Characteristics of paravertebral muscles – fibre type distribution pattern in the pika, Ochotona rufescens (Mammalia: Lagomorpha)
Charakterisierung der paraverlebralen Muskulatur-Fasertyp-Verteilungsmuster beim Pfeifhasen, Ochotona rufescens (Mammalia, Lagomorpha).
Abstract
enThe evolution of the locomotor apparatus in vertebrates is marked by major reorganizations in trunk's musculature. The hypothesized functions of mammalian back muscles in the literature are discussed under consideration of the distribution and proportion of oxidative, type-I-fibres, oxidative-glycolytic, type-IIa-fibres and glycolytic, type-IIb-fibres in paravertebral muscles of a small mammal. The fibre type distribution was examined from a complete series of histological sections maintaining topographical relationships between the muscles as well as within the muscle, in order to establish the overall distribution pattern.
The deep and short muscles showed the highest percentage of oxidative fibres. The larger, superficial paravertebral muscles contained the highest percentage of glycolytic fibres. Two muscles were intermediate in their proportion of fibre types. All epaxial muscles together can be interpreted as an antigravity muscle–complex counteracting enduringly against the rebound tendency caused by gravitation, comparable with antigravity muscles in limbs. A gradient from deep to superficial, or a clear regionalization of oxidative muscle fibres in central deep regions around a large intramuscular tendon was found in the m. spinalis and the m. quadratus lumborum, respectively. Concepts of the function of human back muscles as those of A. Bergmark (1989: Acta Orthop. Scand.230, 1) or S.G.T. Gibbons & M.J. Comerford (2001: Orthop. Division Rev. March/April, 21) were exposed to be more general within mammals. Functional specializations of different muscles and muscle parts are discussed under the consideration of evolutionary reorganization of the paravertebral musculature in tetrapods.
Along the cranio-caudal axis, the percentage of oxidative fibres was decreased in caudal direction within the same muscles, whereas the proportion of glycolytic fibres was increased. Therefore, classifications of muscles as ‘glycolytic’ or ‘oxidative’ based on biopsies or analyses of single cross-sections may result in wrong interpretations. Changes in the proportions of the fibre type distribution pattern were mostly due to oxidative and glycolytic fibre types, whereas the percentage of oxidative-glycolytic fibres had only minor influence. A significant positive correlation between the cross-sectional area of the single fibre and its percentage in the area investigated were observed for oxidative fibres, whereby the size was positive correlated to the proportion of the oxidative fibres.
Zusammenfassung
deIn der Evolution der Wirbeltiere fanden wesentliche Umbauten an der zentralen Körperachse, insbesondere der paravertebralen Muskulatur statt. Den einzelnen Muskeln bzw. Muskelgruppen wurde in der Vergangenheit eine unterschiedliche Bedeutung bei der Bewegung und Stabilisierung der Wirbelsäule zugeschrieben. Die aktuellen Hypothesen zur Funktionsweise der Rückenmuskulatur von Säugetieren werden vor dem Hintergrund des 3D-Verteilungsmuster der oxidativen Typ-I-Fasern, oxidativ-glykolytischen Typ-IIa-Fasern und der glykolytischen Typ-IIb-Fasern am Beispiel des Pfeifhasen diskutiert. Dazu wurde eine komplette Schnittserie des Rückens im Muskel-Knochen-Verband und damit unter Erhalt der topographischen Beziehungen angefertigt für die Fasertypisierung wurden die Schnitte der alkalischen kombinationsreaktion nach Ziegan (1979) unterzogen. Eine Einordnung der erhobenen Befunde in die Evolutionsgeschichte der Tetrapoda wird vorgenommen.
Die tiefen und kurzen Faserzüge wiesen den höchsten Anteil an oxidativen Fasern auf. Große und oberflächliche Muskeln waren überwiegend aus glykolytischen Fasern aufgebaut. Durch eine Regionalisierung – eine Anhäufung von oxidativen Fasern in der Tiefe bzw. um intramuskuläre Sehnenplatten – zeichneten sich zwei der untersuchten Muskeln aus. Aufgrund des beobachteten Fasertypenverteilungsmusters kann der gesamte epaxiale Komplex als Anti-Gravitations-Muskel verstanden werden, der ausdauernd dem Durchsacken des Rückens aufgrund der Schwerkraft entgegenwirkt. Die von A. Bergmark (1989: Acta Orthop. Scand.230, 1) und S.G.T. Gibbons & M.J. Comerford (2001: Orthop. Division Rev. March/April, 21) aufgestellten Konzepte zur Funktion der menschlichen Rückenmuskulatur werden durch das an einem kleinen Säugetier gewonnene metabolische Profil belegt, aber dadurch auch in ihrer Exklusivität als humanspezifische Konzepte in Frage gestellt.
Sowohl entlang der cranio-caudalen Achse als auch von tief nach oberflächlich sind deutliche Verschiebungen in den Anteilen der einzelnen Fasertypen beobachtet worden. Die wenigsten Muskeln zeigen eine homogene 3D-Verteilung der Fasertypen (Streublümchenmuster). Die Klassifikation eines Muskels als ‘glykolytisch’ oder ‘oxidativ’, wie sie z.B. auf der Basis von Biopsien oder Einzelschnitten vorgenommen wird, führt daher in der Regel zu falschen Schlussfolgerungen. Verschiebungen im Fasertypenverteilungsmuster sind auf veränderte Anteile der oxidativen und glykolytischen Fasern zurückzuführen; die oxidativ-glykolytischen Fasern sind gleichmäßig verteilt und haben keinen Einfluß auf das Verteilungsmuster. Eine signifikante, positive Korrelation besteht zwischen dem prozentualem Anteil oxidativer Fasern und ihrer mittleren Querschnittsfläche.
