Biochemical and DNA markers yield strikingly different results regarding variability and differentiation of roe deer (Capreolus capreolus, Artiodactyla: Cervidae) populations from northern Germany
Stark unterschiedliche Ergebnisse zwischen biochemischen und DNA-Markern bei der Analyse von Variabilität und Differenzierung von norddeutschen Rehen (Capreolus capreolus)
Abstract
enThree mainland and two island roe deer (Capreolus capreolus) populations with a total sample size of 105 individuals from Schleswig–Holstein, northern Germany, were analysed with regard to genetic variability within and differentiation among populations as revealed by eight allozyme loci known to be polymorphic in roe deer, eight microsatellite loci and 404 bp of the mitochondrial control region. Surprisingly, the allozymes were completely monomorphic, but microsatellite and control region variability were high. Hypotheses as to demographic reasons for the variability patterns found, including bottlenecks, founder effects and translocations, are put forward. There were no statistically significant differences between the island and the mainland populations in terms of genetic variability as measured by expected heterozygosity, inbreeding coefficient and allelic richness. The correlations of the various variability indices were not statistically significant after Bonferroni correction. Nevertheless, there was a clear tendency for differentiation indices to yield concordant results for microsatellite and mitochondrial markers.
Zusammenfassung
deInsgesamt 105 Rehe (Capreolus capreolus) aus drei Festland- und zwei Inselpopulationen in Schleswig–Holstein, Norddeutschland, wurden im Hinblick auf genetische Variabilität innerhalb und Differenzierung zwischen den Populationen untersucht. Die verwendeten molekularen Marker waren acht Alloenzymloci, acht Mikrosatelliten sowie 404 bp der mitochondrialen Kontrollregion. Die Mikrosatelliten und die Kontrollregion zeigten hohe Variabilitätswerte, aber die Alloenzyme waren überraschenderweise vollkommen monomorph. Mögliche demographische Ursachen für die gefundenen Variabilitätsmuster, darunter Bottlenecks, Gründereffekte und Translokationen, werden diskutiert. Es gab keine statistisch signifikanten Unterschiede in der erwarteten Heterozygotie, dem Inzuchtkoeffizienten oder der Allelic Richness zwischen den Inselpopulationen einerseits und den Festlandbeständen andererseits. Die Korrelationen zwischen den verschiedenen Variabilitätsindizes waren niedrig; dennoch bestand eine deutliche Tendenz zu übereinstimmenden Befunden zur Differenzierung zwischen den Populationen auf der Basis nukleärer und mitochondrialer Marker.
Introduction
The European roe deer (Capreolus capreolus Linnaeus, 1758) is the most common and widespread ungulate species in Europe with about 15 million heads in central Europe alone (Mitchell-Jones et al. 1999). Being a popular game species of the man-shaped cultural landscape – yearly bags in Germany amount to about 1 million (Mitchell-Jones et al. 1999) – the roe deer is suffering a variety of anthropogenic influences such as selective hunting, translocations and habitat fragmentation.
Roe deer genetics has been extensively analysed with regard to genetic variability and population differentiation (e.g. Hartl et al. 1991, 1993; Lorenzini et al. 1997; Milosevic-Zlatanovic et al. 1997; Wang et al. 2002), phylogeography (e.g. Vernesi et al. 2002; Lorenzini et al. 2003; Randi et al. 2004), conservation and management (e.g. Lorenzini et al. 2002; Randi et al. 2004), behavioural aspects (e.g. Wang and Schreiber 2001; Nies et al. 2005) and habitat fragmentation (e.g. Wang and Schreiber 2001; Coulon et al. 2004). The molecular markers most frequently used in these studies were allozymes, microsatellites and sequences of the mitochondrial control region, all known to be powerful tools of comparisons at the population level. While in a couple of roe deer studies two of these markers were analysed (allozymes and microsatellites: Wang and Schreiber 2001; allozymes and sequences: Nies et al. 2005; microsatellites and sequences: Randi et al. 2004), to our knowledge, there is as yet no analysis of roe deer populations (and only relatively few dealing with other taxa, for a study on red and sika deer see Abernethy 1994) using both nuclear allozymes and microsatellites and mitochondrial markers. However, this would be a useful approach perhaps uncovering the different capacities of the three marker systems with regard to detecting genetic variability within and differentiation among populations. The general comparability of nuclear and mitochondrial data has been questioned by studies yielding contradictory results for these markers (e.g. Cathey et al. 1998; Zachos et al. 2003; Feulner et al. 2004). Game species like cervids could be affected particularly strongly by these differences because, apart from selective hunting of males, translocations aiming at trophy improvement may have been male-biased, which would have led to an even greater gap between diploid nuclear and maternally inherited haploid mitochondrial markers.
