Serum-specific stimulation of proliferation and mineralization of fish bone-derived cells
Summary
Teleost fish have recently been implemented as suitable model organisms to study vertebrate development, in particular skeletogenesis. In vitro cell systems derived from fish bone have been successfully established, although their development has been hampered by the limited availability of fish serum to supplement culture medium. Commercially available sera are mostly of mammalian origin and thus not necessarily adequate to fish cell growth. The main objective of this work was to compare proliferative and mineralogenic potential of bovine and fish sera using fish bone-derived cell lines VSa13 and VSa16. Fish serum was shown to (i) strongly stimulate cell proliferation in an apparent dose-dependent and cell type-specific manner, (ii) induce morphological changes, and (iii) enhance extracellular matrix mineralization of bone cells, although cytotoxic for fish osteoblast-like cells at the concentration tested. To better understand mechanisms underlying mineralogenic effect of fish serum in fish chondrocytes, expression of several mineralization-related genes was evaluated by qPCR. Regulation of matrix Gla protein (MGP) and bone morphogenetic protein 2 (BMP2) gene expression was modified upon culture with fish serum in a way compatible with an early onset and an increase in mineralization. In conclusion, fish serum was shown to be more adequate to proliferation and differentiation/mineralization of fish bone-derived cells.
Introduction
Diseases and disorders affecting skeletal development concern millions of people worldwide and despite decades of research, molecular determinants and mechanisms involved in skeletogenesis are still not fully understood. Most of the studies aiming at deciphering mechanisms of vertebrate development, in particular skeletogenesis, have used mammalian systems. However, they have some clear technical limitation, e.g. internal and slow development, limited number of offspring. Instead, fish have become a popular model for developmental studies because their embryos develop externally and rapidly, are transparent, can be manipulated at all stages and can be obtained in large numbers, (Hightower and Renfro, 1998; Kelly et al., 1998; McGonnell and Fowkes, 2006). Recently, fish have also emerged as suitable models for human diseases affecting skeletal development (Fisher et al., 2003; Nissen et al., 2006) and numerous genome-based tools and resources have been developed towards the study of fish skeletogenesis (Ortiz-Delgado et al., 2006; Rafael et al., 2006; Fonseca et al., 2007; Canário et al., 2008; Conceição et al., 2008). Fish bone-derived in vitro cell systems have also recently been established (Pombinho et al., 2004; Marques et al., 2007; Rafael et al., 2010) and used to investigate cellular mechanisms and signalling pathways regulating mineralization in fish (Tiago et al., 2008). However, in vitro studies involving fish bone-derived cell lines have been largely hampered by the lack of appropriate culture conditions. In particular, serum is a source of nutrients, growth factors and hormones routinely added to cell culture medium that not only assures survival of cells but also proliferation and ultimately intrinsic processes. Despite being routinely used for culturing fish cell lines, mammalian serum (mostly bovine but also, to a lesser extent, horse, porcine and lamb) may not be appropriate as it may promote unexpected behaviours, prevent cell differentiation and even provoke cell death (Ganassin and Bols, 1992). On the contrary, fish serum logically improves the growth performance of fish cells most probably due to stronger interactions of serum factors with their receptors in homologous systems (Clark et al., 1987; Hashimoto et al., 1997). It is clear that growing fish cells in bovine serum is financially advantageous; however it should not be to the detriment of cell differentiation towards the study of a particular process. In this work, we propose to compare the proliferative and mineralogenic potential of seabream and bovine sera using two fish bone-derived cell lines.
Materials and methods
Fish culture
An homogenous group of 24 gilthead seabream were obtained from a local aquaculture facility (body weight 180–200 g) then reared for 12 months in 1000-L circular tanks containing gravel-filtered, aerated seawater (salinity: 35 ppt; temperature: 18–25°C; oxygen content: > 80% saturation) until they reached a mean body weight of 680 ± 4 g. During rearing period, fish were submitted to natural photoperiod and hand fed a commercial seabream diet (AquaGold, crude protein 45%, crude fat 16%, Sorgal) to apparent satiety in two daily meals. Fish were starved for 24 h prior to blood sampling.
