Tertiary plotopterids (Aves, Plotopteridae) and a novel hypothesis on the phylogenetic relationships of penguins (Spheniscidae)
Die tertiären Plotopteriden (Aves, Plotopteridae) und die Verwandtschaftsbeziehungen der Pinguine (Spheniscidae)
Abstract
enPlotopterids (Aves: Plotopteridae) are extinct wing-propelled diving birds which exhibit a strikingly similar wing morphology to penguins (Spheniscidae), but also share derived characters with ‘pelecaniform’ birds that are absent in penguins. The similarities between Plotopteridae and Spheniscidae have hitherto been attributed to convergence, and plotopterids were considered to be most closely related to the ‘pelecaniform’ Phalacrocoracidae (cormorants) and Anhingidae (anhingas). However, here I show that assignment of plotopterids to ‘pelecaniform’ birds does not necessarily preclude them from being the sister taxon of penguins. Cladistic analysis of 68 morphological characters resulted in sister group relationship between Plotopteridae and Spheniscidae, and the clade (Plotopteridae + Spheniscidae) was shown to be the sister taxon of the Suloidea, i.e. a clade including Sulidae (boobies and gannets), Phalacrocoracidae, and Anhingidae. Derived characters are discussed which support this novel hypothesis. Paedomorphosis probably accounts for the absence of derived characters in penguins that are shared by Plotopteridae and members of the Steganopodes. Plotopterids exemplify the importance of fossil birds for analyzing the phylogenetic relationships of modern taxa that exhibit a highly apomorphic morphology.
Zusammenfassung
deDie Plotopteriden (Aves: Plotopteridae) sind eine ausgestorbene Gruppe von Flügeltauchern, deren Flügelbau dem von Pinguinen (Spheniscidae) erstaunlich ähnelt. Plotopteridae teilen aber auch abgeleitete Merkmale mit ‘pelikaniformen’ Vögeln, welche bei Pinguinen nicht vorkommen. Die gemeinsamen Merkmale von Plotopteriden und Pinguinen wurden bisher Konvergenz zugeschrieben und die Plotopteridae wurden als nächste Verwandte der ‘pelikanartigen’ Kormorane (Phalacrocoracidae) und Schlangenhalsvögel (Anhingidae) angesehen. Zuordnung der Plotopteriden zu den ‘pelikanartigen’ Vögeln steht allerdings nicht im Widerspruch dazu, daß die Plotopteriden das Schwestertaxon der Pinguine sind. Eine kladistische Analyse von 68 morphologischen Merkmalen resultierte in einem Schwestergruppenverhältnis zwischen Plotopteridae und Spheniscidae und die monophyletische Gruppe (Plotopteridae + Spheniscidae) resultierte als Schwestertaxon der Suloidea, d.h. einer monophyletischen Gruppe welche Sulidae (Tölpel), Phalacrocoracidae, und Anhingidae umfaßt. Abgeleitete Merkmale werden diskutiert, welche diese neue Hypothese stützen. Das Fehlen von abgeleiteten Merkmalen von Plotopteriden und Taxa der Steganopodes in Pinguinen ist vermutlich auf Paedomorphose zurückzuführen. Die Plotopteriden veranschaulichen die Bedeutung fossiler Vögel in der Analyse der Verwandtschaftsbeziehungen moderner Taxa mit stark apomorpher Morphologie.
Introduction
Plotopterids (Aves, Plotopteridae) are extinct, flightless and wing-propelled seabirds that combine a striking mosaic of derived features characteristic for penguins (Spheniscidae) and the ‘pelecaniform’ Suloidea [gannets and boobies (Sulidae), cormorants (Phalacrocoracidae), and anhingas (Anhingidae)] (Olson and Hasegawa 1979). The original description of these birds was based on a proximal end of a coracoid from the early Miocene of California (Howard 1969), but meanwhile partial skeletons were found in late Eocene to early Miocene deposits of Japan and North America (e.g. Hasegawa et al. 1979; Olson 1980; Olson and Hasegawa 1985, 1996; Goedert and Cornish 2002).
The wing of plotopterids is remarkably similar to the flipper of penguins, to which it shows ‘numerous parallels’ (Olson and Hasegawa 1979, p. 689). Similarities to penguins were even noted by Howard (1969) who only knew the proximal coracoid of plotopterids, but Olson (1980) and Olson and Hasegawa (1979, 1996) also reported a number of presumably derived features that are shared by plotopterids and extant ‘pelecaniform’ birds but are absent in penguins. The similarities between plotopterids and penguins were considered to be due to convergence by Olson (1980, p. 56) who noted that only ‘blind adherence to cladistic methodology’ could bring together plotopterids and penguins. (Olson 1980, p. 56) assumed that plotopterids ‘not only belong in the Pelecaniformes, but are clearly derived from members of the suborder Sulae [=Suloidea]’, and (Olson and Hasegawa 1996, p. 742) noted that within that group they ‘definitely cluster with the Anhingidae and Phalacrocoracidae’.
The fossil record of penguins is fairly extensive, dating back into the earliest Tertiary (e.g. Simpson 1946, 1971, 1975; Fordyce and Jones 1990; Clarke et al. 2003 and references therein). However, except for the proximal end of the humerus (Olson 1980, Fig. 2), plotopterids have so far been compared only with modern penguins and not with early Tertiary spheniscids that exhibit a less apomorphic morphology than their modern relatives. Published comparisons between plotopterids and penguins are further restricted to the wing skeleton and the shared similarities in the hind limb have not been mentioned.
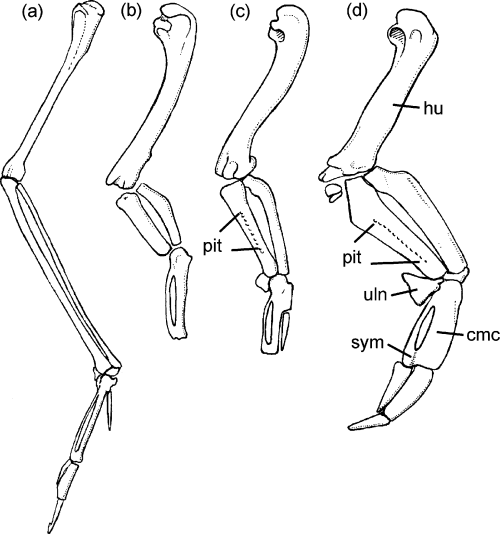
Right wing in comparison: (a) Phalacrocorax carbo (Phalacrocoracidae); (b) Mancalla cedrosensis (Mancallinae, Charadriiformes, after Howard 1971); (c) Copepteryx hexeris (Plotopteridae, after Olson and Hasegawa 1979, 1996; Goedert and Cornish 2002 [carpometacarpus]); (d) Eudyptula minor (recent, Spheniscidae). Abbreviations: cmc, carpometacarpus; hu, humerus; pit, row of pits on ulna for attachment of feather quills; sym, symphysis metacarpalis distalis; uln, os carpi ulnare. Not to scale and slightly schematic; in (b) and (c) the distal phalanges are not shown
Here it is shown that assignment of Plotopteridae to the ‘Pelecaniformes’ does not necessarily preclude them from being the sister taxon of penguins, and for the first time the relationships of the Plotopteridae are subjected to a cladistic analysis.
