from the lateral wall of the anterior labium like the transverse muscle (see following character) and is ventrally attached to the salivary duct. It is conceivable that the transverse muscle of the anterior labium is derived from this muscle. The prementosalivary muscle is absent in most endopterygote lineages, but is present in Micropterix, Osmylus and Raphidia (Wundt 1961; Hasenfuss and Kristensen 2003; Beutel and Ge in press).
40. (tml) Transverse muscle of the anterior labium: (0) absent; (1) present. The muscle passes directly below the salivary duct. Like the previous muscle it helps to hold the duct in its position. It is well developed in Xyela (3, 4), Janus and Xiphydria, but is absent in the other symhytan larvae examined so far (Parker 1935). A muscle arising from the lateral premental wall in Neodiprion is attached to the ventral wall of the salivary duct (M. prementosalivaris) (coded as 0). The transverse muscle is also present in few groups of Coleoptera (Beutel and Friedrich 2005) and Sialis (Das 1937; Röber 1942), but is absent in most groups of beetles (e.g. Beutel 1993, 1999; Beutel and Hörnschemeyer 2002a,b) and in Strepsiptera (Pohl 2000), Neuroptera (Korn 1943; Wundt 1961), Raphidioptera (Beutel and Ge in press), Trichoptera (Winkler 1959), Lepidoptera (Hasenfuss and Kristensen 2003) and in Antliophora [Bierbrodt 1942; Cook 1949; Kramer 1954; Hennig 1973; R. Beutel et al., unpublished data; Siphonaptera (Sharif 1937)].
41. (msp) Muscle of the silk press: (0) absent; (1) present. The longitudinal muscle of the salivarium or muscle of the silk press is absent in Xyela and Xiphydria, but present in the other symphytan larvae examined (see also Parker 1935). It is not described for larvae of other groups of Endopterygota (Bierbrodt 1942; Kramer 1954; Wundt 1961; Widhalm-Finke 1974; Hasenfuss and Kristensen 2003; Beutel and Ge in press). The presence is either an autapomorphy of Hymenoptera or an apomorphy of Hymenoptera excl. Xyelidae. It is unclear whether the muscle is present or absent in the groundplan of Xyelidae and reversal cannot be excluded.
42. (dlm) Size of dorsal longitudinal muscle of prepharynx and anterior pharynx: (0) moderately sized; (1) very strong. The presence of an exceptionally strongly developed longitudinal muscle is a derived condition found in larvae (Fig. 3) and adults of Xyelidae (Beutel and Vilhelmsen in press) and in most other symphytan larvae examined. The muscle is inconspicuous in Xiphydria.
Discussion
Several plesiomorphic groundplan features of Endopterygota are preserved in Xyelidae and other symphytan larvae: the coronal and frontal sutures are distinctly developed; the tentorium is complete, with distinctly developed dorsal arms and posterior grooves adjacent to the foramen occipitale; a ventral closure of the head capsule is absent; the clypeus is divided into an anteclypeus and postclypeus; the labrum is free and a complete set of intrinsic and extrinsic labral muscles is present; M. tentoriomandibularis is present; the labial palp is usually three-segmented; the maxilla is in a retracted position; the salivary duct is well developed. Several features are shared with Mecoptera (or Mecoptera excl. Nannochoristidae), i.e. the globular orthognathous head, the X-shaped tentorium, the very broad tentorial bridge, the occipital furrows and the presence of modified compound eyes. We assume that at least some of these character states belong to the groundplan of Endopterygota. Yet, the polarity is ambiguous, and it is also conceivable that some of these features are derived and have independently evolved in the two orders. The lateral eyes of hymenopteran and mecopteran immature stages differ distinctly. It is a unicornean compound eye with many ommatidia in larvae of Hymenoptera with well developed light sense organs (e.g. Neodiprion; reduced in Xyela and others), but composed of few ommatidia with individual corneal lenses in Mecoptera (corneal lenses reduced in Nannochorista; Melzer et al. 1994). A globular, orthognathous head is also present in many groups of Lepidoptera, but not in the groundplan of this order (the head is prognathous in Micropterigidae). The orthognathous condition is likely correlated with phytophagous habits.
The absence of intrinsic antennal muscles and of glossae and paraglossae and associated muscles are potential autapomorphies of holometabolous insects. Yet, the outgroup comparison is problematic when the entire endopterygote lineage is under consideration. We chose Zoraptera for a preliminary polarity determination in this study. However, Truman and Riddiford (1999) argued that immature stages of hemimetabolous and holometabolous insects are not corresponding stages as suggested by Hinton (1955) and Sehnal et al. (1996) (see discussion in Grimaldi and Engel 2005).