Blood collection and serum preparation
Fish were submitted prior to blood collection to a lethal anesthesia (10 min in seawater containing 400 μl/L of AQUI-S, New Zealand Ltd.). Blood was collected into glass tubes by puncture of the caudal vein then clotted at room temperature for 2 h. Clot was contracted for 4 h at 4°C then samples were centrifuged at 1590 g (10 min, 4°C). Serum, i.e. supernatant fraction, was transferred to a new tube and stored at −80°C.
Cell culture and extracellular matrix mineralization
VSa13 and VSa16 cells, both developed from seabream vertebra, were routinely cultured in Dulbecco’s modified Eagle medium (DMEM) supplemented with 1% penicillin-streptomycin, 1% fungizone, 2 mm l-glutamine and 10% FBS (fetal bovine serum) as described previously (Pombinho et al., 2004). Extracellular matrix (ECM) mineralization was induced in confluent cultures by supplementing culture medium with 50 μg/ml of l-ascorbic acid, 10 mmβ-glycerophosphate and 4 mm CaCl2. Culture medium was renewed twice a week. At appropriate times, mineral deposition was revealed as described by von Kossa (1901) and quantified through densitometry analysis. Cell culture reagents and dishes were purchased from Invitrogen and Sarstedt, respectively.
Cell proliferation assay
Cell proliferation was determined from cultures seeded in 96-well plates at 1.5 × 103 cells per well using the CellTiter 96 non-radioactive proliferation assay kit (Promega) according to manufacturer’s instructions. At appropriate times, cells were incubated for 1 h with 20 μl of reagent mixture then cell proliferation was determined from absorbance at 490 nm. In some cases, cultures were trypsinized (1 min at room temperature using 0.2% trypsin-EDTA solution) and cell number was determined using a Neubauer counting chamber.
RNA extraction and quantitative real-time PCR
Total RNA was extracted from cell cultures as described by Chomczynski and Sacchi (1987) and its quality and quantity assessed on agarose-formaldehyde gels and by UV spectrophotometry, respectively. Total RNA (1 μg) was reverse-transcribed at 37°C for 1 h using the Moloney-murine leukemia virus reverse transcriptase (Invitrogen), RNase Out (Invitrogen) and oligo-d(T) universal primer [5′-ACGCGTCGACCTCGAGATCGATG(T)13-3′]. Quantitative real-time PCR (qPCR) assays were performed using the iCycler PCR system (Bio-Rad). Reaction mixture containing 1× iQ SYBR Green I mix (Bio-Rad), 0.2 μm of forward and reverse primers and 10 ng of reverse-transcribed RNA was submitted to the following PCR conditions: 4 min at 95°C, 50 × [30 s at 95°C] then 30 s at 68°C. Relative gene expression of MGP, COL1A1, OC1, TNAP, OP, ON and BMP2 genes was normalized using RPL27a (primer list in Table 1).