In species or populations relevant for conservation or (economical) management, it is of high importance to correctly assess the genetic variability and similarity of management units. Variability, however, may be strongly marker-dependent as shown by Feulner et al. (2004) for Serbian red deer where 53 out of 54 individuals showed the same mitochondrial control region haplotype whereas in terms of microsatellites, variability was not or only slightly reduced relative to the other populations studied.
These results show the value of comparative approaches based on the various marker systems frequently used in population genetic research. In the present study, we analysed 105 roe deer from five German populations with regard to their variability and differentiation at eight allozyme and eight microsatellite loci as well as 404 bp of the mitochondrial control region and carried out correlation analyses of genetic distances and variability indices based on nuclear and mitochondrial data to document the extent to which the different methodological approaches yield matching results.
Materials and Methods
Populations studied
Altogether 105 roe deer specimens from five different populations in Schleswig–Holstein, northern Germany, were analysed (Fig. 1, for sample sizes see Table 1). Two of the populations were island stocks (Foehr, FO; Fehmarn, FM) while the other three were mainland populations (Nordfriesland, NF; Schleswig, SL; Rantzau, RA). The two island populations were artificially founded in the 1930s with five specimens from an area near Cologne and Dueren in western Germany on Foehr and with eight Danish founders (three males and five females) on Fehmarn. On Foehr, however, one roe buck and a doe died shortly after their arrival, leaving a founder population of only two bucks and one doe (Rieck 1956; Niethammer 1963; Zachos et al. in press). According to local authorities and hunters, there have never been any additional introductions to Fehmarn while on Foehr, 15 Danish roe deer (seven males and eight females) were released in the 1950s and 1970s, two of which, however, died shortly after their introduction to the island.
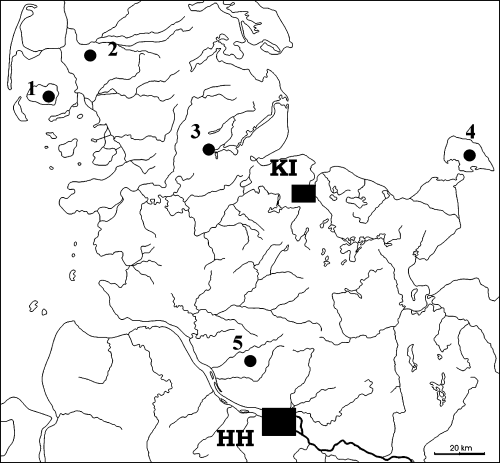
Map of northern Germany showing the geographical location of the populations studied. FO: Foehr; NF: Nordfriesland; SL: Schleswig; FM: Fehmarn; RA: Rantzau; HH: Hamburg; KI: Kiel
| Population | N | H E | H O | F IS | AR | HD | π (%) |
|---|---|---|---|---|---|---|---|
| FO | 18 (16) | 0.74 | 0.55 | 0.239 | 6.1 | 0.892 | 1.533 |
| NF | 22 (23) | 0.78 | 0.64 | 0.180 | 7.8 | 0.834 | 0.547 |
| SL | 16 (16) | 0.76 | 0.55 | 0.271 | 6.4 | 0.650 | 0.223 |
| FM | 26 (26) | 0.74 | 0.58 | 0.213 | 6.5 | 0.729 | 0.723 |
| RA | 23 (23) | 0.79 | 0.64 | 0.187 | 7.9 | 0.636 | 0.253 |
- H E and HO: expected and observed heterozygosity; FIS: inbreeding coefficient; AR: allelic richness; HD: haplotype diversity; π: nucleotide diversity. HE, HO, FIS and AR refer to microsatellite loci, HD and π to the mitochondrial sequences. HE, HO and AR are calculated over all eight loci. N: sample size for microsatellite and (in parentheses) mitochondrial sequence analyses.
Allozymes
Allozymes are enzyme variants encoded by different alleles at the same locus (Harris 1980). Eight loci known to be polymorphic in roe deer from earlier studies (e.g. Hartl et al. 1991,1993; Hewison 1995; Lorenzini et al. 1997; Wang and Schreiber 2001) were analysed. Preparation of tissue extracts, horizontal starch gel electrophoresis and enzyme-specific staining procedures were performed as described in Hartl and Höger (1986) and Grillitsch et al. (1992). The eight loci studied were (abbreviation and EC number given in parentheses): acid phosphatase (Acp, 3.1.3.2), adenylate kinase (Ak, 2.7.4.3), phosphoglucose isomerase (Pgi or Gpi, 5.3.1.9), isocitrate dehydrogenase (Idh, 1.1.1.42), lactate dehydrogenase (Ldh, 1.1.1.27), malate dehydrogenase (Mdh, 1.1.1.37), mannose-6-phosphate isomerase (Mpi, 5.3.1.8) and phosphoglucomutase (Pgm, 2.7.5.1). Due to the complete lack of any polymorphisms (see Section ‘Results’), no further analysis of the data was carried out.