Introduction
In the evolution of tetrapods, major reorganizations in the locomotor apparatus and especially the trunk musculature occurred. But, the plesiomorphic separation of hypaxial and epaxial musculature by the Septum horizontale was maintained. In tetrapods, the originally segmental organization of trunk muscles was dissolved and the epaxial muscles were modified into three large, multi-segmental muscular tracts (transversospinal, longissimus, iliocostalis). Profound modifications occurred in the lumbar region of tetrapods, where the originally hypaxial muscle – m. quadratus lumborum shifted onto the vertebral column and became, together with the former limb muscle – m. psoas major involved as subvertebral muscles. In this new position, these muscles act now as antagonists of the epaxial muscles (Starck 1978).
In mammals, the three tracts allow for high mobility in all three rotational degrees of freedom of the vertebral column, but particularly in horizontal and sagittal planes. The transversospinal group consists of a large number of short and long fibre bundles close to the vertebral column (mm. rotatores, multifidi et semispinales). Former segmental organization is retained in the deeper, short muscle bundles. The transversospinal group functions primarily in stabilization, but also in torsion movements of the spine. The longissimus group, lying more laterally, spans from the cranium to the sacrum by long muscle fibre tracts. This muscle is attributed to extend the spine mainly. The iliocostalis group, as the most lateral tract, is also composed of long muscle tracts and is ascribed to be primarily recruited in lateral bending movements (Starck 1978).
All spinal movements are the outcome of small, additive intervertebral movements, which result in the apparent ‘pelvic movement’; the iliosacral joint itself is rigid. During slow, symmetrical gaits, such as walk and trot, lateral and rotational movements dominate the motion of the vertebral column in mammals (tilting, lateral bending). At faster, asymmetrical gaits, such as gallop or bound, the main functional axis rotates and extensive sagittal bending movements occur along the spine (e.g. Rockwell et al. 1938; Hildebrand 1959; Schilling and Fischer 1999; Fischer et al. 2002). These additive sagittal flexions take place mainly between the last 7 ± 1 presacral vertebrae in several small mammals examined such as, Ochotona rufescens (Schilling et al. 1999). Sagittal bending movements of the spine are used to increase the animals stability as well as their step length by inserting an extensive suspension phase in the locomotor cycle (Hildebrand 1959, 1974; Howell 1965; Goslow et al. 1973; English 1980). Sagittal bending contributes up to about half of the step length at asymmetrical gaits in small mammalian locomotion (Fischer et al. 2002). Self-stabilization is achieved by shifting the centre of mass of the body as an effect of ‘pelvic movement’ (Hackert and Fischer in press). Within the muscles and the vertebral column itself, but especially in the large dorsal aponeurosis, about 50% of the metabolic energy can be stored as elastic energy (Alexander et al. 1985; Alexander 1988; Bennett 1989; Koob and Long 2000; Hackert and Fischer in press). Sagittal bending movements support the ventilation of the lungs by oscillatory movements of the visceral organs (Bramble and Jenkins 1993; Carrier 1996; Bramble 1999).
In general, the role of epaxial muscles is assumed to produce sagittal movement during asymmetrical gaits (one of the characteristics of mammals only), to extend and to stabilize the vertebral column against torsions movements (Tokuriki 1973, 1974; Carlson et al. 1979; English 1980; Shapiro and Jungers 1988, 1994). Most recently, a series of EMG-studies were done on trunk muscles in trotting dogs to understand their role in locomotion and respiration more in detail (Carrier 1996; Fife et al. 2001; Ritter et al. 2001; Deban and Carrier 2002). Despite the assumed function as extensor of the back, all three investigated epaxial muscles (m. multifidus, longissimus et iliocostalis) were active during flexion movements of the body in the second half of stance (Ritter et al. 2001). Ritter et al. hypothesized that the function of epaxial muscles in trotting dogs was mainly to counteract flexions of the body and to stabilize the trunk against its tendency to rebound passively in the sagittal plane as a result of flexion movements of the body. Following this, fibre type distribution in epaxial muscles should be comparable with that of ‘antigravity muscles’ (see below) in limbs, which counteract enduringly against gravitation.
A considerable number of studies on fibre type distribution were carried out on mammalian limb muscles (e.g. Stein and Padykula 1962; Ariano et al. 1973; Collatos et al. 1977; Armstrong 1980; Sickles and Pinkstaff 1981a,b; Armstrong et al. 1982; Brasseur et al. 1987; Braund et al. 1995; Suzuki 1995; Ustunel and Demir 1997; Fuentes et al. 1998; Fischer 1999; von Mering and Fischer 1999). It was shown, that limb muscles have different fibre type distribution patterns related to their function. Muscles mainly involved in propulsory functions consisted predominantly of glycolytic fibre types with only few oxidative fibres distributed over the muscle cross section (‘salt and pepper’ pattern), whereas muscles responsible for maintaining the limb's posture against gravity contained a regionalization of oxidative fibres in deeper muscle parts or near to intramuscular tendons (‘antigravity muscles’: Collatos et al. 1977; Armstrong 1980; Burke 1981; Armstrong et al. 1982; Lexell et al. 1983; Armstrong and Phelbs 1984; Brasseur et al. 1987; Braund et al. 1995; Fischer 1999; von Mering and Fischer 1999). In contrast to limb muscles, only restricted information is available on fibre type distribution in paravertebral muscles in mammals. In most relevant studies only parts of the back were examined, some of them by biopsy (Carlson 1978; Yokoyama 1982; Ford et al. 1986; Thorstensson and Carlson 1987; Kojima and Okada 1996; Mannion et al. 1998; Gellman et al. 2002). This may be partly because of methodological problems, but the main reason is probably that back functions in mammals are not as well understood as limb functions.
Since metabolic profiles of muscles relate to their function (Burke 1981; Fischer 1999; Scholle et al. 2001) it can serve as indicator of function integrated with additional anatomical details. In order to develop hypotheses of functions in the paravertebral muscles in small mammals, the objectives of the current study are: (1) to investigate the fibre type distribution pattern between paravertebral muscles of a small mammalian species, the pika; (2) to determine the number of different fibre types, as well as their cross sectional area, from deep to superficial regions in cross sections of paravertebral musculature; (3) to determine the overall distribution pattern of muscle fibre types and possible correlations of the respective fibre type to the overall distribution pattern; and (4) to assess the relationships between fibre thickness and distribution pattern.