Phylogenetic relationships of penguins
Penguins exhibit a highly apomorphic morphology and strongly differ from all other extant birds, which aggravates analysis of their phylogenetic affinities. There is, however, consensus that they derive from flying ancestors, and virtually all modern authors considered the closest relatives of penguins to be aquatic birds of the taxa Procellariiformes (albatrosses, petrels, shearwaters and allies), Gaviiformes (loons), or ‘Pelecaniformes’ (tropicbirds, frigatebirds, pelicans, gannets, cormorants and allies) (see Sibley and Ahlquist 1990 for a review of the history of avian classification).
Simpson (1946) considered penguins to be most closely related to procellariiform birds and noted that, concerning some osteological features, early Tertiary penguins exhibit a less derived morphology and resemble procellariiform birds. He did, however, not list derived characters that are shared by all penguins and procellariiform birds and only few comparisons were made with non-procellariiform taxa.
Cladistic analyses of hindlimb myology characters by McKitrick (1991) and of cranial and vertebral characters by Livezey and Zusi (2001) resulted in sister group relationship between Spheniscidae and Procellariiformes, although for computational limitations both analyses had to be terminated before the most parsimonious tree was found; character support was not discussed and Livezey and Zusi (2001) explicitly considered their study preliminary.
Cracraft (1985, 1988) considered Spheniscidae to be the sister taxon of the clade (Gaviidae + Podicipedidae) but regarded this clade ill-defined (Cracraft 1988, p. 349). An analysis of 148 morphological characters for 43 neornithine taxa by Mayr and Clarke (2003) resulted in sister group relationship between Spheniscidae and a clade including Procellariiformes, Podicipedidae and Gaviidae. Character support for this node (Mayr and Clarke 2003, Fig. 1) is, however, also weak as three of the nine recovered synapomorphies are reversals into the primitive condition for Neornithes, one is not present in Podicipedidae, and three are related to the reduced hallux of these birds. There is both molecular and morphological evidence that Podicipedidae are the sister group of flamingos (Phoenicopteridae) (van Tuinen et al. 2001; Chubb 2004; Mayr 2004).
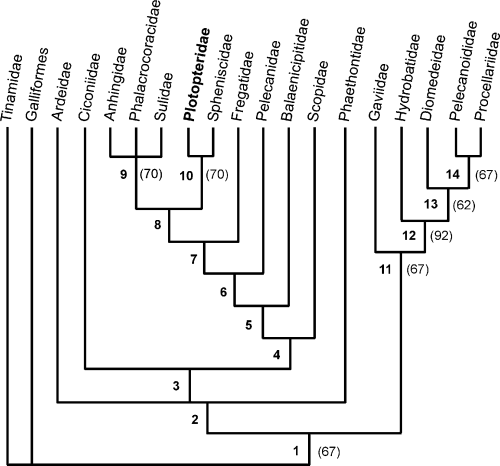
Strict consensus tree of six most parsimonious trees (length = 176, CI = 0.44, RC = 0.29) resulting from an analysis of the character matrix in Appendix 2. The nodes are characterized by the following synapomorphies that were found in all most parsimonious trees (if not indicated otherwise, character transformation is 0→1; asterisked characters have CI = 1.0; superscript letters indicate character that were only found in either the ACCTRAN or DELTRAN transformation mode): 1– 13, 19A, 29A, 31 (1→0); 2– 6, 38, 51A, 53; 3– 7, 8, 9, 34, 61; 4– 1, 5D, 10, 27, 47, 62*; 5– 3, 4 (0→2)*, 25A, 28 (0→2), 35A, 39 (0→2/1→2); 6– 16A, 19 (1→0)A, 26A*, 31A, 33A, 45A, 52, 54 (1→2)A, 56 (0→2), 57, 59, 63, 64; 7– 8 (1→0), 9 (1→0), 16D, 25 (1→0)A, 26D*, 29 (1→0)A, 35D, 46A, 47 (1→0), 49, 51 (1→0); 8– 7 (1→0)A, 15A, 19A, 21A*, 28 (2→1), 33D, 34 (1→0)A, 43*, 45D; 9– 11*, 12, 14, 20*, 21D*, 22A, 25A, 31D, 46 (1→0)A, 65, 66*, 67*, 68A; 10– 3 (1→0)A, 6 (1→0)A, 10 (1→0)A, 32*, 36*, 37*, 41*, 42*, 46D, 48*, 50*, 53 (1→0)A, 56 (2→0)A, 58A, 63 (1→0)A; 11– 18, 29D, 44*, 52, 54 (1→4), 57D; 12– 1, 4*, 5, 30*, 40*, 58 (0→2/1→2); 13– 24, 39A; 14– 13 (1→0), 17. Bootstrap values above 50% are indicated in parentheses to the right of the internodes
Molecular studies, too, did not yield congruent results concerning the position of penguins. The DNA–DNA hybridization studies of Sibley and Ahlquist (1990), that have repeatedly been criticized for methodological reasons (e.g. Houde 1987; Lanyon 1992; Harshman 1994), supported sister group relationship between Spheniscidae and the clade (Gaviidae + Procellariiformes).
Analysis of mitochondrial DNA sequences by Hedges and Sibley (1994) showed penguins to be within a clade including the shoebill (Balaenicipitidae), pelicans (Pelecanidae), storks (Ciconiidae), loons and procellariiform birds, but did not resolve their exact position within that clade; reanalysis of the data by Farris et al. (1999) showed this grouping to be poorly supported.
An analysis combining mitochondrial DNA data, isozymes, and behavioral characters by Paterson et al. (1995) resulted in monophyly of a clade including Spheniscidae and Phalacrocoracidae, the only ‘pelecaniform’ group included in this study.
Analysis of nuclear and mitochondrial DNA sequences of a small taxon sample by Cooper and Penny (1997) resulted in monophyly of the clade (Spheniscidae + (Gaviidae + Procellariiformes)). Analysis of DNA–DNA hybridization data, as well as nuclear and mitochondrial DNA sequences by van Tuinen et al. (2001) supported monophyly of the clade (Spheniscidae + Procellariiformes), which in turn was shown to be the sister taxon of the Gaviidae in the analysis of the sequence data (the Gaviidae were omitted from the analysis of the DNA–DNA hybridization data). Sister group relationship between penguins and procellariiform birds was, however, not supported in the analyses of Siegel-Causey (1997, mitochondrial DNA), Chubb (2004, ZENK gene), and Simon et al. (2004, prepoinsulin gene), although the results of these studies are not congruent concerning the position of penguins.
Materials and Methods
Skeletons of the following extant taxa were examined in the collections of Forschungsinstitut Senckenberg and Museum für Naturkunde, Berlin (sequence of taxa as in Appendix 2): Tinamidae: Crypturellus, Rhynchotus, Tinamus. Galliformes: Megapodius, Crax, Gallus, Meleagris, Numida, Phasianus. Ardeidae: Agamia, Ardea, Ardeola, Botaurus, Butorides, Cochlearius, Egretta, Ixobrychus, Nycticorax. Ciconiidae: Anastomus, Ciconia, Leptoptilus, Mycteria. Gaviidae: Gavia. Diomedeidae: Diomedea. Hydrobatidae: Oceanodroma. Pelecanoididae: Pelecanoides. Procellariidae: Bulweria, Calonectris, Daption, Fulmarus, Procellaria, Pterodroma, Puffinus. Scopidae: Scopus. Balaenicipitidae: Balaeniceps. Phaethontidae: Phaethon. Fregatidae: Fregata. Pelecanidae: Pelecanus. Spheniscidae: Aptenodytes, Eudyptes, Eudyptula, Pygoscelis, Spheniscus. Anhingidae: Anhinga. Phalacrocoracidae: Phalacrocorax. Sulidae: Sula.