Microsatellites
Microsatellites are short tandem-repetitive DNA units with repeat lengths of 1–6 bp. Mutation rates are very high leading to high allele numbers with the various alleles differing in repeat unit number and hence in length (see Goldstein and Schlötterer 1999). The 105 roe deer were genotyped for eight polymorphic microsatellite loci: OarFCB304 (Buchanan and Crawford 1993), RT1, RT7 (Wilson et al. 1997), ILSTS008, ILSTS058 (Kemp et al. 1995), NVHRT16, NVHRT21 and NVHRT24 (Røed and Midthjell 1998). DNA extraction, PCR amplification and length analysis were conducted as described in Zachos et al. (2003). Annealing temperatures for the PCR were as given in the cited literature but were reduced by 2°C in case of initial PCR failure. The lowered temperature results in an increased probability of primer annealing even in the case of mutations in the binding site sequence. The allele data were tested for linkage disequilibria between any two of the loci studied using the programme Fstat (Goudet 1995). The probability of null alleles being present in frequencies deleterious to our analyses was considered to be low because of the lack of complete PCR failures. Such failures occur in individuals homozygous for a null allele, and their absence suggests low null allele frequencies if there are any at all. Expected (HE) and observed (HO) heterozygosities as well as significant deviations from Hardy–Weinberg equilibrium (HWE) for single loci were calculated and tested with Arlequin (Schneider et al. 2000). Arlequin was also used for calculating the overall fixation index FST and RST (a microsatellite-specific analogue of FST), yielding the proportion of the total genetic diversity accounted for by the differentiation among (as opposed to variation within) populations, as well as for testing population differentiation on the basis of pairwise FST values. Population differentiation was further quantified with Nei's (1978) genetic distances adjusted to small sample sizes and chord distances (Cavalli-Sforza and Edwards 1967), both calculated with the software Genetix (Belkhir 2000), and by counting private alleles (alleles exclusive to only one of the populations studied, Slatkin 1985). In order to obtain a measure of allelic diversity (mean number of alleles per locus) independent of sample sizes, allelic richness values were calculated with Fstat by adjusting measures of alleles per locus to the smallest number of individuals typed for a locus in a sample (in our case, 16). Fstat was also used to calculate the inbreeding coefficient FIS for each of the five populations and to test for significant differences in FIS, HE and allelic richness between the island group (FO and FM) and the mainland group of populations (NF, SL and RA). Tests for recent bottlenecks in the populations studied were carried out with the Bottleneck software (Cornuet and Luikart 1996) using, as recommended for microsatellite data by the authors, the Wilcoxon test of deviation from mutation-drift equilibrium under the two-phase-model of microsatellite evolution with 70% of the mutations following the strict Stepwise Mutation Model and 30% being multistep changes. This approach is based on the fact that recently bottlenecked populations often show a heterozygosity excess at selectively neutral loci relative to expectations under mutation-drift equilibrium. The reason for this excess is that allelic diversity is reduced faster than heterozygosity during a bottleneck leading to a relative deficiency in allele number.
As for the mitochondrial DNA (mtDNA) and correlation analyses, Bonferroni corrections were applied whenever multiple tests were carried out.
Mitochondrial DNA
The mitochondrial control region, also known as the D-loop, is a part of the mtDNA that comprises a conserved central region flanked by two highly variable peripheral domains which yield sufficiently high numbers of different haplotypes for studies at the intraspecific level (cf. Douzery and Randi 1997). Four hundred and four base pairs of the control region were sequenced in 104 individuals with an ABI 377 automatic sequencer using a modified ProL (5′-CAGTCTCACCATCAACCCCCAAAGC-3′) and a DLH primer (5′-CCTGAAGTAAGAACCAGATG-3′) (cf. Nies et al. 2005). PCR amplification was conducted as described in Zachos et al. (2003) with an annealing temperature of 58°C. Sequences were aligned using BioEdit version 5.0.9 (Hall 1999). Haplotype and Jukes-Cantor corrected nucleotide diversities (the latter taking into account not only the number of different haplotypes but also the amount of difference between them) were calculated with the DnaSP software (Rozas and Rozas 1999). In addition, Jukes-Cantor corrected net nucleotide diversities were calculated, also with DnaSP, as a pairwise measure of genetic distance between populations. This parameter is mathematically similar to the nucleotide diversity and can be regarded as a distance between two populations corrected for the variation within these populations. Overall and pairwise FST-values were calculated with Arlequin considering not only haplotype frequencies but also the differences between them as estimated by Kimura-2-parameters. According to the intrinsic transition-transversion ratio of the combined data set, transversions were weighted 7.66-fold over transitions in these calculations. Since some of the statistical programmes do not consider deletions, they were treated as transversions in all analyses in order not to lose informative sites.
