The unobtrusive majority: mononucleated bone resorbing cells in teleost fish and mammals
Summary
The canonical view of a mammalian (usually shown as human) bone resorbing cell is that of a giant macrophage-like cell (osteoclast) that dissolves bone minerals and digests bone matrix proteins. The cells’ presence and activity is easily recognised based on three distinct morphological features: (i) multinuclearity, (ii) a multiply folded apical cell membrane (ruffled border), and (iii) deep lacunae (Howship’s lacunae) that the cells eroded into the bone surface. Mononucleated osteoclasts without these features are considered to be inactive precursors. We challenge the view that bone resorbing cells must be multinucleated giant cells, based on our comparative studies on the teleost skeleton, on what is currently known – but often disregarded – about mononucleated mammalian osteoclasts, and on what is know about osteocytic osteolysis.
All teleosts have mono- and multinucleated osteoclasts but in advanced teleosts (paracanthopterygians and acanthopterygians) mononucleated osteoclasts are the prevailing cell type. Mononucleated osteoclasts dominate early bone development and scale resorption also in less derived teleosts (like protacanthopterygians). The mononuclear cells can lack a ruffled border and may not create resorption lacunae (non-lacunar, smooth resorption). The situation appears to be different from mammals but classic and recent studies on mammalian osteoclasts correspond with findings on teleost osteoclasts: (i) A large percentage of human osteoclasts are mononucleated; (ii) a ruffled border is not a prerequisite for resorption, and (iii) smooth resorption is a common process. Another phenomenon that challenges the exclusiveness of multinucleated osteoclasts is osteocytic osteolysis, which is the removal of bone by osteocytes and which has been observed in several teleost and mammalian species.
As is the case for teleost fish, focussing only on multinucleated bone resorbing cells is insufficient to fully explain bone resorption in mammals or to understand all types of bone loss in humans under both physiological and pathological conditions.
The mammalian osteoclast model
Humans are still the most popular model in fish skeletal research. Thus, the canonical view of a human (mammalian) bone resorbing cell (osteoclast) (Fig. 1a) as a giant macrophage-like cell is often applied to teleostean bone resorbing cells. Cell lineage, mechanisms of activation, and function of mammalian multinucleated osteoclasts are well established. The cells derive from haematopoietic bone marrow cells that also give rise to monocytes and macrophages (Holtrop et al., 1982). Transcription factors and signalling molecules required for macrophage differentiation such as PU.1 and TRAF6 are also essential for osteoclast development (Naito et al., 1999; Stenbeck, 2002). For maturation, mammalian osteoclasts need contact with osteoblasts; osteoblasts express molecules such as M-CSF and RANKL that regulate osteoclast differentiation.. Furthermore, osteoblasts release osteoprotegerin, a RANK ligand (RANKL) decoy receptor that downregulates osteoclasts (Teitelbaum, 2000; Li et al., 2006). Osteoblasts are also the target cells for estrogen, PTH and Vitamin D, all of which regulate osteoclasts via osteoblasts (Rodan and Martin, 1981; Franz-Odendaal et al., 2006).