Coding of the Plotopteridae is based on descriptions and illustrations of Tonsala hildegardae and Copepteryx hexeris in Olson (1980), and Olson and Hasegawa (1996), and on a skull of an as yet unnamed plotopterid taxon in Hasegawa et al. (1979, p. 17).
The terms ‘penguin’ and ‘Spheniscidae’ are here used for all taxa more closely related to modern penguins than to Plotopteridae or any non-spheniscid modern avian taxon.
Comparisons with fossil penguins are based on the descriptions and illustrations in Simpson (1946, 1971, 1975); Fordyce and Jones (1986, 1990) and Clarke et al. (2003). With regard to the characters included in the analysis, and inasmuch as these are observable owing to preservation, the known fossil penguins do not differ from their modern relatives. If not stated otherwise, all characters listed as characteristic for Spheniscidae are further present in the fossil taxa.
Non-osteological characters were taken from the literature, the character matrix is in part based on Cracraft (1985) and Mayr (2003), the only comprehensive analyses of ‘pelecaniform’ relationships that are based on osteological characters. Anatomical terminology follows Baumel et al. (1993).
As the paddle-shaped wing of plotopterids and penguins certainly is an adaptation to a special mode of wing-propelled diving, characters relating to the wing and pectoral girdle of these birds may be functionally correlated. It was thus tried to limit the number of characters relating to the wing and pectoral girdle morphology of these birds and to exclude characters that are obviously correlated, i.e. flattening of ulna and of radius and carpometacarpus. Characters for which such a correlation was not obvious, i.e. the flattening of the ulna and the widening of the scapula, were included as separate characters.
A phylogenetic analysis with paup 3.1 (Swofford 1993) was performed using a data set of 68 anatomical characters (see Appendices 1 and 2). All characters were treated as unordered. The shortest tree was found with the ‘branch-and-bound’ search option. Character transformation was evaluated with both the delayed transformation (DELTRAN) and accelerated transformation (ACCTRAN) mode. Consistency index (CI) and rescaled consistency index (RC) were calculated, and the robustness of the tree was evaluated with a bootstrap analysis of 1000 replicates.
Outgroup comparisons were made with the palaeognathous Tinamidae, and with Galliformes which are among the most basal lineages of neognathous birds and generally considered to be outside the clade including penguins and the other ingroup taxa (e.g. Sibley and Ahlquist 1990; Groth and Barrowclough 1999; Livezey and Zusi 2001; Mayr and Clarke 2003).
Results of phylogenetic analysis
Analysis of the character matrix in Appendix 2 yielded six most parsimonious trees (length = 176, CI = 0.44, RC = 0.29). The strict consensus cladogram of these trees is shown in Fig. 1.
The analysis showed Plotopteridae and Spheniscidae to be sister taxa and this clade is supported by a bootstrap support value of 70%. The clade (Plotopteridae + Spheniscidae) was shown to be the sister group of the Suloidea but this grouping was not retained in the bootstrap analysis. Monophyly of the Suloidea to the exclusion of the Plotopteridae received a bootstrap value of 70%. Gaviiformes and Procellariiformes were shown to be sister taxa, although this clade was retained in only 57% of the bootstrap replicates.
Relationships of the other ingroup taxa is in concordance with the analysis of Mayr (2003), except that in the present study Fregatidae are shown to be more closely related to Suloidea than are Pelecanidae, whereas the reverse was the case in the analysis of Mayr (2003). The relative position of Fregatidae and Pelecanidae received, however, no bootstrap support in either analysis.
Characters supporting sister group relationship between Plotopteridae and Spheniscidae (Fig. 1, node 10)
The following characters are shared by plotopterids and penguins and were optimized as synapomorphies of node 10 in Fig. 1; characters in Appendix 1 are referenced by the numbers given in parentheses:
(32) Scapula forming a thin, sheetlike, greatly expanded blade. The scapula of plotopterids is ‘unlike that of any other birds except penguins’ (Olson 1980, p. 55).
(36) Proximal end of humerus with a deep, rounded head and a ventrally directed caput humeri. The proximal humerus of plotopterids has ‘a resemblance among known birds only to penguins’ (Olson 1980, p. 54).
(37) Distal end of humerus strongly flattened and ventrally protruding, sulci scapulotricipitalis et humerotricipitalis forming two deep furrows and shifted towards ventral margin of bone. This character, that occurs in a variety of wing-propelled divers, is considered to be functionally independent from the previous one, that is restricted to plotopterids and penguins (see also comments in Appendix 1).
(41) Ulna and radius flattened and greatly expanded (Fig. 2). Although they are less flattened and widened than in modern penguins, ulna and radius of plotopterids strongly resemble the corresponding elements of a late Paleocene/early Eocene penguin figured by Fordyce and Jones (1986, 1990).
A highly characteristic derived peculiarity of the ulna of plotopterids is the presence of a row of marked pits for the attachment of feather quills (Olson 1980, Fig. 3; Olson and Hasegawa 1996, Fig. 6D). Olson (1980, p. 55) considered this to be ‘a unique condition in birds, and quite unlike that in penguins in which the remiges can no longer be differentiated from other feathers of the wing’. However, a similar row of pits, although less marked, is found in some modern penguins (Eudyptes, Eudyptula, Fig. 3).
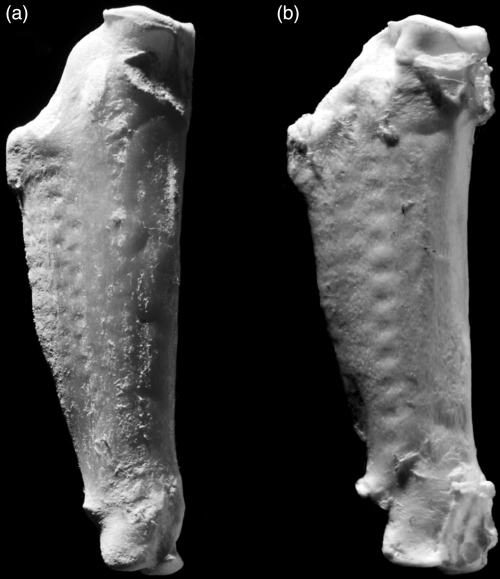
Right ulna of modern penguins, showing distinct rows of pits for the attachment of feather quills similar to the, though more strongly marked, pits in plotopterids (Olson and Hasegawa 1996, Fig. 6D). (a) Rockhopper Penguin (Eudyptes chrysocome); (b) Blue Penguin (Eudyptula minor). Not to scale
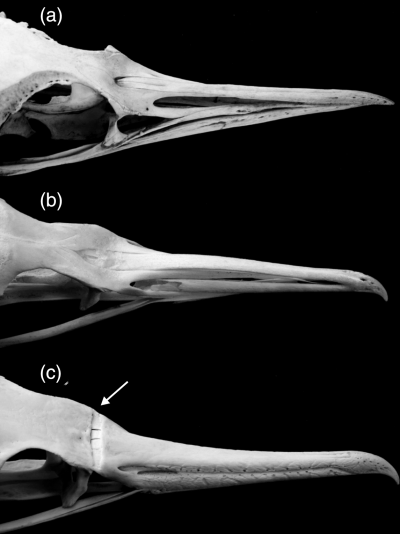
Skull in comparison. (a) Adult Aptenodytes patagonicus (Spheniscidae); (b) juvenile Phalacrocorax carbo (specimen SMF 2482 in collection of Forschungsinstitut Senckenberg); (c) adult P. carbo (Phalacrocoracidae). The arrow points to the naso-frontal hinge in P. carbo. Not to scale
(42) Os carpi ulnare flattened, with large caudal expansion, in plotopterids ‘foreshadowing the even more specialized structure of penguins’ (Olson 1980, p. 55). This character is unique to Plotopteridae and Spheniscidae.