Possible autapomorphies of Hymenoptera are the process of the posterior tentorial arms, the bifurcated tendon of M. craniomandibularis, the lateral lobe of the cardo, the presence of the cranial retractor of the prelabio-hypopharyngeal lobe, the large size of the dorsal longitudinal muscle of the pharynx (also developed in the adult; see Beutel and Vilhelmsen in press) and the muscle of the silk press (reversal in Xyela and Xiphydria, see above). The polarity of the presence or absence of the mola is unclear. The presence of small ganglia at the base of the head appendages is a feature not described for other endopterygote larvae so far. They are also present in other symphytan larvae examined, but smaller in relation to the head size. More study of these structures is required to reveal its phylogenetic significance.
Hymenopteran larvae are characterized by a tendency to reduce the antennae. A basal position of Xyelidae within the order is supported by the high number of antennomeres and the presence of well developed extrinsic antennal muscles. The strongly reduced condition or absence of the antennal musculature is a potential synapomorphy of all hymenopteran groups excl. Xyelidae, being vestigial even in taxa with comparatively well developed antennae (Dolerus, Janus); it would be important to examine representatives of the Pamphilioidea to confirm this assertion, as they have even higher numbers of antennomeres than Xyelidae. The reduction of the frontoclypeal suture is another apomorphic condition found in non-xyelid Hymenoptera.
Potential autapomorphies of Xyelidae are the complete absence of M. craniocardinalis and the absence of the muscles of the salivarium. The latter condition may be correlated with the unusually large diameter of the salivary duct. The reduced compound eye in Xyela is likely correlated with endophytic habits and apparently not a groundplan feature of the family. Distinctly developed eyes are present in Macroxyela (Smith and Middlekauff 1987).
The vestigial unsegmented antennae, the reduction of the lacinia and the unsegmented palps are potential synapomorphies of Apocrita and Orussidae (paralleled within Tenthredinoidea, see Vilhelmsen 2001). The absence of the cardo is a shared derived feature of these two lineages and Siricidae.
Our results do not support a basal position of Hymenoptera within Endopterygota as it was suggested by Rasnitsyn and Quicke (2002) and Kukalová-Peck and Lawrence (2004). In contrast to most other groups more than four antennomeres are present in the groundplan of Hymenoptera (5–7 in Xyelidae; see above). Yet, multisegmented antennae do occur in Neuroptera (New 1991) and are arguably a groundplan condition of Endopterygota.
A sistergroup relationship between Hymenoptera and Mecopterida (e.g. Mickoleit 1966; Hennig 1969, Kristensen 1999; Ronquist 1999) is supported by the presence of a maxillolabial complex in Hymenoptera (Fig. 1E), Amphiesmenoptera (Hasenfuss and Kistensen 2003) and in Mecoptera (e.g. Bierbrodt 1942). The absence in Siphonaptera and Diptera (e.g. Sharif 1937; Cook 1949), i.e. the clearly separated maxillae and labium, is likely a secondary condition, related to the anterior shift of the ventral mouthparts. Another feature suggesting mecopterid affinities of Hymenoptera is the presence of the prelabio-hypopharyngeal lobe. This structure is distinctly developed in Hymenoptera and Amphiesmenoptera (Hasenfuss and Kistensen 2003), but substantially modified or absent in the antliophoran orders (e.g. Bierbrodt 1942; Kramer 1954). Another potential synapomorphy of Hymenoptera and Mecopterida is the vestigial condition or loss of M. craniocardinalis. This is likely correlated with the formation of the maxillolabial complex.
The results of our study provide further evidence for the monophyly of Hymenoptera, they support a basal position of Xyelidae within the order, and they tentatively support a clade comprising Hymenoptera and Mecopterida. The morphological data presented here will be integrated in a more comprehensive character state in a future study (see above). The analysis of a large combined data set will likely contribute to a better understanding to hymenopteran and endopterygote evolution in the future.
Acknowledgements
We are very grateful to E. Altenhofer (Groß Gerungs, Austria) for the generous gift of numerous valuable specimens. This study would not have been possible without his support. N. P. Kristensen (Natural History Museum of Denmark, Copenhagen, Denmark) and A. P. Rasnitsyn (Palaeontological Institute RAS, Moscow, Russia) provided valuable comments on earlier versions of the manuscript.




