Molecular and morphometric assessment of the taxonomic status of Ectemnorhinus weevil species (Coleoptera: Curculionidae, Entiminae) from the sub-Antarctic Prince Edward Islands
Molekulare und morphometrische Bewertung des taxonomischen Status der Ectemnorhinus Rüsselkäfer-Arten (Coleoptera: Curculionidae, Entiminae) der sub-antarktischen Prinz-Edward-Inseln
Abstract
enThere are long-standing controversies on the taxonomic status of Ectemnorhinus weevil species occurring on the sub-Antarctic Prince Edward Islands. Since the two islands that constitute the Prince Edward Islands archipelago (PEIA), Marion Island (MI) and Prince Edward Island (PEI), differ in terms of alien invasive species such as the introduced house mouse Mus musculus and conservation management strategies, it is important to consider inter-island dynamics when investigating inter-specific relationships. Using a combined molecular phylogenetic and morphometric approach, we attempted to resolve the taxonomic status of the PEIA Ectemnorhinus weevil species. A COI gene phylogeny was inferred following the genetic characterization of 52 Ectemnorhinus weevils from both islands, and morphometric assessment using a set of 15 linear, external measurements was used to differentiate between the two currently recognized species, Ectemnorhinus similis and Ectemnorhinus marioni. Analyses revealed the presence of two genetically and morphometrically distinct species on PEI, whilst evidence for a single species, comprising diverse genetically discrete populations was found on MI. Based on these results, the species unique to PEI has been designated Ectemnorhinus kuscheli n. sp. whilst we confirm the synonymy between E. similis and E. marioni, the two species originally described from MI. E. kuscheli appears to be restricted to PEI, whereas E. similis occurs on both MI and PEI.
Zusammenfassung
deDer taxonomische Status der Ectemnorhinus Rüsselkäfer-Arten von den subantarktischen Prinz-Edward-Inseln ist seit langem eine Kontroverse. Das Prinz-Edward-Insel-Archipelago (PEIA) besteht aus zwei Inseln, der Marion-Insel (MI) und der Prinz-Edward-Insel (PEI), die sich bezüglich gebietsfremder, eingewanderter Arten und Naturschutz Management Strategien unterscheiden. Bei der Untersuchung zwischenartlicher Verwandtschaften der Rüsselkäfer ist es daher wichtig, die Dynamik zwischen den Inseln zu berücksichtigen. Wir versuchen hier, durch die Kombination molecular phylogenetischer und morphometrischer Methoden den taxonomischen Status der PEIA Ectemnorhinus Rüsselkäfer-Arten zu ermitteln. Eine phylogenetische Hypothese wurde von COI Gen-Sequenzen von 52 Ectemnorhinus Rüsselkäfern von beiden Inseln abgeleitet. Eine morphometrische Beurteilung basierend auf 15 linearen Messungen äusserer Merkmale wurde benutzt um zwischen den zwei zur Zeit anerkannten Arten E. similis und E. marioni zu unterscheiden. Unsere Analysen ergaben, dass sich auf PEI zwei genetisch und morphometrisch diskrete Arten befinden, während nur eine einzige Art, jedoch bestehend aus diversen genetisch voneinander abgegrenzten Populationen, auf MI vorkommt. Von diesen Ergebnissen abgeleitet wurde die Art, die ausschliesslich auf PEI vorkommt, als E. kuscheli n. sp. identifiziert, während wir bestätigen die Synonymie der beiden Arten E. similis und E. marioni, die ursprünglich für MI beschrieben wurden. Das Verbreitungsgebiet von E. kuscheli scheint auf PEI begrenzt zu sein, während E. similis sowohl auf MI als auch auf PEI zu finden ist.
Introduction
The weevils of the South Indian Ocean province of the Southern Ocean belong to a single, monophyletic unit centred around the genus Ectemnorhinus G.R. Waterhouse, 1853 (Kuschel and Chown 1995). There are approximately 36 species in the group, and they have proven to be taxonomically difficult (Brown 1964; Kuschel 1970; Dreux and Voisin 1989; Chown 1991). In particular, the taxonomic status of Ectemnorhinus marioni and Ectemnorhinus similis from the sub-Antarctic Prince Edward Islands (PEI) has long been controversial. E. similis C.O. Waterhouse, 1885 was the first Ectemnorhinus species described from Marion Island (MI). Subsequently, Jeannel, (1940) described E. marioniJeannel 1940, which was distinguished from E. similis based on the form of the humeri, and interstrial and strial morphology. However, Kuschel (1971) synonymized the two species due to the lack of consistent differences in either internal or external morphology. Subsequently, Dreux and Voisin (1986) continued to recognise the two species, noting that they differed in the form of their elytral striae and interstriae, and the elytral punctuation.
Crafford et al. (1986) recognized three distinct ecotypes within E. similis based on body size and colour. Following a detailed investigation of habitat use, feeding biology, life history, morphology and mating preferences, Chown (1990), however, noted that the use of vestiture colour and body length to distinguish between ecotypes was not justified. Rather, he argued that the species complex should be separated into two morphologically similar, but ecologically distinct species. Small-sized (3.77 mm–7.79 mm; median: 5.53 mm) bryophyte-feeding individuals associated with vegetation types dominated by the plants Azorella selago Hook. f. and Agrostis magellanica Lam, and including bryophytes such as Campylopus spp., Ptychomnion ringianum Broth. and Kaal., Ditrichum strictum (Hook. f. and Wils) Hampe and others, were referred to as E. marioni (Chown 1990). The larger (4.51 mm–8.69 mm; median: 6.44 mm) angiosperm-feeding individuals associated with Acaena magellanica (Lam.) Vahl. herbfields, Callitriche antarctica Engelm. ex Hegelm., Pringlea antiscorbutica R.Br. ex Hook. f., Poa cookii Hook. f. and A. selago were designated E. similis. Although E. similis feeds mainly on angiosperms, bryophytes and other cryptogams are incorporated into their diet at the end of the growing season when vascular plant foliage deteriorates (Chown 1989,1990; Chown and Scholtz 1989). Both E. marioni and E. similis can be found on A. selago, but the former species feeds only on epiphytic bryophytes growing on this plant species (Chown and Scholtz 1989), whereas the latter species feeds both on the A. selago and on epiphytic species including the grass, A. magellanica, and bryophytes.