Correlation analyses
Relationships between variability indices (expected heterozygosity, inbreeding coefficient, allelic richness, haplotype and nucleotide diversity) were tested by means of Spearman rank correlations using the SPSS software version 10.0. Mantel tests for pairwise correlations among genetic distances based on microsatellite and sequence data were performed with the programme NTSYS (Rohlf 1993).
Results
Allozymes
All scorable allozymes of the eight loci (PGM-2 was not scorable because of unequivocal zymogrammes) were completely monomorphic in the roe deer studied. Any further quantitative comparisons using variability parameters were thus rendered impossible.
Microsatellites
All loci were polymorphic yielding between 9 and 18 alleles. There was no significant linkage disequilibrium between the loci. Significant deviations from HWE occurred in each population for single loci. The values of expected and observed heterozygosity and allelic richness as well as the inbreeding coefficients are shown in Table 1.
The total numbers of alleles ranged from 50 (FO) to 68 (NF), but of course, this number is dependent upon sample size. The number of private alleles was high: 39 (34.2%) out of a total of 114 alleles over all populations. SL showed the lowest proportion of private alleles (5.9% of all its alleles were exclusive), the respective values for the other four populations were as follows: FO 12.0%, NF 10.3%, FM 19.7% and RA 16.9%.
There was no statistically significant difference between the island and the mainland populations with regard to expected heterozygosity, allelic richness or inbreeding coefficient.
Overall genetic differentiation was 7.04 (FST) and 7.68% (RST), respectively, meaning that 7–8% of the total genetic variation was accounted for by differences among populations. All populations were significantly differentiated from one another as shown by pairwise FST-values significantly different from 0 (Table 2). Nei and chord distances are shown in Table 3.
| FO | NF | SL | FM | RA | |
|---|---|---|---|---|---|
| FO | – | 9.868 | 8.757 | 7.713 | 9.064 |
| NF | 48.408 (0.876) | – | 4.044 | 4.116 | 6.944 |
| SL | 54.521 (1.051) | 8.775* (0.043) | – | 6.605 | 7.324 |
| FM | 42.615 (0.750) | 24.913 (0.212) | 37.454 (0.326) | – | 6.954 |
| RA | 57.942 (1.049) | 25.742 (0.139) | 21.221 (0.065) | 41.899 (0.363) | – |
- The asterisk (*) denotes the only non-significant FST-value.
| FO | NF | SL | FM | RA | |
|---|---|---|---|---|---|
| FO | – | 0.381 | 0.298 | 0.247 | 0.345 |
| NF | 0.078 | – | 0.141 | 0.134 | 0.298 |
| SL | 0.082 | 0.060 | – | 0.219 | 0.290 |
| FM | 0.079 | 0.056 | 0.075 | – | 0.254 |
| RA | 0.087 | 0.074 | 0.079 | 0.070 | – |
The results of the bottleneck tests did not show any significant heterozygosity excess for the five populations (p-values ranged from 0.125 to 0.727).
Mitochondrial DNA
The 104 roe deer analysed yielded 27 different haplotypes with 26 variable sites: 23 transitions, one transversion and two deletions (Table 4). Haplotypes 1 and 3 were the most common, both present in four of five populations, at least partly in high numbers (Table 5). None of the haplotypes found occurred in all populations but 23 of the 27 haplotypes (85.2%) were exclusive to one of the five populations (private haplotypes). In the FO population, nine haplotypes were found eight of which (88.9%) were private haplotypes. The respective values for the other populations were as follows: NF: five (55.6%) private out of nine haplotypes, SL: two out of five (40%), FM: six out of eight (75%) and RA: two out of four (50%). It has to be stated, however, that most of the private haplotypes occurred in low frequencies (16 of the 23 were only found once), which makes haplotype 2 even more remarkable. This haplotype, exclusive to RA, was found in 11 of the 23 sampled individuals of that population.