(46) Tarsometatarsus greatly abbreviated, measuring only about 1/4 of the length of the tibiotarsus (Fig. 4). An equally abbreviated tarsometatarsus to that of penguins and plotopterids only occurs in Fregatidae (frigatebirds), Nyctibiidae (potoos) and Steatornithidae (oilbird). This character was optimized as a synapomorphy of node 7 if the character transformation was evaluated with the ACCTRAN mode.
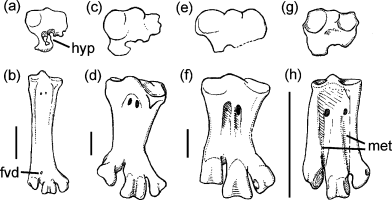
Dorsal aspect (b, d, f, h) and proximal end in proximal view (a, c, e, g) of right tarsometatarsus in comparison; (a, b) Miocene giant anhinga Macranhinga paranensis (Anhingidae, after Noriega 1992, p. 1); (c, d) plotopterid Copepteryx hexeris (Plotopteridae, after Olson and Hasegawa 1979, Fig. 2; Olson and Hasegawa 1996, Fig. 10); (e, f) late Eocene penguin Palaeeudyptes marplesi (Spheniscidae, after Simpson 1971, Fig. 3, reversed to facilitate comparison); (g, h) extant Eudyptula minor (Spheniscidae). Abbreviations: fvd, foramen vasculare distale; hyp, hypotarsus; met, furrows between incompletely fused metatarsalia in modern penguins. Scale bars = 2 mm
(48) Tarsometatarsus, foramen vasculare distale distally open or completely absent (Fig. 4). Among modern birds, the author found this character only in Fregatidae and Nyctibiidae (a foramen vasculare distale is, however, present in Eocene stem group Fregatidae and Nyctibiidae, see Olson 1977; Mayr 1999, 2001); a foramen vasculare distale is further absent in moas (Dinornithidae).
A closed foramen vasculare distale is present in the putative plotopterid Phocavis maritimus from the late Eocene of North America (Goedert 1988). However, the position of this taxon, which is known from the tarsometatarsus only, to be considered uncertain. Apart from being larger, the tarsometatarsus of Phocavis resembles the corresponding bone of early Eocene frigatebirds (Olson 1977) with which it has not yet been compared. If a closer relationship to plotopterids can be confirmed by more material, the closed foramen vasculare suggests that Phocavis is the sister taxon of the clade (Plotopteridae + Spheniscidae).
(50) Limb bones heavily pachyostotic, i.e. bone walls greatly thickened. See Hasegawa et al. (1979, p. 16) concerning pachyostosis in the Plotopteridae.
Among other wing-propelled diving birds only the extinct Lucas auks (Mancallinae, e.g. Howard 1966, 1970, 1971) exhibit a wing morphology comparable to that of plotopterids and penguins (Fig. 2). However, in the case of Lucas auks the similarities concern only the distal end of the humerus and the shape of ulna and radius, the latter two bones being much more abbreviated than in plotopterids and penguins with the grotesquely shortened ulna measuring less than half the length of the humerus and being even shorter than the carpometacarpus (Fig. 2).
Three of the above-listed characters (32, 36 and 42) are not found in any other avian taxon except plotopterids and penguins, including other wing propelled diving birds, such as auks (Charadriiformes, Alcidae), Lucas auks, and diving petrels (Procellariiformes, Pelecanoididae). It is thus likely that these characters are related to a special mode of wing-propelled diving that penguins and plotopterids inherited from a common ancestor, rather than wing-propelled diving per se.
Plotopteridae and Spheniscidae further share a highly derived tarsometatarsal morphology, whereas this bone is very different in all other wing-propelled diving birds including Lucas auks.
Characters supporting inclusion of Spheniscidae into the ‘pelecaniform’ Steganopodes (Fregatidae, Pelecanidae, and Suloidea, Fig. 1, node 8)
The full character support of each node is given in the legend of Fig. 1. Some of the recovered synapomorphies are homoplastic and in the following only those characters are discussed that have a limited distribution among birds and that are present in Spheniscidae; note that the characters were optimized as synapomorphies of different hierarchical levels (characters in Appendix 1 are referenced by the numbers given in parentheses).
(4) External narial openings greatly reduced. This character was optimized as a synapomorphy of node 5 in Fig. 1.
(16) Recessus tympanicus dorsalis greatly enlarged and situated rostrally to the articular facets of the quadrate. This character was shown to be a synapomorphy of node 7 (DELTRAN) or 6 (ACCTRAN) in Fig. 1 and has been secondarily lost in Anhingidae.
(26) Caudalmost thoracic vertebrae opisthocoelous (Fig. 5). This character is shared by Fregatidae, Spheniscidae, Plotopteridae, and Suloidea and was shown to be a synapomorphy of node 7 (DELTRAN) or 6 (ACCTRAN) in Fig. 1. Opisthocoelous thoracic vertebrae otherwise occur in very few other birds, all of which have not been considered closely related to penguins (e.g. some Charadriiformes and Psittaciformes, see Beddard 1898, p. 111; Stresemann 1927–1934, p. 61).
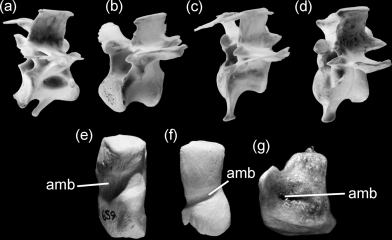
Caudalmost free thoracic vertebra (a, b, c, d) and patella (e, f, g) in comparison. (a) Fulmarus glacialis (Procellariidae); (b, f) Sula bassana (Sulidae); (c, g) Phalacrocorax carbo (Phalacrocoracidae); (d, e) Spheniscus magellanicus (Spheniscidae). Abbreviation: amb, furrow for tendon of musculus ambiens. Note the opisthocoelous thoracic vertebra in Phalacrocoracidae, Sulidae and Spheniscidae. Not to scale
(33) Sternum, apex carinae pointed and projecting far rostrally to sulci coracoidei. This character was optimized as a synapomorphy of node 8 (DELTRAN) or 6 (ACCTRAN) in Fig. 1.
(43) Patella very large, with marked furrow for tendon of musculus ambiens (closed to form a foramen in Plotopteridae, Anhingidae and some Phalacrocoracidae) (Fig. 5). Within Phalacrocoracidae, closure of this foramen may be age dependent (Livezey 1992, p. 203). A perforated patella otherwise occurs in the non-neornithine Hesperornithidae (e.g. Martin and Tate 1976), and according to (Stresemann 1927–1934, p. 800), in the Musk Duck Biziura lobata (Anatidae). This character was optimized as a synapomorphy of node 8 in Fig. 1.