Apart from variation in body size and diet, E. marioni and E. similis also differ in the length of their life cycles and times of emergence (Chown 1990). Ectemnorhinus marioni exhibits a shorter life cycle with fewer instars, and adults are present throughout the year, while adults of E. similis emerge only during summer months, and their emergence appears to be synchronized with the first flushes of angiosperm growth and flowering. Apart from body size, there are neither consistent differences in the male genitalia (Chown 1990), nor consistent differences in either the ovipositor or the spermatheca in females of the two species. Chown (1990) suggested that E. marioni and E. similis evolved sympatrically in a manner similar to that proposed by Rice (1984), with reproductive isolation being induced by size-based assortative mating associated with differences in food preference (Chown 1990; Crafford and Chown 1991). It is important to note that both E. similis and E. marioni were described from MI, while no species was formally based on weevils from PEI. However, because the weevils from PEI appeared to be morphologically and ecologically similar to MI individuals, it was concluded that both species also occur on PEI (Dreux 1971). Many studies have subsequently followed these taxonomic decisions (reviewed by Chown et al. 2002; Klok and Chown 2003), and they have also been applied in conservation management of the PEIs (Anonymous 1996).
Factors influencing population size and density of Ectemnorhinus species on the PEIs have been documented. While the house mouse (Mus musculus Linnaeus, 1758, sensu lato) was introduced by sealers on MI, the larger of the two PEIs, more than 180 years ago (Watkins and Cooper 1986), the smaller PEI has remained mouse-free. Mice feed on a variety of invertebrates and plants on the islands, and especially weevils (Gleeson and Van Rensburg 1982; Smith et al. 2002). In addition, mean temperature in the sub-Antarctic has increased by approximately 1°C in the last 50 years (Smith and Steenkamp 1990), and is believed to have led to an increase in the survival rate of mice during winter months, resulting in an overall population increase (Smith and Steenkamp 1990; Smith 2002). The mean volume contribution of weevil adults found in the guts of mice increased from 7% in 1979/1980 (Gleeson and Van Rensburg 1982) to 11% in 1992/1993 (Smith et al. 2002). House mice are thus considered to be responsible for the significant change in the populations of Ectemnorhinus species on MI, amounting to almost an order of magnitude decline in biomass between 1976 and 1996 (Chown et al. 2002), and a pronounced difference between population densities on MI and PEI (Crafford and Scholtz 1987).
Mice are also thought to have caused a reduction in body size of weevil species on MI relative to PEI, as frequency distributions of the size of the Ectemnorhinus species differ considerably between the islands (Chown and Smith 1993). The authors of this study noted that the situation across the two islands seemed to reflect predation by mice, but also concluded that further investigations were necessary, due particularly to taxonomic difficulties with the genus (see also Chown 1991).
Given the severity of weevil predation on MI and uncertainties on the taxonomic status of the Ectemnorhinus species on both islands (Chown 1990,1991), it is not clear whether the PEI populations alone are sufficient to ensure conservation of the two Ectemnorhinus weevil species, especially if predictions that mice predation is likely to continue escalating (Chown et al. 2002) are realized. If the same species occur on both islands and if the populations are not so dissimilar that they should be considered different management units, then the current management regime (Anonymous 1996) will suffice. An essential assumption of this regime is that PEI serves largely as a mouse-free haven for species occurring on both islands (but see contrary views in Gremmen and Smith 1999; Chown et al. 2002). However, if the species or populations differ, then management practices would have to change, and serious consideration would have to be given to the eradication of mice on MI. Thus, it is clear that resolving the status of the two currently recognized Ectemnorhinus species is important from a conservation perspective.
The aim of the present study was, therefore, to evaluate the taxonomic status of the Ectemnorhinus weevil species occurring on the PEIs, using both molecular and morphometric techniques. The COI gene, which has been successfully used to differentiate between island-bound coleopteran species (Emerson et al. 1999; Sequeira et al. 2000; Trewick 2000; Caccone and Sbordoni 2001), was selected for genetic characterization, whilst a set of 15 linear external measurements (Janse van Rensburg et al. 2003) was used for the morphometric assessment.
Materials and Methods
Study area and samples
Ectemnorhinus weevil specimens were collected over three consecutive years (April 2001–April 2003) from 28 localities (Fig. 1) on MI. Samples from PEI, which has restricted access, were collected along an altitudinal gradient (0–675 m a.s.l.) at 200 m intervals and from an additional locality with vegetation consisting largely of D. strictum, in April 2003. Coordinates for all sampling localities are summarized in Table 1. All specimens were collected by hand and preserved in absolute ethanol.
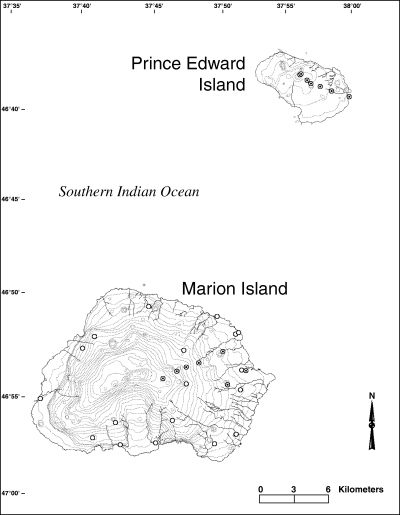
Map indicating Ectemnorhinus weevil sampling localities on Marion Island and Prince Edward Island that correspond to the coordinates summarised in Table 1. Samples collected from all localities were included in the morphometric analyses while only those samples collected from the dotted localities were included in the genetic analyses
| Sampling locality | Coordinates |
|---|---|
| MI 200 m.a.s.l. Junior's Kop | S 46°52.794′E 37°50.083′ |
| MI 400 m.a.s.l. First Red Hill | S 46°53.412′E 37°48.21′ |
| MI 600 m.a.s.l. First Red Hill | S 46°53.647′E 37°47.208′ |
| MI 800 m.a.s.l. Katedraalkrans | S 46°53.896′E 37°46.482′ |
| MI 1000 m.a.s.l. | S 46°54.29′E 37°45.375′ |
| MI Tate's Hill Pringlea | S 46°54.6′E 37°50.478′ |
| MI Albatros Lakes | S 46°53.82′E 37°51.916′ |
| PE Cave Bay | S 46°38.752′E 37°59.780′ |
| PE 200 m.a.s.l. | S 46°38.457′E 37°58.396′ |
| PE 400 m.a.s.l. | S 46°38.211′E 37°57.482′ |
| PE 600 m.a.s.l. | S 46°37.533′E 37°55.985′ |
| PE TvZB1 672 m.a.s.l. | S 46°37.590′E 37°55.891′ |
| PE Ditrichum | S 46°38.057′E 37°56.771′ |
- 1TvZB indicates samples collected at the top of Van Zinderen Bakker Peak; m.a.s.l. = metres above sea level.