Materials and Methods
Two adult males of the pika, O. rufescens (226.1 and 283.8 g) were used for this study. Serial sections were made of the complete backs including the vertebral column to preserve the topographical relationships between the muscles and their intramuscular architecture. For preparation, animals were sacrificed by an overdose of chloroform. Skin, fat, fore limbs, and hind limbs were removed immediately. Forelimbs were separated by removing all muscular connections to the trunk. The hind limbs were exarticulated in the hip joint and pelvic musculature was removed. The sternum was removed by severing all ribs in the middle of their lengths without injuring para- or subvertebral muscles. After the preparation, the back preparations were cut into two parts between the 16th and the 17th thoracic vertebrae. Only the caudal parts, reaching from the last thoracic vertebrae to the end of the iliosacral joint were investigated here (T17–S1). They were quick-frozen in liquid nitrogen cooled isopentan and stored in liquid nitrogen. Sections were made using a kryostat microtome (SLEE MTE, D-knife, 12 μm) and processed for oxidative capacity and muscle fibre type characterization using the alkaline combination reaction based on Ziegan's protocol (Ziegan 1979, for details see von Mering and Fischer 1999). Type-I muscle fibres (slow twitch, oxidative) were stained blue, type-IIa (fast twitch, oxidative-glycolytic) dark brown, and type-IIb (fast twitch, glycolytic) light brown (Fig. 1). Serial sections were analysed using an Zeiss® Axiolab microscope or a Stemi SV 11 (Zeiss, Germany).
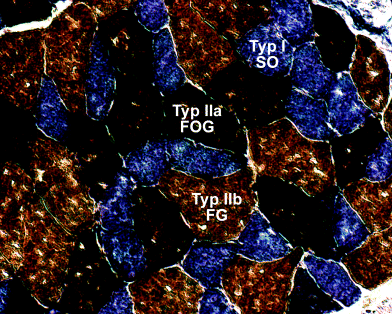
Results of the enzyme histochemical reaction. Oxidative fibres (type-I according to Ziegan 1979) were stained blue, oxidative-glycolytic (IIa) dark brown and glycolytic fibres (IIb) light brown
Drawings were made at 10 different cranio-caudal levels from the serial sections of one of the specimen. Only the levels reaching from T17 onto L4 could be analysed, because in the more caudal region, the proportion of skeletal material (pelvis, iliosacral joint, sacrum) led to damage in the sections. At the same 10 levels, fibre type distributions of m. sacrospinalis, quadratus lumborum, spinalis, and psoas major as well as mm. multifidi, rotatores et semispinales were measured by counting the number of the muscle fibres of the respective type.
Photos of areas reaching from the deep portion near the bone to the muscle surface were taken with a mounted digital camera and analySIS® software on a Zeiss® Axiolab microscope (grey arrows in Fig. 3). The object table of the microscope was motor driven and software controlled, which allowed to take images of adjacent areas with some overlap. Each image contained at least 100 and at most 150 muscle fibres. All fibres of the same type were counted and marked automatically by the software, thus preventing double counting. Proportion of fibre types was calculated as a percentage of the sum of all fibres seen in one image.
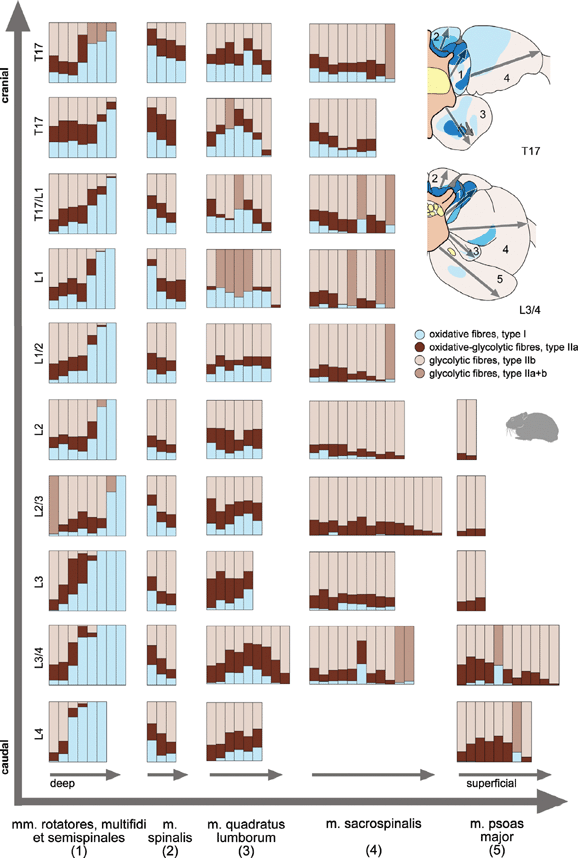
Proportion of different muscle fibres types in the defined regions examined for each paravertebral muscle reaching from deep to superficial parts of the muscle belly (grey arrows) in different cranio-caudal levels. Each column represents the percentages of different fibre types in one image along the region of interest. Columns in middle brown represent all glycolytic fibres (IIa + b), if both types could not be identified separately
Shifts in the distribution pattern can be the result of changing the proportion of all three fibre types or only two of them. To determine the influence of the different fibre types on the composition per image, Pearson's correlation coefficient (bilateral) was calculated using the software SPSS® (10.0.7.). Images, in which oxidative-glycolytic and glycolytic fibres could not be separated clearly, were omitted from the statistical analysis. Only measurements with clear identification of all three fibre types were used for this analysis.
Cross-sectional areas of about 10 selected muscle fibres of each type were measured in each image. The student's t-test for paired samples and Pearson's correlation coefficient (bilateral) in SPSS® (10.0.7) was applied to assess differences in size of the fibre types and correlations between proportion of fibre types and size of the fibre. All proportions and cross-sectional areas presented in the text are based on a serial section of one of the O. rufescens specimens. But the general distribution pattern was highly similar between both specimens.