(61) Glandula nasalis (‘salt gland’) single-lobed and with only a single efferent ductus (Technau 1936; Herbert 1975). The presence of this character is especially remarkable because of the completely different way of living of the shoebill, penguins and ‘pelecaniform’ birds, ranging from exclusively terrestrial to limnic and marine. This character, which is unknown for Plotopteridae, occurs in few other taxa that have not been considered closely related to penguins, i.e. Struthionidae (ostriches), Galliformes (landfowl), Podicipedidae, Bucerotidae (hornbills), and Trochilidae (hummingbirds) (Technau 1936); it was optimized as a synapomorphy of node 3 in Fig. 1.
(62) Eggshell covered with a layer of amorphous calcium carbonate. This character otherwise only occurs in some cuckoos (Cuculidae), some herons (Ardeidae), and, possibly, some Galloanseres (in which the chemical composition of the layer is unknown, Mikhailov 1995); it is unknown for Plotopteridae and was optimized as a synapomorphy of node 4 in Fig. 1. In Podicipedidae and Phoenicopteridae the eggshell is covered with a layer of amorphous calcium phosphate (Mikhailov 1995).
(64) Young fed down gullet of adults. This character is unique to penguins and the traditional (sensu Wetmore 1960) ‘Pelecaniformes’ (van Tets 1965; Cracraft 1985). It was listed as a synapomorphy of ‘Pelecaniformes’ by Cracraft (1985, p. 841) who considered it ‘a good synapomorphy for the order’. The character is of course unknown for the Plotopteridae and was optimized as a synapomorphy of node 6 in Fig. 1.
In penguins, plotopterids (Olson and Hasegawa 1996, Fig. 7), and steganopod taxa the alae praeacetabulares ilii are further oriented in a markedly horizontal plane (character 25 of Cracraft 1985). This character was listed as a synapomorphy of ‘Pelecaniformes’ by Cracraft (1985, p. 841) who considered it a ‘good synapomorphy for the order’ but noted its presence in penguins; it was not included in the present analysis as it is difficult to code it into discrete character states.
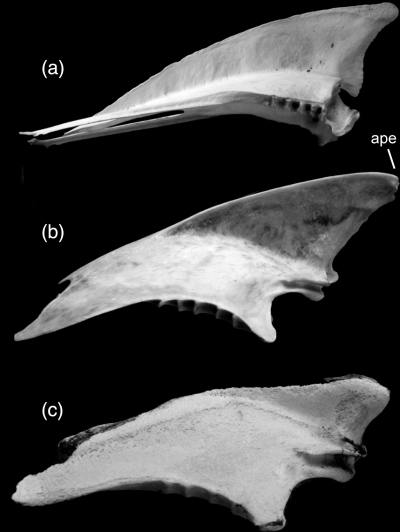
Sternum in comparison; (a) adult Spheniscus magellanicus (Spheniscidae); (b) adult Phalacrocorax carbo (Phalacrocoracidae); (c) juvenile P. carbo (specimen SMF 2482). Abbreviation: ape, apex carinae. Not to scale
Characters listed by Olson (1980) as evidence for monophyly of (Plotopteridae + ‘Pelecaniformes’) to the exclusion of Spheniscidae
Olson (1980, p. 51) listed ten characters in which ‘the Plotopteridae resemble the Pelecaniformes and differ from Sphenisciformes and Charadriiformes’ (the latter being mentioned to exclude affinities between Plotopteridae and flightless auks):
- 1
Skull without ‘supraorbital furrows for salt glands’. The position and size of the narial gland is highly variable within closely related birds (see Technau 1936), as exemplified by Charadriiformes (e.g. well developed in Haematopus ostralegus versus poorly developed in Scolopax rusticola) or Anseriformes (e.g. well developed in Somateria mollissima versus poorly developed in Anas platyrhynchos). The absence of supraorbital furrows for narial glands unquestionably is a primitive feature and their presence in penguins may well be autapomorphic for the Spheniscidae. As noted above, the narial gland of penguins agrees with that of the Steganopodes (Pelecanidae, Fregatidae and Suloidea) but differs from Procellariiformes and Gaviiformes in the presence of a single efferent ductus (Technau 1936; Herbert 1975). Herbert (1975, p. 85) noted that narial glands tend to be well-developed in ‘marine birds, especially penguins and petrels, which feed on invertebrates with a high salt content’, whereas they are small in marine birds which mainly feed on fish, as do ‘pelecaniform’ birds.
- 2
Skull with ‘deep transverse naso-frontal hinge’ (Fig. 6). See discussion concerning the absence of this character in penguins.
- 3
Sternum with ‘large, pointed carina projecting far anterior [=cranial] to coracoidal sulci’. This character does not distinguish plotopterids from penguins in which the apex carinae does point markedly cranially (Fig. 7).
- 4
Furcula ‘articulating solidly by a large rounded facet with apex of carina’ (Fig. 8). See discussion concerning the absence of this character in penguins.
- 5
Scapula ‘with very large acromion projecting anteriorly [=rostrally] well beyond coracoidal articulation’. This character is also present in Mesozoic non-neornithine birds (e.g. Clarke and Norell 2002), palaeognathous Tinamidae, and basal Neognathae such as Galliformes, and thus probably primitive within neornithine birds. Its absence in modern penguins may be related to the extreme specialization of the wing for underwater propulsion.
- 6
Coracoid ‘with large flat furcular facet’. This character does not distinguish plotopterids from penguins in which the facies articularis scapularis of the coracoid also is flat. The coracoid of plotopterids is distinctive because of its widely flared sternal extremity (Olson and Hasegawa 1996, Fig. 1), a feature which is, however, also present in a penguin from the Late Oligocene of New Zealand (Fordyce and Jones 1990, Fig. 18.6).
- 7
Coracoid with ‘procoracoid process simple, without foramen’. The foramen in the coracoid of modern penguins probably is not homologous to a true foramen nervi supracoracoidei as found in, e.g. Gaviidae and Procellariiformes. This foramen, which is incompletely closed in some modern penguins (e.g. Zusi 1975; Stephan 1979), is absent in the ‘Waipara bird’, the earliest known penguin (Fordyce and Jones 1986, 1990). It is further absent in juvenile penguins, whereas it is present in juveniles of other avian taxa in which the adults exhibit a foramen nervi supracoracoidei (Fig. 9). Both, the fossil record and the ontogeny of penguins thus indicate that it evolved in the stem lineage of the Spheniscidae, probably by ossification of a part of the membrana sternocoracoclavicularis.
- 8
Femur ‘with proximal and distal ends proportionately broader, neck elongate’. The femur of the plotopterid Copepteryx resembles that of early Tertiary penguins (compare Olson and Hasegawa 1996, Fig. 11 with Simpson 1971, Fig. 18 or Clarke et al. 2003, Fig. 5).
- 9
Tibiotarsus, ‘internal condyle (…) with marked medial deflection, and tendinal groove and openings wide’. This character does not distinguish plotopterids from penguins (see also Cracraft 1985, p. 841).