| Haplotype | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 3 | 3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 5 | 6 | 8 | 8 | 1 | 5 | 5 | 6 | 1 | 1 | 2 | 2 | 2 | 3 | 3 | 4 | 4 | 5 | 5 | 6 | 8 | 1 | 3 | |
| 0 | 1 | 2 | 9 | 2 | 1 | 6 | 9 | 0 | 9 | 2 | 5 | 8 | 2 | 3 | 4 | 1 | 7 | 1 | 3 | 3 | 7 | 9 | 5 | 0 | 2 | |
| HT 1 | A | G | A | A | A | A | A | G | G | T | G | G | C | G | A | A | A | A | A | T | T | G | C | T | T | C |
| HT 2 | . | . | . | . | . | . | . | . | . | . | A | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| HT 3 | . | . | . | . | . | . | . | . | . | . | . | A | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| HT 4 | . | . | . | . | . | . | . | . | . | . | . | A | T | . | . | . | . | . | . | . | . | A | . | . | C | . |
| HT 5 | . | . | . | . | . | . | . | . | . | . | . | A | . | T | . | . | . | . | . | C | . | . | . | . | C | . |
| HT 6 | . | . | . | . | . | . | . | . | A | . | . | A | T | . | G | . | G | . | . | . | C | . | . | C | . | T |
| HT 7 | . | . | . | G | . | . | . | . | . | . | . | A | . | T | . | . | . | . | . | C | . | . | . | . | C | . |
| HT 8 | . | . | G | . | . | . | G | . | A | . | . | A | . | . | G | . | G | . | . | . | C | . | . | C | . | T |
| HT 9 | – | – | . | . | . | . | . | . | A | . | A | T | . | G | . | G | . | . | . | C | . | . | C | . | T | |
| HT 10 | . | . | G | . | . | . | G | . | A | . | . | A | T | . | G | . | G | . | . | . | C | . | . | C | . | T |
| HT 11 | . | . | . | . | . | . | G | . | A | . | . | A | T | . | G | . | G | . | . | . | C | . | . | C | . | T |
| HT 12 | . | . | G | . | G | G | G | . | A | . | . | A | T | . | G | G | . | . | . | C | . | . | C | . | T | |
| HT 13 | . | . | . | . | . | . | . | A | . | . | . | A | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| HT 14 | . | . | . | . | . | . | . | A | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| HT 15 | . | . | . | . | . | . | G | . | . | . | . | . | . | . | . | . | G | . | . | . | . | . | . | C | . | . |
| HT 16 | . | . | . | . | . | . | . | . | . | C | . | . | T | . | . | . | . | . | . | . | . | . | . | C | . | . |
| HT 17 | . | . | . | . | . | . | G | . | . | C | . | . | T | . | . | . | . | . | . | . | . | . | T | C | . | . |
| HT 18 | . | . | . | . | . | . | . | A | . | . | . | . | T | . | . | . | . | . | . | . | . | . | . | C | . | . |
| HT 19 | A | . | . | . | . | . | . | A | . | C | . | . | T | . | . | . | . | . | . | . | . | . | . | C | . | . |
| HT 20 | A | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | G | . | . | . | . | . | . | . |
| HT 21 | – | – | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| HT 22 | A | . | . | . | . | . | G | . | . | . | . | A | . | . | . | G | . | . | . | . | . | . | . | C | C | . |
| HT 23 | A | . | G | G | . | . | G | . | . | . | . | A | . | . | . | G | . | . | . | . | . | . | . | C | C | . |
| HT 24 | A | . | . | . | . | . | . | . | . | . | . | A | . | . | . | G | . | . | . | . | . | . | . | C | C | . |
| HT 25 | A | . | . | . | . | . | . | A | . | . | . | A | . | . | . | G | . | . | . | . | . | . | . | C | C | . |
| HT 26 | A | . | G | . | . | G | G | . | . | . | . | A | . | . | . | G | . | G | . | . | . | . | . | C | . | . |
| HT 27 | A | G | G | . | G | G | . | . | . | . | A | . | . | . | G | . | . | . | . | . | . | . | C | C | . |
- Vertical numbers refer to the aligned sites in the 404-bp data set (only variable sites are shown). Dots indicate identity with HT 1, dashes denote deletions.