For the morphometric component of the study, between 5 and 30 Ectemnorhinus specimens per locality were measured. Due to the uncertainty regarding the taxonomic status of Ectemnorhinus species on both MI and PEI, individuals were only identified as belonging to the genus, based on the generic descriptions provided by Kuschel and Chown (1995), with a priori rather than a posteriori multivariate morphometric analyses (Sneath and Sokal 1973) being used to define phenetic groupings.
Fifty-two Ectemnorhinus individuals from seven localities on both MI and PEI (Fig. 1) were analysed for the molecular component of the study. In an attempt to ensure adequate representation of species-associated feeding preferences as indicated by Chown (1990), Ectemnorhinus individuals were collected from D. strictum, A. selago and P. antiscorbutica. Individuals collected from D. strictum on MI were from an A. selago-free polar desert site near Albatross Lakes, whereas those collected from D. strictum on PEI were from a site comprising mainly of D. strictum (coordinates given in Table 1). As gut contents were not evaluated, we were, however, mindful that sampling from a particular plant species did not necessarily imply feeding preference for that plant species. Body size variation, another criterion used by Chown (1990) to distinguish between E. marioni and E. similis, was accommodated by including the extreme size classes (largest two and the smallest two individuals) per locality. A priori assignment into different species was not taken into account in subsequent molecular analyses. Ectemnorhinus viridis (Waterhouse, 1853) from Heard Island was selected as an outgroup, since it is a congener of E. marioni (Kuschel and Chown 1995).
Molecular characterization
Following rehydration of ethanol-preserved weevils with water, one to two weevil legs per specimen were frozen in liquid nitrogen before being ground and mixed with phosphate-buffered saline. DNA was extracted using a modified guanidinium thiocyanate (GuSCN)/silica-based method (Boom et al. 1990).
Published primers C1-J-1718 and TL2-N-3014 (Simon et al. 1994) were initially used to generate partial sequence data for representatives of all six weevil species currently considered to occur on MI namely, Bothrometopus elongatus (Jeannel 1953), Bothrometopus parvulus (Waterhouse 1885), Bothrometopus randi (Jeannel 1953), E. marioni (Jeannel 1940), E. similis (Waterhouse 1885) and Palirhoeus eatoni (Waterhouse 1879). As these primers generally resulted in poor quality sequences, two MI weevil-specific COI primers were designed from the aligned partial sequences, following the guidelines of Rychlik (1993). These MI weevil-specific COI primers termed GF and GR1 (Table 2) amplified a 1059 bp PCR product under the following conditions: 1× Buffer, 0.2 mM dNTP, 0.4 μM of each primer and 1 U Taq polymerase in a final volume of 50 μl containing 200 ng of template DNA. A typical temperature profile consisted of an initial denaturation step at 94°C for 90 s, followed by 40 cycles at 94°C for 22 s, 46°C for 30 s and 72°C for 1 min. PCR products were purified and DNA sequences were determined by automated cycle sequencing on an abi prismTM 3100 Analyser using the abi prism Big DyeTM Terminator version 3.0 sequencing standard.
| Name | Orientation | Sequence | T m |
|---|---|---|---|
| C1-J-1718 | Forward | 5′ GGAGGATTTGGAAATTGATTAGTTCC 3′ | 60°C |
| TL2-N-3014 | Reverse | 5′ ATTATACCGTCTAATCACGTAACCT 3′ | 58°C |
| GF-1858 | Forward | 5′ GGGACAGGTTGAACAGTTTATC 3′ | 58°C |
| GR1-2938 | Reverse | 5′ ATGTTGTTATTCTTGAAGATGAAAG 3′ | 54°C |
| GF3-2206 | Forward | 5′ GGTCACCCAGAAGTATATAT 3′ | 53°C |
| GF4-2662 | Forward | 5′ GCTGGAATAGTACAATGATT 3′ | 53°C |
| GF5-1940 | Forward | 5′ TACATATAGCAGGTGTATCATC 3′ | 54°C |
| GR5-2935 | Reverse | 5′ GTTATTCTTGAAGATGAAAGATT 3′ | 51°C |
- T m: Melting temperature, calculated using the formula; Tm = [69.3 + (0.41 × %GC)] − 650/primer length.
Internal primers termed GF3, GF4, GF5 and GR5 (Table 2) were designed from the sequences initially generated with the MI weevil-specific primers. The latter two primers were used in all subsequent cycle-sequencing reactions to generate a homologous 885 bp region of sequence data. The sequences were viewed and edited in Chromas version 1.43 (McCarthy 1996–1997) and aligned with dapsa version 4.9 (Harley 2000).