Results
Anatomy
The vertebral column of O. rufescens is composed of seven cervical, 17 thoracic, four lumbar, two sacral and approximately 10 caudal vertebrae. The 12th thoracic is the diaphragmatic and anticline vertebra. The fifth or sixth posterior ribs do not connect to the sternum.
Autochthon, epaxial muscles (m. erector spinae)
Lateral tract: m. sacrospinalis (sacrospinal group), mm. intertransversarii (intertransversal group).
The m. sacrospinalis consists of a medial (m. longissimus) and a lateral subunit (m. iliocostalis). The m. longissimus (thoracolumbar region) originates from the crista iliaca and inserts at the procc. transversi of all lumbar and of the last two thoracic vertebrae. The muscle also attaches to the spinous processes of the lumbar vertebrae by means of strong tendons and onto the fascia of subjacent muscles of the medial tract. The m. iliocostalis emerges from the fascia thoracolumbalis and inserts at the caudal margins of the posterior ribs. The pars thoracalis and the pars lumbalis could not be separated from each other.
The mm. intertransversarii are a series of small muscle bundles between adjacent transverse processes of all vertebrae.
Medial tract: m. spinalis, mm. interspinales (spinal group); mm. rotatores, mm. multifidi, mm. semispinales (transversospinal group).
The m. spinalis originates from the fascia thoracolumbalis and the procc. spinosi of all thoracic and lumbar vertebrae and inserts at the procc. spinosi of the more cranial vertebrae. The mm. interspinales are a series of small muscles connecting adjacent procc. spinosi.
The mm. rotatores are mono-segmental muscles originating from the proc. transversus and inserting at the proc. spinosus of the cranially next vertebra. The mm. multifidi connect the procc. transversi, mammillares et articulares and the procc. spinosi of the more cranially lying vertebrae skipping 2–4 vertebrae. In a similar way, the mm. semispinales connect transverse and spinous processes over a distance of five or more vertebrae. Areas of origin of mm. multifidi et semispinales extended to the fascia thoracolumbalis.
Deep, ventral muscles: m. quadratus thoracis et lumborum.
The mm. quadratus thoracis et lumborum belongs to the subvertebral muscles and its two parts, thoracic and lumbal could not be separated clearly from each other. Therefore, both parts will be subsumed as m. quadratus lumborum in the current study. The m. quadratus lumborum originates from the ventral border of the ilium and the procc. transversi of the vertebrae L3–L4. It inserts at the procc. transversi and ventrolateral face of the centra of T7–L4.
Hip muscles: m. iliopsoas (m. psoas major, m. iliacus), m. psoas minor.
The m. psoas major originates from the vertebral centra and the procc. transversi of L1–4 and inserts together with the m. psoas minor and the m. iliacus at the trochanter minor femoris. The two heads of the muscle enclose the m. quadratus lumborum. The m. iliacus and m. psoas minor were not examined, because they originate posterior to the back region under consideration. The m. iliacus originates from the ventral border of the ilium. The psoas minor muscle has its origins on the vertebrae L4 (caudal part) and S1.
Distribution pattern of muscle fibre types
The two O. rufescens specimens were highly similar in their overall distribution patterns. Most of the muscles showed a more or less heterogeneous distribution of the different fibre types over the muscle cross-section, but also in cranio-caudal direction. Oxidative fibres were irregularly spread over the cross-sectional area. Because of the predominant relationship of glycolytic and oxidative fibre types and the more or less independent distribution of oxidative-glycolytic fibres (see below), the following description of the distribution pattern is focused on the oxidative fibres and the glycolytic fibres.
The highest proportion of glycolytic fibres was present in the mm. sacrospinalis et psoas major. Only few individual oxidative fibre were more or less regularly distributed over the cross-sectional areas (‘pepper and salt’ pattern). But, in larger parts of the muscles, oxidative fibres were completely absent. At the level of the 17th thoracic vertebrae, a slight increase of the number of oxidative fibres near to deeper and dorsal muscles parts were observed in m. sacrospinalis (Fig. 2). The m. psoas major was free of oxidative fibres close to its origin and the percentage of oxidative fibres increases only slightly in a small central region in caudal direction (Fig. 2). Details about percentages and their course along the regions of interest are given in Fig. 3.
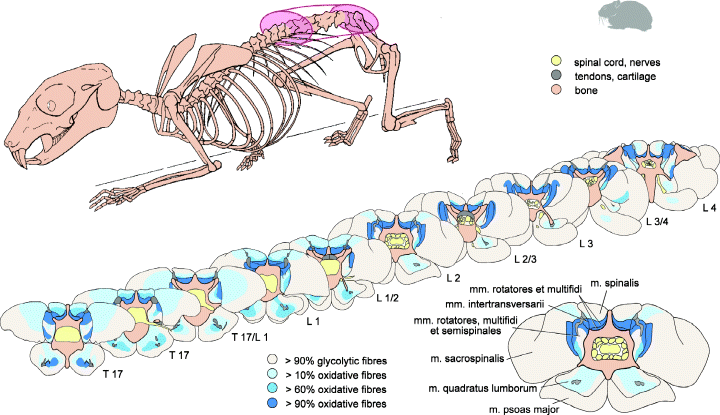
Fibre type distribution pattern of paravertebral muscles in different cranio-caudal levels of the back. Note the higher percentage of oxidative fibres in deeper muscles regions. Oxidative-glycolytic and glycolytic fibres were summarized here as glycolytic fibres
The highest percentages of oxidative fibre types were found in the mm. intertransversarii et interspinales, and in the muscles of the transversospinal group. The mm. intertransversarii and mm. interspinales, contain more than 90% oxidative muscle fibres (Fig. 3, mm. interspinales: level L3/4, mm. intertransversarii: T17, L1, L2, L3, L4). The same high percentage of oxidative fibres (>90%) was determined in the mm. rotatores (Fig. 2). Due to their complex spatial anatomical relationships and their mutual proximity, mm. rotatores, mm. multifidi, and mm. semispinales could not be recognized as individual muscles in the serial sections. Therefore, these muscles were analysed as one group in 2, 3. Laterally and ventrad to the hypapohyses of the vertebral centrum, the percentage of glycolytic muscle fibres was higher. Close to the hypapophyses, oxidative fibre types predominate (Fig. 2). As derived from manual dissection, the deep, dorsal, mono-segmental muscles belong to the mm. rotatores and the mm. multifidi. The corresponding area in cross-sections consisted predominantly of oxidative fibres. The more ventrally originating, multi-segmental parts correspond more likely to the mm. semispinales and contain more glycolytic fibres. In cranio-caudal direction, the percentage of oxidative fibres increased substantially (Fig. 3).