| Gene name (acronym) | Accession number | Primer sequence (5′-3′) |
|---|---|---|
| Matrix Gla protein (MGP) | AY065652 | FW: TGTGTAATTTATGTAGTTGTTCTGTGGCATCTCC |
| RV: CGGGCGGATAGTGTGAAAAATGGTTAGTG | ||
| Collagen type 1, alpha 1 (COL1A1) | DQ324363 | FW: GAGATGGCGGTGATGTGGCGGAGTC |
| RV: GCCTGGTTTGGCTGGATGAAGAGGG | ||
| Osteocalcin 1 (OC1) | AF048703 | FW: TGGACACTGAGGGAATCATCGCTGC |
| RV: CACGCTACTCTACGGGTTAGGGGAAATG | ||
| Alkaline phosphatase (TNAP) | AY266359 | FW: CATCGCAACCCTTTTCACAGTCACCCG |
| RV: AACAGTGCCCAAACAGTGGTCCCATTAGC | ||
| Osteopontin (OP) | AY651247 | FW: AAAACCCAGGAGATAAACTCAAGACAACCCA |
| RV: AGAACCGTGGCAAAGAGCAGAACGAA | ||
| Osteonectin (ON) | AY239014 | FW: AGGAGGAGGTCATCGTGGAAGAGCC |
| RV: GTGGTGGTTCAGGCAGGGATTCTCA | ||
| Bone morphogenetic protein 2 (BMP2) | AY500244 | FW: GCGAAGGGCATGGGCTGTCTTTGGT |
| RV: AGCAGTACCACGAGAGAGCGGACCAC | ||
| Ribosomal protein L27a (RPL27a) | AY188520 | FW: AAGAGGAACACAACTCACTGCCCCAC |
| RV: GCTTGCCTTTGCCCAGAACTTTGTAG |
Statistical analysis of data
Data are presented as the average ± standard deviation of values from at least three separate experiments. Statistical significance of data was evaluated through the Student’s t-test or the analysis of variance (anova). Differences were considered to be significant for P < 0.05.
Results and Discussion
Following the protocol described in the Materials and Methods section, 94 ml of blood were collected from 24 seabream specimens and 36 ml of serum were recovered (efficiency was approximately 38%). Proliferative and mineralogenic effects of seabream serum (GSS) were then tested using two seabream cell lines – VSa13 (chondrocyte-like) and VSa16 (osteoblast-like) – capable of in vitro mineralization.
Fish serum enhances proliferation of fish bone-derived cell lines
Proliferative effects of serum were initially tested on cells cultured in a medium supplemented with 10% of either GSS or FBS (considered as control thereafter) and evaluated at appropriate times using CellTiter proliferation assay kit and/or cell counting. Both methods revealed that proliferation of seabream cell lines was stimulated by GSS after 13 days, although to a lesser extent in VSa13 cells (Fig. 1). Morphological changes were also observed, i.e. cells were typically bipolar and fibroblastic (sub-confluent cultures) then polygonal (confluent cultures) when grown in bovine serum and epithelial-like and sharply outlined when grown in seabream serum (Fig. 1). Effect of different FBS/GSS ratios (final serum concentration was maintained at 10%) were then tested and highest proliferative effect was attained using FBS/GSS ratios of 5/5 and 7.5/2.5 for VSa13 and VSa16 cells, respectively, suggesting that GSS proliferative effect is dose-dependent and cell type-specific (Fig. 2). Using these conditions, proliferation was stimulated approximately 3.5-fold in both cell lines compared with control (Fig. 2). Again, both methods used to assess cell proliferation (i.e. CellTiter proliferation assay and cell counting) indicated similar stimulatory effects although data from CellTiter proliferation assay has a tendency for value compression.