Phylogenetic analyses
Three sequence datasets were compiled, a MI dataset, a PEI dataset and a combined MI and PEI dataset. Neighbour-Joining (Saitou and Nei 1987) and Minimum Evolution (ME; Rzhetsky and Nei 1992) algorithms in mega version 2 (Kumar et al. 2001) were used to construct phylogenies for the combined dataset with nodal support being assessed by 10 000 bootstrap replications.
modeltest version 3.06 (Posada and Crandall 1998) was used to identify the model of evolution that best fits the data with parameters identified under the Akaike Information Criterion (Akaike 1974) being used for subsequent maximum likelihood analyses (ML; Felsenstein 1981). In each case, the TrN + I model with equal rates for all sites that corresponds to the general time-reversible model, GTR + I (Rodriguez et al. 1990), was selected. The proportion of invariable sites (I) and three different substitution types estimated for the dataset was as follows: I = 0.7935, rate [A-G] = 48.19, rate [C-T] = 11.65 and other rates = 1.00.
Maximum likelihood analyses were performed in paup* version 4.0b10 for Macintosh (Swofford 1999) under the above-mentioned model parameters prior to bootstrap re-sampling. Bayesian phylogenetic analyses using MrBayes version 3.0B4 (Huelsenbeck and Ronquist 2001) were performed with the same models and parameters recovered for each of the respective datasets. Analyses were initiated with random starting trees and run for 500 000 generations with Markov chains sampled every 100 generations. Of the 5000 trees obtained, 2000 were discarded as ‘burn-in’.
Parsimony analyses performed with paup* version 4.0b10 included equal weighting and differential weighting schemes such as character weighting where third base positions were given a weight of 1, and first base positions were up-weighted to 9.76923; successive weighting (Farris 1969); 6 parameter parsimony on its own and combined with both character and successive weighting (Williams and Fitch 1990).
The equality of evolutionary rates between lineages was tested using the relative rate test (Li and Bousquet 1992) in phyltest version 2.0 (Kumar 1996). In addition, the likelihood ratio test (Felsenstein 1981,1988) was performed, and log-likelihood scores obtained with and without the molecular clock enforced, were compared. Divergence times were calculated from uncorrected pair-wise values and calibrated using 2.3% nucleotide sequence divergence per million years based on the arthropod mtDNA survey of Brower (1994). Haplotype (h) and nucleotide diversities (π) were estimated for each island individually in dnasp 3.51 (Rozas and Rozas 1999). Differences in total body lengths of individuals between clades were assessed using analysis of variance (anova; Zar 1996) for PEI.
Morphometric analyses
Fifteen morphometric measurements were recorded by a single observer (LJvR) using a stereomicroscope fitted with a calibrated eyepiece micrometer. Measurements, defined and selected based on a morphometric character selection procedure followed by Janse van Rensburg et al. (2003) included: total body length (TL), pronotum breadth (PB), femur length (FL), interocular distance (O), metacoxal distance (MT), maximum breadth of elytra (EW), length of first three tarsal segments (T3), meso/metacoxal distance (MM), interantennal distance (A), mesocoxal distance (MS), femur breadth (FB), funicle segments 1, 2 and 3 (F1, F2 and F3) and rest of funicle (FR). Measurements were recorded to the nearest 0.05 mm (TL and EW), 0.03 mm (PB and FL) and 0.01 mm (O, A, F1, F2, F3, FR, T3, MS, MT, MM and FB).
For multivariate morphometric analyses, the absence of multivariate sexual dimorphism (Janse van Rensburg et al. 2003) permitted pooling of sexes for subsequent analyses. For MI, a total of 807 individuals from 28 localities, which provided adequate geographical coverage of Ectemnorhinus species, were used for morphometric analysis. A total of 240 Ectemnorhinus specimens from six localities on PEI were analyzed. Data screening revealed five outlier specimens, not considered representative of the populations. A re-examination of these specimens revealed outlier values arising from damaged parts, and to avoid the introduction of bias in the sample, they were excluded from subsequent analyses. After determining the absence of multivariate sexual dimorphism using principal components analysis (PCA), these datasets were subjected to a randomization procedure (Manly 1991), where a new dataset with an equal number of individuals as the original dataset were randomly sampled with replacement for each island to assess whether the absence of multivariate sexual dimorphism in the original dataset was significantly different from the randomly selected dataset.
Following Chimimba et al. (1999), sampled localities on MI and PEI were grouped into a number of computationally manageable geographical subsets to accommodate for the unweighted pair-group arithmetic average (UPGMA) cluster analyses for the MI (n = 807) and the combined island (n = 1047) data matrices, since the data matrices were too large for simultaneous specimen-level analyses. The results of the individual-level analyses of the geographical subsets facilitated the grouping of locality mean values in subsequent analyses that accommodated entire island data that were similar to the results of the individual-level analyses. The 28 and 24 genetically identified Ectemnorhinus individuals from MI and PEI, respectively, were included in all morphometric analyses as references in defining phenetically-derived groupings.
Multivariate analyses included PCA and UPGMA cluster analysis to assess whether species could be identified based on morphometric characters (Sneath and Sokal 1973). Canonical variates analysis (CVA) of genetically defined groupings was also undertaken (Pimentel and Smith 1986) to define phenetic groups a posteriori. The CVA was followed by a multivariate anova (manova; Zar 1996) to test for statistically significant differences between pre-defined groups. UPGMA cluster analysis was based on both Euclidean distances and product–moment correlation coefficients among operational taxonomic units (Sneath and Sokal 1973), whereas the PCA was computed from product–moment correlation coefficients among variables (Sneath and Sokal 1973). All statistical procedures were performed using statistica version 5.5 (StatSoft Inc 1995).