- 10
Tarsometatarsus with ‘metatarsals completely fused, hypotarsus with large medial crest, outer trochlea elevated well above others, inner trochlea elongate and at same level as middle trochlea’. Again, this character complex distinguishes plotopterids only from modern penguins but not from early Tertiary taxa, the tarsometatarsus of which is much more similar to that of the Plotopteridae (Fig. 4). Contrary to the tarsometatarsus of all members of the Suloidea, the hypotarsus of plotopterids and penguins lacks ossified canals and exhibits a distally open foramen vasculare distale; it is further much more abbreviated than the tarsometatarsus of all Suloidea, including the giant Miocene Anhingidae that are of comparable size to large plotopterids (e.g. Noriega and Alvarenga 2002; Alvarenga and Guilherme 2003) (Fig. 4).
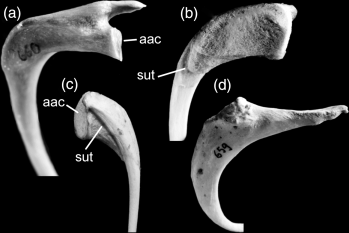
Furcula in comparison. (a) Adult Phalacrocorax carbo (Phalacrocoracidae); (b) juvenile P. carbo, lateral aspect (specimen SMF 2482, reversed to facilitate comparisons); (c) juvenile P. carbo, medial aspect (specimen SMF 2482, reversed to facilitate comparisons); (d) adult Spheniscus magellanicus (Spheniscidae). Abbreviations: aac, facies articularis acrocoracoidea; sut, suture in juvenile cormorants (see text). Not to scale
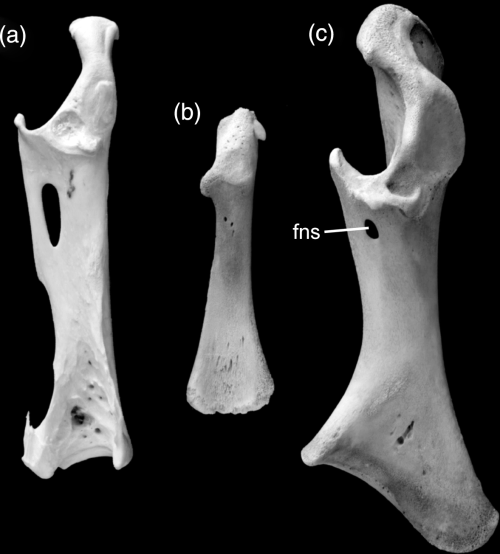
Right coracoid in comparison. (a) Adult Spheniscus magellanicus (Spheniscidae); (b) juvenile Spheniscus humboldti (Spheniscidae); (c) juvenile Bugeranus carunculatus (Gruidae). Abbreviation: fns, foramen nervi supracoracoidei. Not to scale
Another derived character that is shared by plotopterids and most ‘pelecaniform’ birds but absent in Spheniscidae, and that was mentioned by Olson and Hasegawa (1996), is the presence of a strongly developed facies articularis acrocoracoidea on the extremitas omalis of the furcula.
Discussion
The present study does not support monophyly of the traditional (sensu Wetmore 1960) ‘Pelecaniformes’ which is in concordance with most other recent phylogenetic analyses of molecular and morphological data (e.g. Sibley and Ahlquist 1990; Siegel-Causey 1997; van Tuinen et al. 2001; Mayr 2003; Chubb 2004– contra Cracraft 1985; Livezey and Zusi 2001). However, placement of penguins within the ‘pelecaniform’ Steganopodes, i.e. the clade including Pelecanidae, Fregatidae and Suloidea (see Cracraft 1985; Mayr 2003), has not been assumed by earlier authors.
Unquestionably, penguins are very different from modern Steganopodes in many respects, as they strongly differ from any modern avian taxon, and especially the different foot structure may have prevented earlier authors from considering a position of penguins within Steganopodes. Whereas the hallux is long and connected with the second toe by a web in all Steganopodes – thereby forming the so-called ‘totipalmate’ foot – it is greatly reduced in penguins. The toes of plotopterids have not yet been described and it is unknown whether these birds had a well developed hallux, but underlying the phylogeny in Fig. 1, the hallux appears to have been secondarily reduced in the stem lineage of either Spheniscidae or the clade (Spheniscidae + Plotopteridae). It is debatable whether there is a short web between the hallux and the second toe in penguins (Stresemann 1927–1934, p. 799), but the presumably derived (e.g. George and Berger 1966, p. 447) arrangement of the deep flexor tendons of penguins (character 54 in Appendix 2) is identical to that of the totipalmate anhingas and cormorants and differs from Gaviiformes and Procellariiformes, despite the fact that the hallux is also reduced in the latter and the foot thus more similar to that of penguins.
Penguins also lack a number of derived osteological characteristics that are shared by plotopterids and ‘pelecaniform’ birds and that must have been lost in the stem lineage of the Spheniscidae, most notably a distinct naso-frontal hinge, a strongly developed facies articularis acrocoracoidea on the extremitas omalis of the furcula, and a furcular articulation facet on the apex carinae of the sternum.
Reversal into the plesiomorphic state of these presumably functionally independent features in different parts of the skeleton can be explained by the assumption that paedomorphosis, i.e. truncated development of morphological traits owing to neoteny (reduced growth rate) or other ontogenetic mechanisms (e.g. Livezey 1995, p. 179), played a major role in penguin evolution.
Fürbringer (1888) and McDowell (1948), e.g. assumed a neotenic origin for the incompletely fused metatarsal bones of penguins (Fig. 4), and many other characters that were considered by earlier authors (e.g. Lowe 1933) to be primitive traits of penguins occur in the ontogeny of other birds (e.g. Starck 1989) and are thus also likely to be paedomorphic, including the incomplete fusion of cranial sutures in some individuals, the unfused processus uncinati, the incomplete fusion of ilium and synsacrum, and the incomplete fusion of the distal metacarpal symphysis (Fig. 2). That some morphological traits of penguins and flightless auks are due to paedomorphosis was also considered possible by Livezey (1988, 1989, 1995) although it has to be emphasized that paedomorphosis does not necessarily have an equal effect on all parts of the involved organ systems (e.g. Livezey 1995, p. 179).
Juveniles of the Black Cormorant (Phalacrocorax carbo) – the only steganopod taxon of which skeletons of juveniles were available to the author – indeed lack a naso-frontal hinge and exhibit large, open narial openings similar to those of penguins (Fig. 6). Moreover, in juvenile P. carbo, the furcula does not articulate with the sternum, the apex carinae of which thus lacks an articulation facet (Fig. 7).
The proximal end of the furcula of a juvenile P. carbo resembles that of plotopterids in lacking a proximally projecting processus acromialis (Fig. 8) which is well-developed in adult Suloidea. Most notably further, the prominent facies articularis acrocoracoidea is formed by ossification of a separate element (apparently an ossified portion of ligamentum acrocoracoclaviculare superficiale) that attaches to the lateral surface of the extremitas omalis, from which it is however still completely separated by a suture (Fig. 8). Thus, a penguin-like furcular extremitas omalis can be derived from a steganopod-like one by an ontogenetic process which suppresses formation of this accessory bony element. The assumption that the acrocoracoclavicular joint of penguins derives from a steganopod-like one gains further support by the fact that, according to Baumel and Raikow (1993, p. 161f.), it is a synovial joint with freely movable bones as in Steganopodes and not a syndesmosis (articulating bones held together by ligaments) as in most other birds.