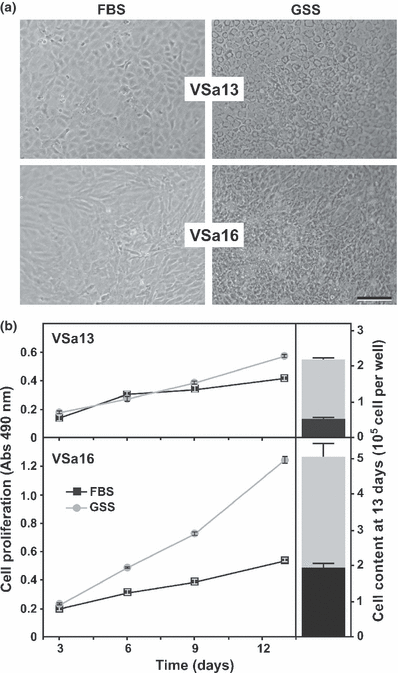
Effect of bovine and seabream sera on cell phenotype (a) and growth performance (b) of VSa13 and VSa16 cells. Cells were cultured for 13 days in DMEM supplemented with 10% FBS (black squares) or GSS (grey circles). Cell proliferation was assessed at appropriate times using CellTiter proliferation assay and after 13 days by cell counting. Values are the mean of at least four measurements ± standard deviation. All values are statistically different (Student’s t-test, P < 0.05) from control (10% FBS)
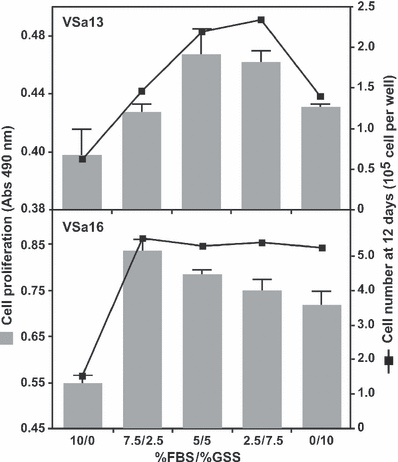
Growth performance of VSa13 and VSa16 cells cultured in DMEM supplemented with different FBS/GSS ratios. Cell proliferation was evaluated after 12 days using CellTiter proliferation assay (grey bars) and cell counting (black squares). Values are the mean of at least four measurements ± standard deviation. All values are statistically different (Student’s t-test, P < 0.05) from control (10% FBS)
Altogether, these results indicate that GSS is more effective than FBS to promote the growth of seabream bone-derived cells and suggest the presence in seabream serum of growth factor(s), which are most probably more effective than their bovine homolog(s). Interestingly, reducing GSS proportion to 50% (VSa13) or 25% (VSa16) while maintaining final serum concentration at 10% by increasing FBS proportion enhanced seabream cell proliferation. Similar experiments using goldfish fin cells revealed that a mixture of carp and bovine sera, 50% each, was more effective in cell growth than carp serum alone and that early passages of these cells responded better to higher proportions of carp serum (Hashimoto et al., 1997). Proliferation data from goldfish and seabream cells suggest that fish cells grow better in the presence of fish serum and that fish cells previously maintained in mammalian serum still require its presence for an optimal growth, probably because of some adaptation versus weaning mechanisms. The clear modification of GSS-treated cell phenotype is in agreement with the previous observation by Hashimoto et al., 1997 who mentioned that cells cultured in medium supplemented with different sera show singular shape responses. This change of phenotype in GSS-treated cells could indicate the capacity of seabream cells to return to their original phenotype (a phenotype lost upon culture in FBS) and the presence of factor(s) in seabream serum capable of restoring innate characteristics of seabream cells. Factors present in seabream serum could be similar to cell adhesion factors identified in rainbow trout serum (Lee and Bols, 1991), and responsible for the stimulation of cell spreading and subsequent morphological changes (Hashimoto et al., 1997). In conclusion, our data indicate that seabream serum contains factors essential for cell growth and phenotype establishment, and support the idea that bovine serum is not totally adequate to culture fish cells.
Fish serum stimulates in vitro mineralization of fish bone-derived cell lines
Effects of serum on extracellular matrix mineralization were tested on cells cultured in mineralogenic medium supplemented with 10% of either GSS, FBS or a combination of both sera. While mineral deposition was only detected after 3.5 weeks in FBS-treated VSa13 cell cultures, it was clearly visible after only 2 weeks in GSS-treated cells and the levels of mineral deposition were much higher (approx. 3 times) after 3.5 weeks when compared to those obtained after an equal period in cells supplemented with FBS (Fig. 3). These results suggest the presence in seabream serum of mineralogenic factor(s), which modify onset and extent of mineralization in chondrocytic VSa13 cells in a dose-dependent manner (effect was noticeable with 5% GSS and higher with 10%). Similarly, mineral deposition was observed in GSS-treated VSa16 cell cultures after 2 weeks, while none was detected in FBS-treated cells until 3.5 weeks (Fig. 3). Unexpectedly, GSS exhibited a strong cytotoxic effect at 3.5 weeks as indicated by the reduced mineralization in cultures grown with 2.5% of GSS and absence of mineralization in cultures grown with 10% of GSS (Fig. 3). Despite being toxic for these cells, GSS also demonstrated mineralogenic effects in osteoblastic VSa16 cells, similar to those observed in VSa13 cells, also in a dose-dependent manner (Fig. 3). Recent studies in which demineralized rat tibia were incubated in different sera (including serum from vertebrates and invertebrates) then evaluated for recalcification, revealed the presence of factors essential for mineralization in vertebrate sera (e.g. rat, bony fish and shark) but not those from invertebrates (Price et al., 2004; Hamlin et al., 2006). Our data also suggest the presence of mineralogenic factors in vertebrate serum and confirm that fish serum is more appropriate than bovine serum to trigger an optimal mineralization of fish bone-derived cells, most probably because of the higher specificity of fish mineralogenic factors for fish cells. Toxicity in VSa16 cell cultures treated for 3.5 weeks with GSS suggest that proportion of fish serum is still not optimal and should be reduced for culturing these cells.