Results
Molecular analyses
A homologous region of 885 bp corresponding to nucleotide positions 514–1399 of the COI gene was generated for 52 PEI archipelago (PEIA) Ectemnorhinus individuals and two E. viridis outgroup specimens. All sequences have been deposited in the GenBank database under accession numbers AY762267–AY762320. For the combined dataset, 775 of the 885 sites were conserved across all 54 sequences, and 98 of the 110 variable sites were parsimony informative. The % A + T was 68.6% and the transition (ti)/transversion (tv) ratio was 6. Third base position substitutions accounted for 88.2% of the variation and the remaining 11.8% was due to the first base substitutions. Mutations at nucleotide level gave rise to five non-synonymous amino acid substitutions at codons 19, 85, 241, 279 and 289. Of the 52 PEIA Ectemnorhinus individuals sequenced, 42 had unique haplotypes. When the individuals collected from MI and PEI were pooled, nucleotide diversity (π) and haplotype diversity (h) of 0.02032 and 0.990, respectively, were obtained. From the 28 MI sequences, 22 unique haplotypes were obtained with a π of 0.01217 and an h of 0.976. The π and h for the PEI was estimated to be 0.01687 and 0.986, respectively, with 21 unique haplotypes being identified from the 24 PEI sequences generated.
Although all major clades (numbered 1–7; Fig. 2) did not have high levels of support across all methods of phylogenetic analysis utilised, clade topology was consistent across all the methods. Clade 5, which has low levels of bootstrap support in ML and ME, had high support (0.9) with Bayesian analysis. Clades 6 and 7 consisted solely of individuals collected on PEI, while clades 1 and 2 incorporated individuals from both islands. In clade 1, MI individuals ranging in size from 4.23 mm to 8.08 mm, and collected on A. selago, P. antiscorbutica and D. strictum grouped together. Similarly in clade 7, individuals collected on both A. selago and D. strictum from PEI grouped together, indicating that assignment of species according to host plant preference does not appear to hold. Clade 7 was consistently basal to clades 1–6 with the grouping of the latter clades having ML and 6-parameter parsimony bootstrap values of 80% and 92%, respectively. When the individuals in clade 7 were compared to the PEI individuals in each of the other clades, it was found that they were significantly larger [anova: F(8,34) = 9.38, P < 0.05]. Sequence divergence values of 2.2% were observed between clade 7 and clades 1–6 when all the individuals from both MI and PEI were pooled, while sequence divergence values of 1.5% were observed between clade 7 and clades 1–6 when only the individuals collected on PEI were used.

Minimum evolution (ME) tree based on 885 base pairs of the mitochondrial COI gene and inferred using the Tamura–Nei distance correction algorithm for the combined data set. For each specimen, the sample number is indicated followed by the body length measurement, plant species it was collected from, locality, sex and the island of origin (where ‘MI’ denotes Marion Island and ‘PEI’ indicates Prince Edward Island). Nodal support was assessed by 10 000 bootstrap replications, with only those bootstrap values ≥50 being indicated next to the relevant nodes. Bootstrap values obtained from maximum likelihood (ML) analysis (based on 100 bootstrap replications) are indicated in brackets. TvZB indicates samples collected at the top of Van Zinderen Bakker Peak at an elevation of 672 m above sea level
No significant rate heterogeneity was found among the substitution rates at P < 0.05 according to both relative rate and likelihood ratio tests. Therefore, it was concluded that Ectemnorhinus individuals from the PEIs do not evolve at markedly different rates and a molecular clock based on that calibrated for arthropods (Brower 1994) could, therefore, be imposed. Consequently, from this simplistic dating approach, it was found that the Ectemnorhinus weevil lineages coalesced at approximately 0.49 million years ago (MYA) and 0.33 MYA on PEI and MI, respectively.
Morphometric analyses
Principal component analyses based on both original and randomly selected data for both MI and PEI, showed no grouping of the sexes indicating the absence of multivariate morphometric sexual dimorphism within datasets.
All multivariate morphometric analyses of both individual and combined island datasets as well as individual-level analyses and those based on mean values were similar, and are best illustrated by PCA results. The PCA of the combined MI and PEI dataset (encompassing all size classes) showed a distinct separation neither with reference to the two currently recognized Ectemnorhinus species nor with regard to island of origin (Fig. 3). Lack of separation, however, seems to be largely confounded by a large degree of body size variation among individuals from the two islands. This is reflected by the high positive loadings of the measurements on the first PCA axis that accounted for 86.08% of the total variance (Table 3). A PCA restricted to the 52 genetically identified specimens from both MI and PEI indicated no separation based on either body size or shape in the MI sample. However, two groups are observed for PEI Ectemnorhinus weevils, with only a single Ectemnorhinus individual from group A (encompassing individuals of clades 1, 2, 3 and 6; Fig. 2c), falling within Ectemnorhinus group B, comprising individuals from clade 7 (Fig. 4a). The 52 genetically identified specimens showed a more pronounced separation between Ectemnorhinus group A, B and the MI Ectemnorhinus specimens on the first CVA axis. A shape related separation was observed on the second CVA axis between the MI Ectemnorhinus species and Ectemnorhinus group A from PEI (Fig. 4b). A manova showed a statistically significant phenetic difference between these pre-defined groupings [F(30,62) = 4.34, P < 0.0001].