| FO | NF | SL | FM | RA | ∑ | |
|---|---|---|---|---|---|---|
| HT 1 | 1 | 7 | 9 | 9 | 26 | |
| HT 2 | 11 | 11 | ||||
| HT 3 | 1 | 1 | 13 | 2 | 17 | |
| HT 4 | 1 | 1 | ||||
| HT 5 | 2 | 2 | ||||
| HT 6 | 5 | 5 | ||||
| HT 7 | 2 | 2 | ||||
| HT 8 | 1 | 1 | ||||
| HT 9 | 1 | 1 | ||||
| HT 10 | 1 | 1 | ||||
| HT 11 | 2 | 2 | ||||
| HT 12 | 1 | 1 | ||||
| HT 13 | 6 | 3 | 9 | |||
| HT 14 | 4 | 4 | 8 | |||
| HT 15 | 1 | 1 | ||||
| HT 16 | 1 | 1 | ||||
| HT 17 | 1 | 1 | ||||
| HT 18 | 1 | 1 | ||||
| HT 19 | 1 | 1 | ||||
| HT 20 | 1 | 1 | ||||
| HT 21 | 1 | 1 | ||||
| HT 22 | 1 | 1 | ||||
| HT 23 | 2 | 2 | ||||
| HT 24 | 1 | 1 | ||||
| HT 25 | 4 | 4 | ||||
| HT 26 | 1 | 1 | ||||
| HT 27 | 1 | 1 | ||||
| ∑ | 16 | 23 | 16 | 26 | 23 | 104 |
Overall FST was 41.38%, the pairwise FST-values are shown in Table 2. All but one pairwise value were significantly different from 0, the exception being the one between SL and NF, which was not significant at the 5%-level after Bonferroni correction.
Correlation analyses
There were no significant Spearman correlations between any two variability indices – neither between indices based on the same marker system nor between indices based on microsatellites and mitochondrial haplotypes. The only p-value below 0.05 was the one for the correlation between expected heterozygosity and allelic richness (rs = 0.821, p = 0.044). This value, however, was not significant after Bonferroni correction (the adjusted significance level with 10 pairwise comparisons was 0.005).
The results of the Mantel tests for matrix comparison are shown in Table 6. None of the correlations was significant after Bonferroni adjustment of the p-values for multiple tests but there was a clear tendency towards low p-values: they ranged from 0.009 to 0.071 with the corrected significance level of the 5%-criterion being 0.005 (10 pairwise comparisons). Expectedly, the correlation coefficients (normalized Z-values) were higher for comparisons of parameters based on the same marker system (microsatellites or mtDNA sequences, respectively), ranging from 0.85 to 0.95, than for comparisons between different markers (0.63 < Z < 0.82).
| Nei78 | Chord | F ST(ms) | NND | F ST(mt) | |
|---|---|---|---|---|---|
| Nei78 | – | 0.011 | 0.009 | 0.062 | 0.042 |
| Chord | 0.85 | – | 0.010 | 0.071 | 0.052 |
| F ST(ms) | 0.95 | 0.90 | – | 0.051 | 0.027 |
| NND | 0.63 | 0.69 | 0.79 | – | 0.011 |
| F ST(mt) | 0.68 | 0.73 | 0.82 | 0.93 | – |
- Nei78: Nei (1978) genetic distance corrected for small sample sizes; Chord: chord distances; FST(ms): pairwise FST-values based on microsatellite allele frequencies; NND: net nucleotide diversities; FST(mt): pairwise FST-values based on mitochondrial haplotypes.
Discussion
The complete lack of polymorphisms at the reportedly polymorphic enzyme loci used in this study is astonishing. Herzog et al. (1993) found a lack of genetic variation in the transferrin system, a protein system highly polymorphic in other cervid species, in German roe deer populations but this monomorphism may be characteristic of the whole species whereas in the present case, the loci studied have been selected because of their known polymorphisms in roe deer. Unfortunately, Wang and Schreiber (2001) did not analyse the two roe deer populations from Schleswig–Holstein (which they genotyped for microsatellites) with regard to the allozymes they studied in other populations. Since these enzyme loci included three loci also examined in the present study (Ak, Mpi, Pgm), it would have been interesting to see if the allozymic homogeneity found by us is confined to our populations or if it is a more common phenomenon in northern Germany. The most probable explanation for this lack of variability is the demographic history of the roe deer in northern Germany: in the middle of the 19th century, the roe deer population was nearly driven to extinction through relentless persecution (Jessen 1988). This bottleneck may still be visible in the allozyme patterns of the species although introductions from other parts of Europe (e.g. Denmark, Poland and Hungary) have been carried out since then (Niethammer 1963; Jessen 1988). This hypothesis, however, stands in clear contrast to the very high variability of the microsatellites and the mitochondrial control region (see below). Alternatively, it cannot be ruled out that our results are an artefact of the eight loci studied and that allozymic variability would increase significantly if further loci were screened as was the case in red foxes and mustelids, which showed no polymorphisms at the loci studied by Simonsen (1982) but were highly polymorphic at the loci studied by Hartl et al. (1988) and Frati et al. (1998). Yet, given that exactly the loci which we analysed proved to be polymorphic in roe deer from very different parts of Europe, we consider this alternative to be rather unlikely.