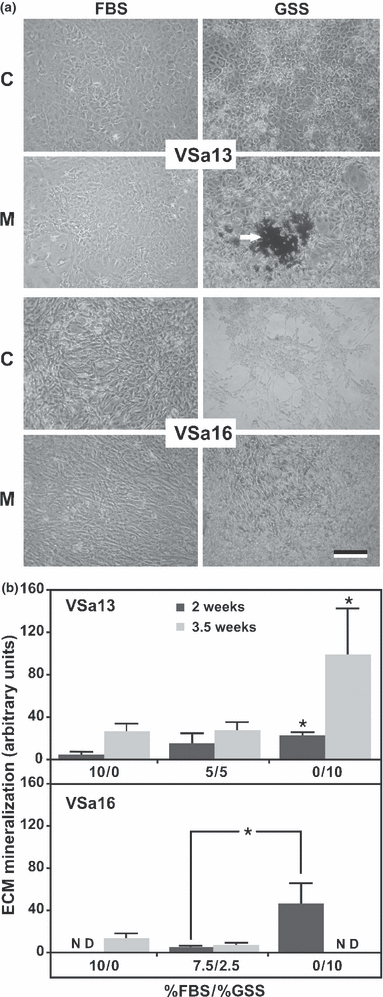
Mineralization capacity of VSa13 and VSa16 cells cultured in DMEM supplemented with different FBS/GSS ratios. (a) Phase-contrast micrographs of von Kossa-stained cultures after 2 weeks in medium containing 10% FBS or GSS, under control (C) or mineralizing conditions (M). White arrow indicates silver-stained mineral nodules. Bar 100 μm. (b) Levels of ECM mineralization evaluated by densitometry and presented as the ratio between individual values and their corresponding controls. Values are the mean of at least four measurements ± standard deviation. Asterisks indicate values significantly different from control (10% FBS) or from indicated conditions (one-way anova; P < 0.05)
Regulation of mineralization-related gene expression by GSS
To better understand mechanisms underlying mineralogenic effect of GSS, expression of several bone-related genes was evaluated by qPCR after 1 week of mineralization in VSa13 cells (VSa16 cells were not tested due to the cytotoxic effect previously observed). Comparative analysis of gene expression in GSS- versus FBS-treated mineralizing VSa13 cells (Fig. 4) revealed a decrease of matrix Gla protein (MGP) expression (approx. 6-fold) and an increase of bone morphogenetic protein 2 (BMP-2) expression (approx. 10-fold). MGP is a vitamin K-dependent protein secreted by chondrocytes and vascular smooth muscle cells into the extracellular compartment where it functions as a physiological inhibitor of mineralization, delaying chondrocyte maturation and blocking endochondral ossification (Luo et al., 1995; Yagami et al., 1999; Murshed et al., 2004). Any mechanism decreasing MGP gene expression and protein production in bone-derived cell lines would remove or reduce its inhibitory action, therefore advancing onset and/or extent of ECM mineralization. BMP-2 is a member of the TGF-β family of growth factors and a potent inducer of bone formation by stimulating osteoblast differentiation (Ducy and Karsenty, 2000; Huang et al., 2002). Any mechanisms increasing BMP-2 gene expression and protein production would stimulate osteogenesis, therefore advancing onset and/or extent of ECM mineralization. We propose that mineralogenic effect of GSS could be attributed to serum factors regulating the transcription of MGP and BMP-2 genes and consequently ECM mineralization. Previous studies (Hamlin et al., 2006) had shown that serum from bony fish and shark contains a potent nucleator of collagen calcification capable of remineralizing a demineralised rat tibia. However, the nature of this serum nucleator and its mode of action are still poorly understood. Changes in expression of other bone-related genes did not provide clear evidence of their participation in the mineralogenic effect of GSS. Expression of osteocalcin 1 (OC1), a Gla-containing protein associated with tissue ossification (Ducy et al., 1996; Hunter et al., 1996) and a marker of osteoblast differentiation (Nieden et al., 2005; Li et al., 