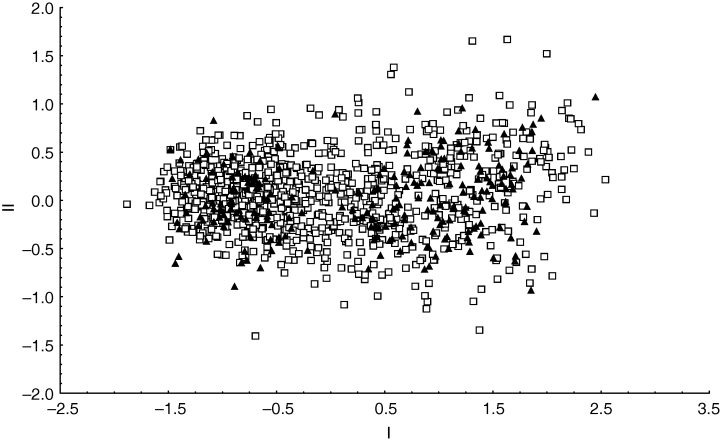
Components I and II from a principal components analysis of Ectemnorhinus species collected from both Marion Island (open squares) and Prince Edward Island (closed triangles), indicating no distinct separation with reference to the two currently recognized Ectemnorhinus species nor with regard to island of origin
| Variable1 | Marion Island | Prince Edward Island | Marion and Prince Edward Island | |||
|---|---|---|---|---|---|---|
| Principal components | Principal components | Principal components | ||||
| I | II | I | II | I | II | |
| TL | 0.974 | −0.029 | 0.989 | 0.057 | 0.976 | −0.012 |
| EW | 0.956 | −0.036 | 0.976 | 0.041 | 0.960 | −0.038 |
| PB | 0.946 | −0.042 | 0.986 | 0.080 | 0.952 | −0.066 |
| O | 0.952 | −0.009 | 0.977 | −0.006 | 0.952 | −0.058 |
| A | 0.897 | −0.012 | 0.957 | 0.090 | 0.906 | −0.065 |
| F1 | 0.940 | −0.047 | 0.959 | −0.020 | 0.944 | −0.041 |
| F2 | 0.938 | −0.059 | 0.961 | −0.051 | 0.941 | −0.062 |
| F3 | 0.884 | −0.052 | 0.921 | −0.115 | 0.890 | −0.093 |
| FR | 0.931 | −0.054 | 0.938 | −0.064 | 0.930 | −0.013 |
| T3 | 0.956 | −0.043 | 0.807 | −0.517 | 0.949 | −0.010 |
| MS | 0.555 | 0.830 | 0.833 | 0.258 | 0.712 | 0.698 |
| MT | 0.936 | 0.044 | 0.945 | 0.071 | 0.937 | 0.082 |
| MM | 0.875 | −0.071 | 0.950 | 0.089 | 0.889 | −0.045 |
| FL | 0.986 | −0.061 | 0.988 | 0.020 | 0.986 | −0.054 |
| FB | 0.958 | −0.023 | 0.973 | 0.011 | 0.960 | −0.055 |
| % trace | 84.23 | 4.78 | 89.37 | 2.58 | 86.08 | 3.54 |
- 1TL, Total body length; PB, pronotum breadth; FL, femur length; O, interocular distance; MT, metacoxal distance; EW, maximum breadth of elytra; T3, length of first three tarsal segments; MM, meso/metacoxal distance; A, interantennal distance; MS, mesocoxal distance; FB, femur breadth; F1, F2 and F3, funicle segments 1, 2 and 3; FR, rest of funicle.
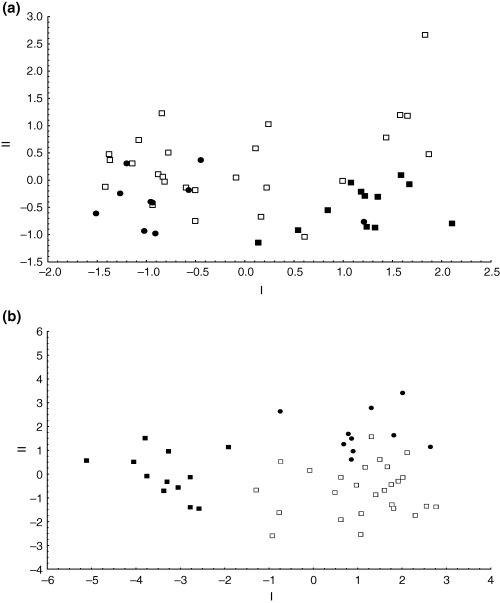
(a) Components I and II from a principal components analysis based on genetically-identified individuals. No separation is observed in the Marion Island sample (open squares), while two groups are observed for samples collected on Prince Edward Island, Ectemnorhinus group A (closed circles; individuals of clades 1, 2, 3 and 6, Fig. 2c) and Ectemnorhinus group B (closed squares; individuals from clade 7). (b) Components I and II from a canonical variates analysis (CVA) showing a more pronounced separation of genetically-identified individuals from Prince Edward Island, Ectemnorhinus group A (closed circles) and Ectemnorhinus group B (closed squares) with no separation in the Marion Island samples (open squares)
A PCA of MI samples (represented by all size classes) showed a considerable degree of phenetic variation, but no separation based on either size or shape variables (Fig. 5a). Principal component I (84.23% of the total variance) had high positive loadings on all measurements (Table 3), highlighting the importance of size variation. No separation was observed on the second (Table 3) and subsequent PCA axes.
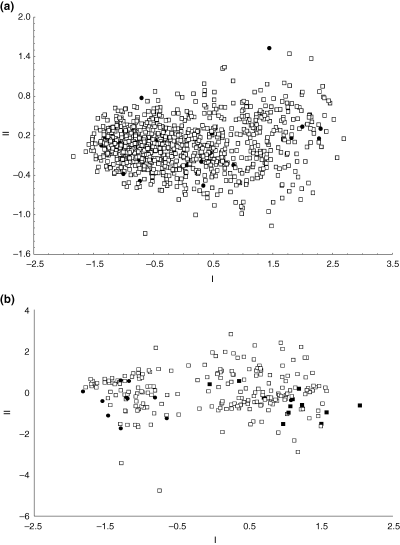
(a) Components I and II from a principal components analysis of Ectemnorhinus species collected from Marion Island indicate no separation based on either size or shape variables. Genetically-identified individuals are indicated by black circles. (b) Components I and II from a principal components analysis of Ectemnorhinus species collected from Prince Edward Island indicate separation between two group based on size variation. Genetically-identified Ectemnorhinus group A (closed circles) and Ectemnorhinus group B (closed squares) are indicated
A PCA, comprising all size classes and including genetically identified PEI samples as references (Fig. 5b), however, showed two phenetic groupings for the Ectemnorhinus species. Only a single genetically identified individual from the Ectemnorhinus group A clustered with individuals of Ectemnorhinus group B. Separation between the two groups is based on a size-related variation rather than a shape-related variation, as is shown by PCA axis I (89.37%), which generally has high positive loadings on measurements analysed (Table 3).