Microsatellite variabilities in terms of expected heterozygosity were much higher in our 5 populations (0.74–0.79) than in the 12 Italian populations analysed by Lorenzini et al. (2002 0.17–0.58) and in the 27 populations from Germany, the Netherlands and France studied by Wang and Schreiber (2001 0.33–0.66; the two populations from Schleswig–Holstein exhibited values of 0.54 and 0.50, respectively). In the latter two studies, the microsatellite loci yielded distinctly lower numbers of alleles than in our study, which is probably the reason for the discrepancies in heterozygosity, as another comprehensive study with an average of 14.1 alleles per locus showed values of expected heterozygosity between 0.53 and 0.79 (Randi et al. 2004). Taken together, the roe deer from northern Germany show a variability not unmatched by other populations but certainly at the upper end of the species’ range.
Even more astonishing than the high variability at the microsatellite loci as compared with the allozymic homogeneity is the very high number of mitochondrial haplotypes in our roe deer which showed, on average, one distinct haplotype over 3.85 individuals (104/27 = 3.85). This is in line with previous results on control region variability: Randi et al. (2004) arrived at a respective ratio of 4.52 and Nies et al. (2005) even at a ratio of 1.71. If one further takes into consideration that the sequences analysed by the two other studies were longer (704 and 453 bp, respectively, as compared with 404 bp in our study) and that the geographic scale was much larger, the variability of the Schleswig–Holstein roe deer is even more remarkable and by far higher than comparable values for red deer as shown by Feulner et al. (2004 582 bp of the control region analysed in 120 individuals from southeastern Europe yielded merely 14 haplotypes) and an unpublished analysis of red deer from the very area that the roe deer of the present study come from (Schleswig–Holstein, northern Germany; here, 437 bp of the control region sequenced in 52 specimens resulted in only four haplotypes). In addition, similarly to the private microsatellite alleles, the number of private haplotypes is large but the very high proportion of 85.2% is due mainly to the high values of the two island populations. For the FO population, the repeated introductions may account for this, and the same almost certainly holds for FM in spite of the hunters’ claim that after the initial introduction in 1935, there have been no further releases. This claim is definitely wrong as the five founder females could have possessed only five different haplotypes but we have found eight showing pairwise differences of 1–8 mutations! Thus, there must have been additional introductions of female roe deer to the island of Fehmarn in later times, a conclusion supported by the bottleneck analyses which found no detectable reduction of effective population size for either of the populations and also backed by the fact that the island populations – although showing on average lower values of genetic variability (Table 1) – did not differ significantly from the mainland in terms of variability as measured by expected heterozygosity, inbreeding coefficient and allelic richness. The negative results of the bottleneck analyses in spite of the population breakdown as a result of overhunting in the 19th century may cautiously be interpreted as evidence that re-introductions were more common than mentioned in the literature.
With regard to genetic variability within populations, microsatellite and control region data are somewhat incongruent, in accordance with the lack of any significant correlation between the variability parameters. FO, e.g., has the lowest heterozygosity and allelic richness values and the second highest inbreeding coefficient but, at the same time, shows the highest haplotype and by far the highest nucleotide diversity. Similarly, RA yields the highest heterozygosity and allelic richness and the second lowest inbreeding coefficient but also shows the lowest haplotype and the second lowest nucleotide diversity, which is far lower than the values of the remaining three populations. In contrast to that, the different microsatellite-based variability indices yield more consistent results in that the populations with the highest heterozygosity (NF and RA) also have the highest allelic richness and the lowest inbreeding coefficients. Yet, this is not mirrored by the results of the Spearman correlations. Differences between the sequence-based indices are larger but this is due to the way they are calculated: low numbers of significantly different haplotypes will result in low haplotype but rather high nucleotide diversities while high numbers of only slightly different haplotypes will yield high haplotype and low nucleotide diversities. A possible reason for the striking differences in the FO population is the repeated introduction of individuals: at least eight females were released after the initial foundation of the population (Zachos et al. in press). Due to the generally much higher number of microsatellite alleles than haplotypes in a population, the introduction of small numbers of females is more influential in the mitochondrial gene pool than in the nuclear one. Furthermore, the effective population size (Ne) for the mitochondrial genome is, due to its being haploid and inherited by one sex only, a priori only one fourth of the nuclear Ne. Thus, the addition of a new haplotype to the gene pool is a relatively larger increase in variability than the addition of two more microsatellite alleles.