An obvious heterogenous distribution of fibre types with a distinct regionalization of oxidative fibres was found in the central region of the m. quadratus lumborum. Oxidative fibres were distinctly arranged around a strong intramuscular tendon plate. The oxidative region in the m. quadratus lumborum was most extensive in the cranial parts of the lumbar vertebral column (Fig. 2, height T17). Towards the caudal region the oxidative region decreased in size and the intramuscular tendon became thinner (Fig. 2, height L3/4).
In the m. spinalis, an accumulation of oxidative fibres is present in the deeper parts near to the vertebral column (Fig. 2). The percentage of oxidative fibres decreases to the more superficial dorsal regions of the muscle from about 50% to approximately 10% (Fig. 3). In caudal direction, the percentage of oxidative fibres was halved.
Shifting of the distribution pattern
A significant correlation (p ≤ 0.01) between the number of oxidative fibres and the number of glycolytic fibres (Table 1) was present in all muscles independent of the distribution pattern of the respective fibre types. A low percentage of glycolytic fibres was present in regions with a high percentage of oxidative fibres, and vice versa, whereas the proportion of oxidative-glycolytic fibres seems to be independent from the percentages of the other types. Significant negative correlations between oxidative and oxidative-glycolytic fibres were only found in the mm. multifidi et semispinales, the muscles with the highest proportion of oxidative fibres. Between oxidative-glycolytic and glycolytic fibres, significant correlations were only found in the m. sacrospinalis and m. psoas major, the muscles with the highest percentage of glycolytic fibres under study. Therefore, proportional changes of fibre types is mainly due to increase or decrease of the percentage of oxidative and glycolytic fibres, and less to changes concerning the oxidative-glycolytic fibres.
| Oxidative-glycolytic (IIa) | Glycolytic (IIb) | |
|---|---|---|
| mm. rotat., multif. et semispinales (n = 67) | ||
| oxidative (I) | −0.669 | −0.903 |
| oxid.-glycolytic (IIa) | – | 0.285 |
| glycolytic (IIb) | – | – |
| m. spinalis (n = 32) | ||
| oxidative (I) | 0.018 | −0.886 |
| oxid.-glycolytic (IIa) | – | −0.479 |
| glycolytic (IIb) | – | – |
| m. quadratus lumb. (n = 80) | ||
| oxidative (I) | −0.196 | −0.656 |
| oxid.-glycolytic (IIa) | – | −0.612 |
| glycolytic (IIb) | – | – |
| m. sacrospinalis (n = 89) | ||
| oxidative (I) | 0.217 | −0.755 |
| oxid.-glycolytic (IIa) | – | −0.803 |
| glycolytic (IIb) | – | – |
| m. psoas major (n = 25) | ||
| oxidative (I) | 0.422 | −0.535 |
| oxid.-glycolytic (IIa) | – | −0.992 |
| glycolytic (IIb) | – | – |
- p ≤ 0.01 for values in bold.
Muscle fibre size and proportion
Independent of the fibre distribution patterns oxidative fibres had the smallest mean cross-sectional area in all muscles reaching from 761 μm2 within the mm. multifidi et rotatores up to 1129 μm2 in the m. psoas major (Table 2). Glycolytic fibres were the largest of all fibres examined (mean cross-sectional area: 1373 μm2 in mm. multifidi et rotatores up to 2633 μm2 in m. sacrospinalis). The oxidative-glycolytic fibres were intermediate measuring 1061 μm2 in the mm. multifidi et rotatores to 1759 μm2 in the m. psoas major. Size differences between the fibre types were highly significant in all muscles and between all types (p ≤ 0.001), except for oxidative and oxidative-glycolytic fibres in the m. psoas major. However, there is a noteworthy, extensive overlap between mean sizes of the fibre types within different muscles. There are no clear cut size classes between the three types. Strong correlations (p ≤ 0.01) between the proportion and the cross-sectional area of the muscle fibre types were determined in two muscles (Table 2). The cross-sectional area of the oxidative muscle fibres increases with the percentage of oxidative fibres in the mm. multifidi et rotatores and the m. spinalis (Pearson's correlation coefficient 0.787 and 0.676, respectively).
| Percentage and csa | |
|---|---|
| mm. rotat., multif. et semispinales (n = 27) | |
| oxidative (I) | 0.787 |
| oxid.-glycolytic (IIa) | 0.183 |
| glycolytic (IIb) | −0.128 |
| m. spinalis (n = 32) | |
| oxidative (I) | 0.676 |
| oxid.-glycolytic (IIa) | 0.301 |
| glycolytic (IIb) | 0.406 |
| m. quadratus lumb. (n = 62) | |
| oxidative (I) | 0.003 |
| oxid.-glycolytic (IIa) | 0.115 |
| glycolytic (IIb) | 0.200 |
| m. sacrospinalis (n = 80) | |
| oxidative (I) | 0.005 |
| oxid.-glycolytic (IIa) | 0.055 |
| glycolytic (IIb) | 0.211 |
| m. psoas major (n = 6) | |
| oxidative (I) | −0.077 |
| oxid.-glycolytic (IIa) | 0.840 |
| glycolytic (IIb) | −0.532 |
- p ≤ 0.01 for values in bold.