Discussion
Assessment of the taxonomic status of weevil species originally described from MI was undertaken using a combined molecular and morphometric approach. The COI gene phylogeny identified seven recently diverged, well-supported clades on the PEIA, but gave no support for the presence of the two species, designated E. marioni and E. similis by Chown (1990) from MI. When investigating individuals collected from MI, it was found that none of the clades in the molecular phylogeny containing MI individuals displayed clustering on the basis of body size or according to plant species from which they were collected. In addition, multivariate analyses of the MI sample showed no separation of Ectemnorhinus individuals according to either body size or body shape variation. The molecular analyses, therefore, suggest that previous morphologically- and ecologically-defined distinguishing characteristics for MI Ectemnorhinus weevils (Crafford et al. 1986; Chown and Scholtz 1989; Chown 1990) do not consistently correspond to the clades identified using genetic markers. Moreover, the current morphometric analysis confirms that size variation is a major source of difficulty when attempting to establish species limits within the genus (see Brown 1964; Kuschel 1970,1971; Crafford et al. 1986; Chown and Scholtz 1989; Chown 1990,1991).
In contrast to the results obtained for the individuals collected on MI, two major, size-distinct clades were discernible for the individuals collected on PEI from both the genetic (clade 7 as opposed to the PEI individuals collectively grouped within clades 1–6) and the morphometric analyses. A sequence divergence value of 1.5% was observed between the two major PEI clades that corresponds well with the intra-generic Kimura-2-parameter genetic distances of 1.5% and 2.1% reported for arthropods from other island systems (Trewick 2000). This, together with the high levels of bootstrap support for the two size- and genetically-distinct clades, suggest that the present recognition of two species distinguishable on the basis of size is supported on PEI. The presence of two size-discrete groupings of Ectemnorhinus individuals on PEI was also confirmed by the morphometric analyses, where all multivariate analyses indicated the presence of two size-related phenetic groupings. The single Ectemnorhinus individual from group A, which clustered within the Ectemnorhinus group B assemblage is indicative of the extent of body size variation within Ectemnorhinus species.
In light of these results, a re-evaluation of the species status of the PEIA Ectemnorhinus weevils is necessary. Because individuals from PEI have not been used in formal taxonomic assessments (i.e. they have not been physically identified and labelled, with a corresponding description or specimen listing in the literature), the larger PEI-restricted weevils that constitute clade 7 have been designated Ectemnorhinus kuscheli (see Appendix for a full species description). Likewise, because neither the phylogenetic nor the morphometric data support the existence of two species on MI, E. similis and E. marioni from MI, together with those weevils from PEI that group within clades 1–6, have been synonymized, under the name E. similis, which has priority.
Although our data suggest the presence of two species on PEI and only one on MI, it is important to note that two species, E. marioni and E. similis, were originally described from MI. The critical question that may now be posed relates to the apparent disappearance of one species of Ectemnorhinus on MI. Was there only one Ectemnorhinus species to begin with on MI or is it possible there were indeed two species 65 years ago when Jeannel (1940) first described E. marioni as a second species distinct from E. similis?
The first hypothesis for the observed difference in weevil assemblage between the two islands is the loss of one of the originally described Ectemnorhinus species from MI. One possible cause for the loss could be that the reduction in body size of E. similis through size-selective predation by mice (Chown and Smith 1993; Smith et al. 2002) which would have removed the size-induced reproductive barrier that was proposed on grounds of the significant relationship between female and male body size in in-copula pairs observed by Chown (1990) on MI. This scenario would allow the two previously recognized species to interbreed. In addition to mice predation, it is also possible that climate change may play a role, as temperature on the PEIs has increased by an average of 0.04°C per year since the late 1960s (Smith 1991). It is well-known that in arthropods, and indeed most invertebrates, increasing developmental temperatures often lead to a decline in body size (Atkinson 1994). Moreover, if temperatures increase to such an extent that generation time is much shorter than season length, then additional declines in body size with increasing temperature can be expected (Kozłowski et al. 2004). Investigations of weevil species on MI have shown that long-term warming (at least since the 1960s) may have led to on-going declines in body size, accompanied by a secondary, significant influence of mouse predation (Janse van Rensburg 2005). These changes in the environment may select for smaller individuals within the populations, leading eventually to introgression.
The second hypothesis is that there was originally only one Ectemnorhinus species on MI that was erroneously described twice. The original descriptions of E. similis by Waterhouse (1885) and E. marioni by Jeannel (1940) indicate that there are distinct differences between the two species. The controversial taxonomic status of these two species is, however, a clear indication of the difficulty taxonomists faced in the past when using morphological characteristics alone. One possible reason for the different status of Ectemnorhinus species between the two islands may also be due to differences in glaciation histories, as MI was extensively glaciated whilst PEI was not (Verwoerd 1971). As a result, weevils on PEI would have had longer exposure to vascular plants as an additional, more nutritious food source to bryophytes, than those on MI. This may have given rise to two species, a smaller one with a preference for bryophytes and a larger one with a preference for angiosperms, as suggested by Chown (1990). Another possible explanation for the dissimilar status of these island species may be due to the differences in coalescence times. Results from this study indicate that the Ectemnorhinus weevils lineages coalesced at approximately 0.49 MYA on PEI, soon after the islands emerged, while coalescence on MI was dated to have occurred approximately 0.16 million years later.
Whatever the underlying cause(s), there is no doubt that a marked difference in the species exists between the islands with PEI harbouring both the newly described E. kuscheli and E. similis and MI harbouring only E. similis. The two islands also differ considerably with respect to the genetic composition of their respective populations of E. similis, with each island having numerous island-unique haplotypes and only one haplotype of E. similis being shared between the islands. PEI may thus not be considered as a safe haven for E. similis populations that are on MI. The Ectemnorhinus weevils from the two islands are distinct and should therefore, be considered as different management units. Even if the PEI populations were not drastically different from those on MI, and the MI populations were completely removed by mice predation, the restocking of an island with specimens from another island may not be appropriate. Management regimes at the PEIs should instead focus on preserving the genetic variation unique to MI. There is an urgent need to explore the possibilities of either eradicating or drastically controlling mice on MI as a long-term goal. In the interim, the current policy of restricting human visits to PEI should be strictly maintained.