As to the pairwise distance values, it is obvious that the population most differentiated from the others is FO, which might be accounted for by its hybrid origin from Danish and west-German animals. The mainland populations exhibit only little differentiation in the mitochondrial control region among each other (see pairwise FST-values and net nucleotide diversities) whereas the microsatellite-based indices do not show marked differences between islands and mainland. This may be either a result of random drift or of a male-biased introduction policy in the process of restocking the north-German populations in the second half of the 19th century. These males would have left their traces in the nuclear but not the mitochondrial genome. Interestingly, the only pairwise FST-value not significantly different from 0 after Bonferroni correction was the mtDNA-based one between SL and NF. This value was much lower than all the other mitochondrial ones and even lower than some of the microsatellite-based FST-values. SL and NF are the only two populations without geographic barrier between them, as RA, the third mainland stock, is separated from SL and NF by the Kiel Canal which is known to be crossed by roe deer sometimes but which nonetheless considerably reduces the gene flow between the northern and the southern parts of Schleswig–Holstein. In our opinion, the pairwise differentiation tests nonetheless suggest that all populations may be regarded as separate stocks, as the microsatellite test showed a significant difference even for SL and NF. For the island populations, the detailedly known history of their origin can be viewed as further evidence of their distinct status.
Although the Mantel tests did not yield significant correlations after Bonferroni corrections, most of the p-values were smaller than 0.05 with the highest one being 0.071. Nies et al. (2005) conducted Mantel tests for detecting correlations between allozyme-based Nei (1978) and modified Rogers distances on the one hand and net nucleotide diversities calculated from control region sequences in roe deer from Switzerland, Austria, Slovenia and Slovakia on the other hand. They found much weaker consistencies between nuclear and mitochondrial distances (Z = 0.15, p = 0.172 for Nei distances and net nucleotide diversities; Z = 0.20, p = 0.128 for Rogers distances and net nucleotide diversities; these values are not given in the original publication), which are, however, not directly comparable with our results because they are based on allozymes and sequences while the values given in Table 6 refer to microsatellites and sequences.
There are a few other studies on roe deer using microsatellites and mtDNA data. Randi et al. (2004) conclude that both maternal and biparental markers describe concordant patterns of population structure with microsatellites being more capable of uncovering recent demographic events. Nevertheless, there are considerable differences in several of their populations between mitochondrial (haplotype diversity and average pairwise sequence divergence) and microsatellite variability (expected heterozygosity). Lorenzini et al. (2003), analysing mitochondrial restriction fragment length polymorphisms (RFLPs) and microsatellites in Spanish roe deer populations, also found ‘highly concordant’ results in their phylogenetic trees. Furthermore, FST-values derived from mitochondrial RFLP data were significantly correlated with both Rho, an unbiased estimator of RST, and FST-values based on microsatellites (p < 0.05). The exact p-values are not given and Bonferroni correction was probably less strict as a result of the smaller number of pairwise tests than in the present analysis. Similarly, Lorenzini et al. (2002), in an analysis of Italian roe deer, arrived at concordant results drawn from mitochondrial RFLPs and microsatellites, both with regard to their phylogenetic analysis and variability indices derived from the two marker systems.
Conclusions
The roe deer populations from northern Germany of this study exhibit a remarkable discrepancy between allozymic monomorphism on the one hand and high or even very high variability at microsatellite loci and the mitochondrial control region on the other hand. As in the case of the population from the island of Foehr, these differences are at least partly explicable when the demographic history is taken into account. In terms of microsatellite diversity, the Schleswig–Holstein roe deer are within the range of values reported in the literature for this species although variability is very high. The presented level of D-loop variability is one of the highest ever found in roe deer and very probably a result of extensive introductions and translocations. In particular, the island population of Fehmarn must have been repeatedly restocked with females, which shows that information from local authorities should be viewed critically when it comes to popular game species. Clearly, the north-German roe deer are genetically much more diverse than their sympatric relatives, the red deer, which have been listed as ‘near threatened’ by human-induced habitat fragmentation and inbreeding in the latest regional Red List.
Concordance of microsatellite- and control-region-based variability indices was low and not significant but genetic distances derived from the two marker systems showed a clear tendency to yield concordant results as judged from the low p-values most of which were below the usual significance level of 5% before Bonferroni correction. These results suggest that the analysis of differentiation among populations may be less marker-dependent than studies of variability within populations. Especially when dealing with small populations, the different effective population sizes for diploid, biparental nuclear and haploid maternally inherited mitochondrial markers may lead to distinctly different or even contradictory assessments concerning genetic variability. Analysis of both nuclear and mitochondrial marker systems is therefore strongly suggested when taxa relevant for conservation or management are examined with regard to genetic diversity within populations.
Acknowledgements
The authors wish to thank all the people and authorities involved in obtaining tissue samples including the Landesjagdverband Schleswig–Holstein, forestry departments and local hunters. S. Herzog and an anonymous referee made helpful comments on an earlier version of the manuscript. F. E. Zachos was getting financial support from the German National Academic Foundation during the time when the study was carried out.