- As only regions containing all three fibre types were selected for this correlations, the number of samples is differing from Table 1.
Discussion
Current concepts on back muscle function
Gross anatomy of the epaxial muscles lead to the assumption that they are involved in extension or lateral bending of the trunk. Despite single results of EMG-studies in dogs, cats or primates on only single muscles or only one body site (Tokuriki 1974; Carlson et al. 1979; English 1980; Shapiro and Jungers 1994), the more complex investigation of Ritter et al. (2001) in trotting dogs showed the epaxial muscles as stabilizers of the trunk to counteract its passive rebound tendency in the sagittal plane. However, several functions for each of the back muscles were not elucidated for quadrupedal mammals.
In spite of the alteration of the preferred used body axis in humans (tilting–torsion), some of the actual concepts on human back muscle function should also discussed here. According to anatomical position, superficial muscle fibre direction, and muscle activity, different functional roles were proposed for human back muscles in stabilizing and mobilizing of the vertebral column. In general, stabilizer muscles were described as being mono-articular or segmental, deep, working eccentrically to control movement, and having static holding capacities. Mobility muscles were thought of as bi-articular or multi-segmental, superficial, working concentrically with the acceleration of the movement, and producing power (Goff 1972; for more references see Gibbons and Comerford 2001).
Bergmark suggested the concept of local and global muscles for human back muscles (Bergmark 1989). In the local system, all muscles have their origin or insertion at the vertebrae. It is responsible for the control of the spine curvature and provides stiffness to maintain mechanical stability of the lumbar spine, e.g. mm. multifidi et rotatores. In the global system, the muscles are more superficial (non-segmental) and connect the thorax to the pelvis. These muscles produce strong forces and are responsible for the balancing of high loads, e.g. m. psoas major.
Based on the concepts of mobilizers and stabilizers, and global and local muscles a new functional classification was proposed by Gibbons and Comerford (2001) for human back muscles. Their classification includes local and global stability muscles as well as global mobility muscles. According to them, the particular role of the local stability muscles was maintenance of segmental stability. Based on a former study (Panjabi et al. 1989), Gibbons and Comerford (2001) proposed, that the mm. multifidi, together with mm. rotatores et interspinales as well as the m. psoas major (posterior fascicles), were best suited to control segmental movement, and that they act as spinal stabilizers. Local stability muscles were characterized by a continuous activity. The global stability muscles of the lumbar spine would control the range of movement of the back and their activity was non-continuous (e.g. m. spinalis, m. obliquus abdominis). Global mobility muscles, as Gibbons and Comerford (2001) supposed, would generate forces to produce power and speed for large ranges of movement and high loads (e.g. m. iliocostalis, m. rectus abdominis) by non-continuous activity (summarized in Table 3, upper part). Unfortunately, none of the named studies discussed developmental changes in the back muscles related to bipedality during human evolution.
| Local Stabilizer | Global stabilizer | Global mobilizer |
|---|---|---|
| Control segmental motion | Control range of movement | Produce range of movement |
| Direction in-dependent activity | Direction dependent activity | Direction dependent activity |
| Continuous activity | Non-continuous activity | Non-continuous activity |
| Working eccentrically | Working concentrically | |
| Deep | Superficial | |
| Origin or insertion at vertebrae | Connect thorax and pelvis | |
| Mono-articular or mono-segmental | Bi-articular or multi-segmental | |
| Low contraction force | Intermediate contraction force | High contraction force |
| Slow contraction speed | Intermediate contraction speed | Fast contraction speed |
| Fatigue resistant | Middle fatigue resistance | Fast fatiguing |
| Predominant oxidative | Oxidative and glycolytic (regionalized) | Predominant glycolytic |
| mm. multifidi | mm. semispinales | m. sacrospinalis (mm. Longissimus et iliocostalis), m. psoas major |
| mm. rotatores, mm. interspinales, mm. intertransversarii | m. spinalis, m. quadratus lumborum |
- Upper part is based on the concepts of Bergmark (1989) and Gibbons and Comerford (2001). Complemented by current results on fibre type distribution in the lower part.
Distribution pattern of muscle fibre types
As inferred from their anatomical position and their high percentage of fatigue resistant, oxidative fibres, the muscles of the transversospinal group (mm. rotatores et multifidi) appeared best suited to maintain segmental stability and to act as spinal stabilizers. According to their metabolic profile, these muscles are local stabilizers, best suited to resist small movements for a long-time. They maintain the body posture, control segmental motions, and control the neutral position in the intervertebral joints (Panjabi 1992a,b). The mm. interspinales et intertransversarii, which connect adjacent vertebrae, are characterized by a high percentage of oxidative fibres, and are, therefore, also local stabilizers. A postural function was also suggested for the mm. intertransversarii based on enzyme-histochemical investigations in the rabbit (McFadden et al. 1984). In the Japanese macaque, the mm. intermammilares as well as the mm. mammiloaccessorii contained 69–88% of type-I-fibres (Kojima and Okada 1996). High percentages of oxidative fibres in the mm. multifidi, interspinales, and intertransversarii in comparison with all other back muscles were also described for the cat (Carlson 1978). Because of the most medial anatomical position of the transversospinal muscle group and their high percentage of oxidative fibres, those muscles are suggested as stabilizing muscles to resist movements and counteract the rebound tendency of the trunk enduringly, hypothesized by Ritter et al. (2001).