Acknowledgements
The authors wish to thank C. Lyal of the Natural History Museum for provision of the Ectemnorhinus lectotype image, and U. Kryger and C.H. Scholtz (University of Pretoria) for translation of the abstract and assistance with the species description, respectively. The referees are thanked for their constructive comments. The Directorate Antarctica and Islands of the South African Department of Environmental Affairs and Tourism funded this work and provided logistic support at MI via the South African National Antarctic Programme. This material is based on the work supported by the National Research Foundation South African National Antarctic Programme under Grant number GUN2068301. Any opinions, findings and conclusions or recommendations expressed in this material are those of the author(s) and therefore the NRF does not accept any liability in regard thereto.
Appendix
A Description of Ectemnorhinus kuscheli n. sp.
Description: Length: 4.69–8.15 mm (holotype: 7.69 mm with left front leg missing). A large Ectemnorhinus species. Brown to black; antennae, abdomen and base of femora brown to black. Rostrum medially depressed or sulcate. Scrobes below antennal tubercle narrowly exposed. Epistome distinctly asymmetrical due to right side projecting further forward. Mandibles rather slender, moderately curved on outer edge, lacking cuSPS or scar. Labial palp 2-segmented. Pronotum medially not sulcate. Each elytron rounded at apex leaving part of tergites exposed. Interstriae 1, 3 and 5 not subcostate or uneven. Elytra with distinct erect setae on dorsum although setae sometimes very short. Hind wings very rudimentary, less than 0.1× length of elytron. Inner flange of elytra rudimentary. Hind margin of prothorax behind coxae truncate. Metepisternal structure obsolete. Tibiae without mucro. Claw segment shorter than other segments combined. Tarsomeres 1–3 with adhesive pads on sole. Lobes of tarsomere 3 of unequal size. Tergite 8 of female exposed beyond 7. Blade of sternite 8 of female not transversely quadrangular. Internal sac with an elongate appendage on each side of gonopore. Gonopore basal on internal sac. Proximal hemisternites with struts. Setae on distal hemisternites long. Bursa without sclerite. Spermathecal duct insertion on bursa median or terminal. Spermathecal gland very long, much longer than duct. Proventricular blades rudimentary, without bristles.
Diagnosis: Identical to E. similis in external morphology and internal structure (See Chown 1990). Statistically larger than E. similis. Ecology, habitat and feeding preference identical to E. similis. Significant difference between E. similis and E. kuscheli are to be found in genetic evidence (Fig. 2). Four nucleotide positions can be used as diagnostic characters to distinguish between the two species, namely:
- (i)
Position 568 of the COI gene: E. kuscheli has a T while E. similis has an A.
- (ii)
Position 997 of the COI gene: E. kuscheli has a T while E. similis has a C.
- (iii)
Position 1063 of the COI gene: E. kuscheli has a C while E. similis has a T.
- (iv)
Position 1297 of the COI gene: E. kuscheli has either an A or G (R) while E. similis has either a C or T (Y).
Distribution: Prince Edward Island.
Etymology: This new species is named after G. (Willy) Kuschel in recognition of his work on sub-Antarctic weevils.
Type material examined:
Holotype:♀, South Africa, Prince Edward Island, on Azorella selago, 600 m above sea level, 46°37.533′S 37°55.985′E, GenBank no. AY762310, voucher no. 311-7, date collected April 2003, collector G.C. Grobler. Deposited in the Natural History Museum, London, United Kingdom.
Paratypes:♂, South Africa, Prince Edward Island, on Azorella selago, Top of Van Zinderen Bakker Peak, 46°37.590′S 37°55.891′E, GenBank no. AY762315, voucher no. 321-2, date collected April 2003, collector G.C. Grobler. Deposited in the Natural History Museum, London, United Kingdom.
♀, South Africa, Prince Edward Island, on Azorella selago, 400 m above sea level, 46°38.211′S 37°57.482′E, GenBank no. AY762318, voucher no. 310-3, date collected April 2003, collector G.C. Grobler. Deposited in the Natural History Museum, London, United Kingdom.
♀, South Africa, Prince Edward Island, on Azorella selago, 400 m above sea level, 46°38.211′S 37°57.482′E, GenBank no. AY762316, voucher no. 309-7, date collected April 2003, collector G.C. Grobler. Deposited in the Natural History Museum, London, United Kingdom.
♀, South Africa, Prince Edward Island, on Azorella selago, 600 m above sea level, 46°37.533′S 37°55.985′E, GenBank no. AY762309, voucher no. 311-10, date collected April 2003, collector G.C. Grobler. Deposited in the Transvaal Museum, Pretoria, South Africa.
♀, South Africa, Prince Edward Island, on Azorella selago, 200 m above sea level, 46°38.457′S 37°58.396′E, GenBank no. AY762311, voucher no. 316-6, date collected April 2003, collector G.C. Grobler. Deposited in the Transvaal Museum, Pretoria, South Africa.
♂, South Africa, Prince Edward Island, on Poa cookii, Cave Bay, 46°38.752′S 37°59.780′E, GenBank no. AY762312, voucher no. 304-13, date collected April 2003, collector G.C. Grobler. Deposited in the Transvaal Museum, Pretoria, South Africa.
♀, South Africa, Prince Edward Island, on Azorella selago, 200 m above sea level, 46°38.457′S 37°58.396′E, GenBank no. AY762314, voucher no. 316-15, date collected April 2003, collector G.C. Grobler. Deposited in the National Insect Collection (SANC), Pretoria, South Africa.
♀, South Africa, Prince Edward Island, on Ditrichum strictum, 46°38.057′S 37°56.771′E, GenBank no. AY762313, voucher no. 305-1, date collected April 2003, collector G.C. Grobler. Deposited in the National Insect Collection (SANC), Pretoria, South Africa.
♀, South Africa, Prince Edward Island, on Azorella selago, 400 m above sea level, 46°38.211′S 37°57.482′E, GenBank no. AY762317, voucher no. 310-4, date collected April 2003, collector G.C. Grobler. Deposited in the National Insect Collection (SANC), Pretoria, South Africa.