Taxa which exhibit many paedomorphic, ‘pseudo-primitive’ traits pose severe limitations to numerical cladistic analyses. However, although sister group relationship between Suloidea and the clade (Spheniscidae + Plotopteridae) was not retained in the bootstrap analysis, placement of penguins within Steganopodes is better supported by derived morphological characters than alternative hypotheses on the relationships of penguins. The above listed derived characters that are shared between modern Spheniscidae and Steganopodes are further complemented by a mosaic distribution of highly characteristic derived spheniscid and ‘pelecaniform’ characters in the Plotopteridae, and plotopterids exhibit a remarkably similar osteology to the earliest known penguin, an as yet unnamed taxon from the late Paleocene/early Eocene of New Zealand (Fordyce and Jones 1986, 1990; Jones and Mannering 1997).
True penguins, i.e. members of the Spheniscidae, have not yet been found outside the Southern Hemisphere, but recognition of the Northern Hemisphere Plotopteridae as sister taxon of Spheniscidae may indicate that at least their earliest evolution was not restricted to the Southern Hemisphere.
Acknowledgements
I thank S. Tränkner (Forschungsinstitut Senckenberg) for taking the photographs, and Julia Clarke for critical comments that improved the manuscript.
Appendix
Appendices
Appendix 1. Character descriptions
1. Upper beak, praemaxilla with sharply hooked tip: absent (0), present (1). Note that coding of Sulidae differs from Mayr (2003).
2. Distinct naso-frontal hinge: absent (0), present (1).
3. Osseous narial openings extremely reduced or completely absent: no (0), yes (1).
4. External narial openings: not as follows (0), tubular (1), greatly reduced (2).
5. Upper beak, marked furrow distal of nasal opening: no (0), yes (1). The furrow of Ardeidae is much wider and shallower than that of the other taxa.
6. Ventral surface of upper beak strongly ossified, processus maxillares of palatinum and processus maxillopalatini of maxillare largely fused: no (0), yes (1).
7. Os ectethmoidale vestigial or completely reduced: no (0), yes (1). The Ardeidae show a great variation in the size of the os ectethmoidale (see Payne and Risley 1976, 6, 7, 8); a well-developed ectethmoid bone is probably primitive for the taxon and was coded it accordingly.
8. Ossa maxillaria, processus maxillopalatini greatly enlarged and spongy: no (0), yes (1).
9. Os palatinum, pars choanalis very deep in dorso-ventral direction: no (0), yes (1).
10. Ossa palatina fused along their midline: no (0), yes (1).
11. Os palatinum a flat horizontal plate: no (0), yes (1).
12. Vomer: present (0), absent (1).
13. Well-developed processus basipterygoidei that articulate with the ossa pterygoidea: present (0), absent (1).
14. Processus paroccipitales protruding in caudal direction: no (0), yes (1).
15. Lamina parasphenoidalis (‘basitemporal plate’) essentially flat and rostrolaterally bordered by marked osseous walls, tubercula basilaria strongly developed: absent (0), present (1). In the Sulidae the lamina parasphenoidalis is strongly pneumatic.
16. Recessus tympanicus dorsalis: not as follows (0), greatly enlarged and situated rostrally to the articular facets of the quadrate (1), enlarged and situated laterally to the articular facets of the quadrate (2) (see Saiff 1976, 1978). Usually the recessus tympanicus is small and situated between the articular facets of the quadrate.
17. Fossae temporales very marked and extending to midline of cranium: no (0), yes (1).
18. Fossae glandularum nasales very marked and situated on dorsal surface of supraorbital margin of orbitae: no (0), yes (1).
19. Quadratum, condylus medialis with marked, rostrally or laterally projecting concave articular surface: no (0), yes (1).
20. Quadratum, processus orbitalis reduced: no (0), yes (1).
21. Number of scleral ossicles: 14 or more (0), 13 or less (1); (after Lemmrich 1931; Warheit et al. 1989, and pers. obs.).
22. Styliform process at caudal end of cranium (e.g. Stresemann 1927–1934, Fig. in p. 851, Owre 1967): absent (0), present (1).
23. Columella tubular (Feduccia 1977, Fig. 1): no (0), yes (1).
24. Third cervical vertebra, osseous bridge from processus transversus to processus articularis caudalis (Mayr and Clarke 2003, Fig. 6D): absent (0), present (1).
25. Eighth–11th cervical vertebrae: processus carotici ankylozed along midline, forming an osseous canal: no (0), yes (1).
26. Caudalmost thoracic vertebrae opisthocoelous: no (0), yes (1). See Goedert and Cornish (2002) concerning the presence of opisthocoelous thoracic vertebrae in the Plotopteridae. The condition in Pelecanidae is uncertain as the caudalmost thoracic vertebrae are fused to a notarium.
27. Furcula, extremitas omalis with strongly developed, laterally protruding facies articularis acrocoracoidea which articulates with a distinct ovoid facies articularis clavicularis of the coracoid: no (0), yes (1). The extremitates omales of furcula and coracoid are fused in the Fregatidae.
28. Furcula, apophysis furculae: not as follows (0), abutting with an articular facet at the apex carinae of the carina sterni (1), fused with the apex carinae of the carina sterni (2).
29. Coracoid, foramen nervi supracoracoidei: absent (0), present (1), condition in penguins (2). For the reasons detailed above, the author considers the foramen in the coracoid of penguins to be non-homologous to that of the other ingroup taxa (see above and Fig. 9).
30. Coracoid, extremitas sternalis, processus lateralis greatly elongated: no (0), yes (1).
31. Scapula, acromion very large and projecting rostrally well beyond coracoidal articulation: no (0), yes (1).
32. Scapula forming a thin, sheetlike, greatly expanded blade: no (0), yes (1).
33. Sternum, apex carinae pointed and projecting far rostrally to sulci coracoidei: no (0), yes (1).
34. Sternum, dorsal surface with numerous pneumatic foramina along midline and lateral margins: no (0), yes (1).
35. Sternum, caudal margin, trabecula mediana very short, reaching much less far distally than trabeculae laterales: no (0), yes (1).
36. Humerus, proximal end with a deep, rounded head and a ventrally directed caput humeri: no (0), yes (1).
37. Humerus, distal end strongly flattened and ventrally protruding, sulci scapulotricipitalis et humerotricipitalis forming two deep furrows and shifted towards ventral margin of bone: no (0), yes (1). This character complex is also found on the distal humerus of the Great Auk (Pinguinus impennis) and in Lucas Auks (Mancallinae), the humeri of which otherwise, however, distinctly differ from those of plotopterids and penguins. It appears to be functionally independent of the previous character which is absent in Pinguinus and Mancallinae.
38. Humerus, proximal end, sulcus transversus very deep, long, and rectangular-shaped: no (0), yes (1).
39. Humerus, crista deltopectoralis: not as follows (0), strongly protruding and triangular (1), reduced (2).
40. Humerus, large and strongly protruding processus supracondylaris dorsalis present: no (0), yes (1).
41. Ulna and radius greatly expanded and flattened: no (0), yes (1).
42. Os carpi ulnare flattened, with large caudal expansion: no (0), yes (1).
43. Patella very large and with marked sulcus for tendon of musculus ambiens (closed to form a canal in Plotopteridae, some Phalacrocoracidae, and Anhingidae): no (0); yes (1).