2009), was apparently significantly reduced in mineralizing GSS-treated VSa13 cells but was found at levels close to qPCR detection limits in all conditions. Previous expression data showing its absence in VSa13 cells (Pombinho et al., 2004) suggest that the observed decrease in osteocalcin expression in GSS-treated cells may be negligible. Similar conclusions applied to osteopontin (OP) while changes in the expression of osteonectin (ON) and tissue non-specific alkaline phosphatase (TNAP) genes were not significant. Expression of collagen type 1 (COL1A1), an early marker of osteoblast commitment and the major protein in bone extracellular matrix (Karsenty and Park, 1995), was increased in both mineralizing GSS- and FBS-treated cells, although this increase was more pronounced in cells cultured in FBS (approx. 1.5 times). Any mechanism increasing type 1 collagen gene expression should enhance extracellular matrix formation and probably mineralization. However, levels of mineralization were lower in GSS-treated cells. There is no obvious explanation for this result and future experiments (e.g. measurement of total collagen content) will be needed to understand the relationship between decreased COL1A1 expression and increased ECM mineralization. In general, our results suggest the presence of factor(s) in GSS serum capable of regulating specific bone-related gene expression in a specific manner towards VSa13 cell differentiation/mineralization.
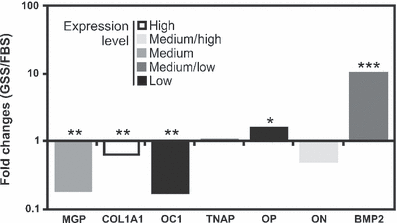
Comparative analysis of serum effect on specific bone-related gene expression during ECM mineralization of VSa13 cells. Expression values are presented as the ratio between fold changes in GSS- versus FBS-treated cells and are the mean of three biological replicates. Housekeeping gene RPL27a was used to normalize gene expression. Gene name acronyms are defined in Table 1. Asterisks indicate values statistically different from corresponding controls: *P < 0.05; **P < 0.01; ***P < 0.001 (Student’s t-test)
Conclusions
The differential effect of bovine and gilthead seabream sera on cell growth and ECM mineralization clearly indicates the presence in the serum of proliferative and mineralogenic factors, which are logically more potent in serum used to grow cells from the same taxon (e.g. fish serum with fish cells and mammalian serum with mammalian cells). Therefore, it supports the idea that mammalian serum is not totally adequate to culture fish cells and that the successful development of fish in vitro cell systems to study vertebrate development will ideally require the availability of commercial fish serum. Future experiments should aim at identifying these serum factors and determining their mechanisms of action.
Acknowledgements
This work was partially funded by grant PTDC/MAR/70855/2006 from the Portuguese Science and Technology foundation (FCT). JR and DMT were supported by doctoral (SFRH/BD/47433/2008) and postdoctoral (SFRH/BPD/45034/2008) fellowships, respectively.