Despite of the high percentage of oxidative fibres in the transversospinal group in the pika, the muscles of the sacrospinal group (mm. longissimus et iliocostalis) and also the m. psoas major consisted mainly of glycolytic fibres. This is in accordance to results from the sacrospinal muscle in the rat (Schwartz-Giblin et al. 1983), the rabbit (McFadden et al. 1984), the cat (Carlson 1978) and the Japanese macaque (Kojima and Okada 1996) as well as the sheep (Peinado et al. 2004). More cranially, a slightly higher percentage of oxidative fibres in the deep and central region of the m. sacrospinalis than in the more caudally levels was found in the present study. A segregation of slow twitch, oxidative fibres was located superficially from L2 to L6 and in the deep medial region of L5 of the rat (Schwartz-Giblin et al. 1983). Such a cranio-caudal distribution pattern was also described in primates in a comparative study, but surprisingly not found in the other species such as rats, mice, cats, or dogs in the same study (Yokoyama 1982). The fibre type distribution pattern of mm. sacrospinalis et psoas major make them suitable to generate forces and speed for a wide range of movements, e.g. for shock absorption and high loads. The hypothesized function as global mobility muscles is in accord with their metabolic profile observed here. As it was shown earlier by EMG studies in trotting dogs, the main function of the m. longissimus dorsi is in locomotion. The muscle is not involved in respiration (Deban and Carrier 2002).
The spatial integration and the higher percentage of glycolytic fibres in the mm. semispinales (as compared with the other muscles in the transversospinal group), suggest that they are global stabilizers. The m. spinalis is also considered to be a global stabilizer. Its gradient with deep lying oxidative fibres and more superficially positioned glycolytic fibres allows the muscle to act with intermediate contraction speed and contraction force. These muscles are well suited to control the range of movements of the back. The same fibre type distribution pattern as in the pika with a higher percentage of oxidative fibres in deeper parts of muscles and more glycolytic fibres in superficial regions were described for the Japanese macaque in the m. spinalis and also in the m. transversospinalis (Kojima and Okada 1996).
The m. quadratus lumborum attracted attention, because of its particular regionalization of oxidative fibres around a strong intramuscular tendon. A similar condition is well known for limb muscles in several mammalian species (antigravity muscles). These muscles (e.g., m. triceps brachii, mm. gastrocnemius et soleus) counteract passive limb flexions, that is gravitational forces. Deep oxidative regions were shown to be activated separately from the rest of the muscle belly dependent on temporal requirements and the performance of the animal (Chanaud et al. 1991; Scholle et al. 2001). The m. quadratus lumborum is active synchronously to the second third of the inspiration cycle and supports the inspiration process by widening the pleural cavity in addition to the dilation by diaphragm contraction in the first third of the cycle (Boyd et al. 1965). The muscle also stabilizes the posterior ribs during inspiration, so that the ribs can resist the pulling from the diaphragm contraction (Boyd et al. 1965). Because of the central region of oxidative, fatigue resistant muscle fibres within the m. quadratus lumborum this region could be responsible for this continuous action during respiration. It is supposed that, the oxidative fibre rich region within the m. quadratus lumborum is activated separately from the rest of the muscle's belly, similar to the deep oxidative region in limb muscles, for instance in the m. triceps brachii caput longum (Scholle et al. 2001). Based on the metabolic profile containing oxidative and glycolytic fibres, this muscle is also considered to be a global stability muscle.
As it was shown in the current study (Fig. 2), the distribution pattern of different fibre types was heterogeneous within the serial sections. The proportions of oxidative and glycolytic fibres changed in cranio-caudal direction and in areas within a given section. Therefore, classifications of muscles as ‘glycolytic’ or ‘oxidative’ based on biopsies or on analyses of a single cross sections representing a supposed ‘typical distribution pattern’ of the muscle will not reflect the true fibre composition of a whole muscle. For instance, Thorstensson & Carlson or Rantanen et al. found no difference in fibre type proportions between the m. multifidus and the m. longissimus or m. iliocostalis resp. in humans (Thorstensson and Carlson 1987; Rantanen et al. 1994). McFadden et al. described the Mm. multifidi in the rabbit as ‘white muscle’ (McFadden et al. 1984). However, these results were based on local samples only and in comparison with the results represented here and previous results on other small mammalian species, it would be very surprisingly if mm. multifidi in humans and rabbits would be composed predominantly of glycolytic fibres. Beside this, the inferred distribution pattern depended very much on the region and depth in which the sample was taken. A nearly homogenous distribution pattern with more than 90% of the same fibre type was found in only few muscles (e.g. mm. interspinales, intertransversarii or psoas). Distribution pattern along the entire muscle in all orientations should be determined, before any statement on the muscle's metabolic profile is made.
In most of the muscles examined, the percentage of oxidative fibres decreased in caudal direction and the percentage of glycolytic fibres increased inversely. Only in the spinal stabilizers (mm. rotatores, multifidi et semispinales), the percentage of oxidative fibres doubled in caudal direction. Since metabolic profiles of muscles relate to their function, the cranio-caudal gradient in fibre type distribution suggested a functional transition along the longitudinal body axis. Much of the change in fibre type distribution occurred around level L1, the same level, were the m. psoas major arose in the serial section. Because of its higher percentage of glycolytic fibres, the lumbar part of back region investigated is probably more involved in the extensive, fast and forceful sagittal bending movements at asymmetrical gaits (amplitudes of additive mean ‘pelvic movement’ ranged from 27° to 31°, as compared with 10° at symmetrical gaits in O. rufescens; Fischer et al. 2002). The more cranial part of the region investigated with the higher percentage of oxidative fibres is probable more responsible for the rotational and lateral bending movements at symmetrical gaits. Preliminary results on diverse small mammals including O. rufescens, suggest a cranio-caudal raise of intervertebral movement amplitudes (Schilling unpublished). At asymmetrical gaits, the highest amplitudes of sagittal spinal bending were observed in the presacral intervertebral joint (Schilling et al. 1999).
Shifting of the distribution pattern
Alterations of the distribution pattern of fibre types in the cranio-caudal or transverse direction can be the result of a degree of increase or decrease of all fibre types or of only some of them. It was demonstrated in this study that oxidative and glycolytic fibres had the strongest influence on the general distribution pattern, whereas the percentages of oxidative-glycolytic fibres had no effect. Changes in the distribution pattern mainly based on percentages of oxidative and glycolytic fibres, with a constant proportion of oxidative-glycolytic fibres, were also found in back muscles of the cat (Carlson 1978). In the current study, only in muscles mainly consisted of one fibre type (oxidative or glycolytic), the proportion of oxidative-glycolytic fibres was also correlated to the dominant fibre type – the oxidative in mm. rotatores et multifidi or the glycolytic in mm. sacrospinalis et psoas major, respectively.