44. Tibiotarsus, proximal end, cristae cnemiales greatly enlarged and strongly proximally protruding: no (0), yes (1).
45. Tibiotarsus, distal end bent medially, condylus medialis protruding farther distally than condylus lateralis: no (0), yes (1).
46. Tarsometatarsus greatly abbreviated, measuring only about 1/4 of the length of the tibiotarsus: no (0), yes (1).
47. Tarsometatarsus, hypotarsus with tendon of musculus flexor digitorum longus and m. flexor hallucis longus enclosed in bony canal: no (0), yes (1).
48. Tarsometatarsus, foramen vasculare distale distally open or completely absent: no (0), yes (1). Although a foramen vasculare distale is absent in modern Fregatidae, it is present in early Tertiary stem group frigatebirds (Olson 1977) and apparently was lost in the stem lineage of the crown group; accordingly it has been coded as present for Fregatidae.
49. Tarsometatarsus, trochlea metatarsi II markedly longer than trochlea metatarsi IV, reaching as far distally as trochlea metatarsi III: no (0), yes (1).
50. Limb bones heavily pachyostotic, i.e. with greatly thickened bone walls: absent (0), present (1). See Hasegawa et al. (1979, p. 16) concerning pachyostosis in the Plotopteridae.
51. Musculus ambiens: present (0), extremely vestigial or absent (1); (after McKitrick 1991).
52. Musculus flexor cruris lateralis, pars accessoria (‘Y’ muscle in the formula of George and Berger 1966, Table IX.1): present (0), absent (1); (after McKitrick 1991).
53. Musculus caudofemoralis, pars pelvica (‘B’ muscle in the formula of George and Berger 1966, Table IX.1): present (0), absent (1); (after McKitrick 1991).
54. Musculus flexor hallucis longus and musculus flexor digitorum longus, type of arrangement; see George and Berger (1966, p. 447) for description of types I–VIII; (after McKitrick 1991).
55. Powder down patches on back of rump: absent (0), present (1); (after Beddard 1898; Mitchell 1913; Stresemann 1927–1934).
56. Gular pouch: absent (0), very inconspicuous and feathered (1), large and naked (2).
57. Three anterior toes webbed: no (0), yes (1).
58. Hallux: not as follows (0), greatly reduced (1), as before, consisting of a single phalanx only (2). Within Galliformes, a long hallux is present in stem lineage representatives (Mayr 2000) and presumably basal (e.g. Mayr 2000) extant taxa like Megapodiidae and Cracidae, and the author considers a long hallux to be primitive within the taxon.
59. Hallux included in webbed foot: no (0), yes (1). This character is generally considered to be a synapomorphy of the ‘Pelecaniformes’, it has been coded as unknown in Spheniscidae and Procellariiformes in which the hallux is greatly reduced.
60. Claw of third toe distinctly pectinated on its medial side: no (0), yes (1).
61. Glandula nasalis (‘salt gland’) single-lobed and with only a single efferent ductus: no (0), yes (1); after Technau (1936). The Balaenicipitidae lack a glandula nasalis.
62. Eggshell covered with layer of amorphous calcium carbonate: no (0), yes (1) (Mikhailov 1995).
63. Young at hatching: downy (0), naked (1).
64. Young fed down gullet of adults (Cracraft 1985); no (0), yes (1).
65. Eggs incubated beneath feet: no (0), yes (1) (after Cracraft 1985; del Hoyo et al. 1992).
66. ‘Sky-pointing’/’wing waving’ display: absent (0), present (1); (after van Tets 1965; Cracraft 1985; del Hoyo et al. 1992).
67. Hop-display: absent (0), present (1); (after van Tets 1965; Cracraft 1985; del Hoyo et al. 1992).
68. ‘Kink-throating’ display: absent (0), present (1); (after van Tets 1965; Cracraft 1985; del Hoyo et al. 1992).
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tinamidae | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ? | 0 | 0 | 0 | 0 |
| Galliformes | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Ardeidae | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Ciconiidae | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 |
| Gaviidae | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Diomedeidae | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 |
| Hydrobatidae | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pelecanoididae | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | ? | 0 | 0 | 1 | 0 |
| Procellariidae | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 01 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 01 | 0 |
| Scopidae | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | ? | 0 | 0 | 0 | 0 |
| Balaenicipitidae | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | ? | 0 | 1 | 0 | 1 |
| Phaethontidae | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | ? | 1 | 0 |
| Fregatidae | 1 | 0 | 1 | 2 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Pelecanidae | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | ? | 0 | 1 |
| Plotopteridae | ? | 1 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | 0 | 0 | ? | ? | ? | ? | ? | ? | ? |
| Spheniscidae | 01 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 01 | 0 | 0 | 0 | 0 |
| Anhingidae | 0 | 1 | 1 | 2 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 |
| Phalacrocoracidae | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 0 | 0 | 01 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 |
| Sulidae | 0 | 1 | 1 | 2 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 |
| 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | 40 | 41 | 42 | 43 | 44 | 45 | 46 | 47 | 48 | 49 | 50 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tinamidae | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Galliformes | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ardeidae | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Ciconiidae | 0 | 0 | 1 | 01 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gaviidae | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Diomedeidae | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hydrobatidae | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pelecanoididae | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Procellariidae | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 01 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 01 | 0 | 0 | 0 |
| Scopidae | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Balaenicipitidae | 0 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Phaethontidae | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fregatidae | 1 | ? | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 |
| Pelecanidae | ? | 1 | 2 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 |
| Plotopteridae | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | ? | ? | 1 | 1 | 1 | 2 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 |
| Spheniscidae | 1 | 0 | 0 | 2 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 2 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 |
| Anhingidae | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Phalacrocoracidae | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Sulidae | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 |
| 51 | 52 | 53 | 54 | 55 | 56 | 57 | 58 | 59 | 60 | 61 | 62 | 63 | 64 | 65 | 66 | 67 | 68 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tinamidae | 0 | 0 | 0 | 12 | 01 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Galliformes | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ardeidae | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 01 | 1 | 0 | 0 | 0 | 0 | 0 |
| Ciconiidae | 01 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 01 | 0 | 0 | 0 | 0 | 0 |
| Gaviidae | 0 | 1 | 0 | 4 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Diomedeidae | 0 | 1 | 0 | 4 | 0 | 0 | 1 | 2 | ? | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hydrobatidae | 0 | 01 | 0 | 4 | 0 | 0 | 1 | 2 | ? | 0 | ? | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pelecanoididae | 1 | 1 | 1 | 4 | 0 | 0 | 1 | 2 | ? | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Procellariidae | 0 | 1 | 0 | 4 | 0 | 0 | 1 | 2 | ? | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Scopidae | 1 | 0 | 1 | ? | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Balaenicipitidae | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | ? | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Phaethontidae | ? | 0 | 1 | ? | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Fregatidae | 0 | 1 | 1 | 5 | 0 | 2 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Pelecanidae | 1 | 1 | 1 | ? | 0 | 2 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 |
| Plotopteridae | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| Spheniscidae | 0 | 1 | 0 | 2 | 0 | 0 | 1 | 1 | ? | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| Anhingidae | 0 | 1 | 01 | 2 | 0 | 2 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Phalacrocoracidae | 0 | 1 | 1 | 2 | 0 | 2 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Sulidae | 0 | 1 | 1 | 1 | 0 | 2 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 |