Muscle fibre size and proportion
In all muscles, oxidative fibres stand out as smallest and glycolytic fibres as largest in their cross-sectional area. Oxidative-glycolytic fibres were intermediate in their size. But, the mean size depends on the muscle, because the ranges of cross-sectional areas overlap between all three muscle fibre types. Sharp size limits were not found between fibre types in the muscles under study and also described in sheep limb and back muscles (Suzuki 1971a,b; Suzuki and Cassens 1983; Peinado et al. 2004).
Location dependent size were described by for back muscles (Carlson 1978) and for limb muscles in the cat (Braund et al. 1995), and the rat (Punkt et al. 1998). In general, oxidative fibres were smaller than the glycolytic ones in superficial muscle regions, whereas in deeper regions with a higher percentage of oxidative fibres, all fibre types were nearly of the same size. A strong positive correlation between proportion and cross-sectional area was found in the mm. semispinales, rotatores et multifidi, and also in m. spinalis for oxidative fibres in the current study.
Evolution of paravertebral musculature
The anatomy of the trunk musculature is highly interesting from an evolutionary point of view. During the evolution of tetrapods major reorganizations of the trunk musculature took place. Homonomous myomeres of fish-like ancestors (Winterbottom 1974) fused and formed longer functional tracts along the trunk extending over multiple segments. For example, in salamanders multisegmental tracts are present in the hypaxial musculature of the trunk whereas the epaxial muscles are segmental (Francis 1934; Carrier 1993). In amniotes, also the epaxial myomeres fused to multisegmental functional units and formed the transversospinalis, longissimus and iliocostalis muscle systems as in lizards (Oldham and Smith 1975; Ritter 1995) or crocodilians (Frey 1988). But also in frogs, epaxial multisegmental tracts are essential for jumping (Jenkins and Shubin 1998). The most derived trunk musculature has evolved in mammals, both in the hyp- and epaxial portions (Bolk et al. 1938; Starck 1978; Fischer 2004). Especially, the epaxial musculature as the longissimus and iliocostalis muscles were strengthen (in comparison with other amniotes) and this strengthening is suggested to be connected to the evolution of dorso-ventral mobility. Mammals are the only animals, that regularly use asymmetrical gaits during locomotion, such as gallop or halfbound, with extensive sagittal spine movement.
It is particularly evident from the results of this study that the reorganization in mammals (as compared with the hypothetical fish-tetrapod transition) not only includes remarkable macroscopic changes (tracts) but also intramuscular fibre type patterns. These derived features are particularly complex in the rib-free and adjacent rib-bearing presacral region of the spine. This pattern suggests that it evolved in connection with the sagittal mobility of the spine in this region. The functional separation of the muscles and even muscle parts as indicated by their fibre type distribution pattern (this study), the mobility and reduction of ribs in the presacral region, appear to be interdependent and a key derived feature in mammalian evolution. Thus far, reconstructing even preliminary transformation series that led to this key feature in mammals is hardly possible, because of the lack of detailed comparative data from extant amniote representatives.
Conclusions
Characteristics of mammalian paravertebral muscles supplemented by the results from the present study were summarized in Table 3. Concepts, derived on humans and thought as human specific were proved to be more general within mammals by the investigation of the metabolic profile of a mammalian species. The metabolic profiles of the back muscles examined suggested clear functional specializations of different paravertebral muscles, their intramuscular regions and even in cranio-caudal direction. But all together (as m. erector spinae) can be interpreted as an antigravity muscle–complex counteracting enduringly against the rebound tendency caused by gravitation. The observed fibre type distribution pattern is suggested to be related to the major reorganizations of the axial musculature in tetrapods and especially to the evolution of sagittal mobility in mammals.
Mm. rotatores, multifidi, interspinales et intertransversarii appeared best suited to fulfil the assumed function of local stabilizers and were comparable with the deep oxidative region shown for antigravity muscles in limbs (Ariano et al. 1973; Collatos et al. 1977; Armstrong 1980; Burke 1981; Armstrong et al. 1982; Braund et al. 1995; Fischer 1999). Mm. longissimus, iliocostalis et psoas major were considered as global mobilizer muscles and were equivalent to the more superficial glycolytic muscle parts in anti-gravity muscles. The intermediate physiological characteristics of mm. semispinales, spinalis et quadratus lumborum suggested them as global stabilizers.
Due to the heterogeneous distribution pattern of muscle fibre types in cranio-caudal direction as well as in the transverse plane, a general classification of a muscle as ‘oxidative’ or ‘glycolytic’ is not warranted. Biopsy results are unsuitable for generalizing fibre type characterizations for complete muscles. The smallest cross-sectional area of fibres was found in oxidative ones, the largest in glycolytic fibres; oxidative-glycolytic fibres were intermediate in size in all muscles. The size ranges were overlapping. Sharp size limits did not exist between fibre types in the material examined. The mean cross-sectional area of fibre types depended on the muscle and in some muscles on the fibre type composition in the very location of a given fibre. The percentage of oxidative and of glycolytic fibres correlated with the distribution pattern of muscle fibre types, whereas the proportion of oxidative-glycolytic fibres was more or less homogeny.
Acknowledgements
I wish to thank M.S. Fischer, D.R. Carrier, R.G. Beutel, and A. Haas for stimulating discussions and valuable comments on the manuscript. The technical assistance by I. Weiß and M. Roser is gratefully acknowledged. The study was generously funded by the Berufsgenossenschaft Nahrungsmittel und Gaststätten (Germany, Erfurt). The pikas were kindly provided by A. Puget (Toulouse, France).