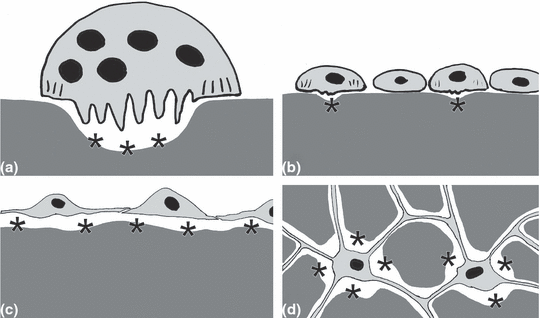
Schematic representation of different modes of bone resorption. Dark grey, mineralised bone matrix; light grey, bodies and processes of bone resorbing cells; black, nuclei. Osteocytes are not shown except in (d) (see Fig. 2 for the presence and absence of osteocytes). Black asterisks indicate locations of bone removal. (a) Typical osteoclast with the three distinct morphological features (Blair et al., 2008): multinuclearity, ruffled border, and a deep resorption lacuna. These cells are easily detected in standard histological preparations as well as in vitro. (b) Mononucleated resorbing cells according to Domon et al. (1994) and Bar-Shavit (2007). The cells have a small ruffled border and may create small shallow resorption lacunae (*). Both features may be easily overlooked in standard histological preparations. (c) Mononucleated osteoclasts according to Witten (1997). The cells are interconnected and may thus create a sealed acidic environment that is required for bone resorption. Like all osteoclasts these cells are TRAP-positive. (d) Osteocytic osteolysis according to Teti and Zallone (2009). Osteocytes are capable of enlarging their osteocyte lacunae by resorbing bone. (a–d) Intermediate stages between all four types of bone resorbing cells may exist
The current literature considers mononucleated osteoclasts as inactive cells that must first fuse into multinucleated precursor cells prior to activation. Consequently, only multinucleated osteoclasts are considered as active bone resorbing cells (Fig. 1).
Multinucleated mammalian osteoclasts are mobile cells that migrate to the site of bone resorption upon which the apical cell membrane develops a peripheral sealing zone (that conceals the subcellular space) and a central ruffled border (multiple deep membrane infoldings) (Fig. 1a) (Parfitt, 1988; Li et al., 2006). The ruffled border is the area of active bone resorption. The sealed space under the ruffled border functions as a giant extracellular phagolysosome (Baron et al., 1993). The extracellular space is acidified by the action of a vacuolar proton pump (H+-ATPase) (Väänänen et al., 1990; Mattsson et al., 1994; Witten et al., 1999) to promote the dissolution of bone minerals and the activity of lysosomal enzymes such as cathepsins, unspecific acid phosphatase and tartrate-resistant acid phosphatase (TRAP), that break down bone matrix components (Lucht, 1971; Witten, 1997; Manolagas, 2000).
Recognising bone resorbing cells
From a mammalian perspective, the presence and activity of bone resorbing cells can be recognised morphologically based on one of three distinct features (Fig. 1a): (i) multinuclearity, (ii) the folded apical cell membrane (ruffled border), and (iii) deep lacunae (Howship’s lacunae) on the bone surface. Mononucleated bone resorbing cells, that lack these features, are considered to be inactive osteoclast precursors (Blair et al., 2008; Schett, 2009). We challenge the view that bone resorbing cells must be multinucleated giant cells, based on (i) our comparative studies on the teleost skeleton, (ii) on what is currently known – but often disregarded – about mononucleated mammalian osteoclasts, and (iii) on what is known about bone resorption by osteocytes (osteocytic osteolysis).
Osteoclasts in teleost fish
All teleosts have mono- and multinucleated osteoclasts, but in advanced teleosts mononucleated osteoclasts are the predominant cell type (Witten and Huysseune, 2009). Mononucleated osteoclasts have also been described for basal osteichthyan taxa, such as the Australian lungfish, Neoceratodus forsteri (Kemp, 2003) and the gray bichir, Polypterus senegalus (Hall and Witten, 2007). There appears to be a relationship between the type of bone resorption (with multinucleated or mononucleated cells) and the type of bone (cellular or acellular) (Fig. 2). Mononucleated osteoclasts are small, may lack a ruffled border and can resorb bone without the creation of Howship’s lacunae (Fig. 1b,c). Developmental studies and mineral deprivation experiments have shown that mononucleated osteoclasts alone can accomplish bone resorption in advanced teleosts, multinucleated osteoclasts not being required (Weiss and Watabe, 1978, 1979; Ekanayake and Hall, 1987; Witten, 1997; Witten and Villwock, 1997; Witten et al., 2004; Takano et al., 2005). The exact cell lineage of mononucleated teleostean osteoclasts has not been established. However, conserved developmental pathways for monocytes and macrophages and the presence of osteoclast key enzymes such as H+-ATPase and TRAP are consistent with these cells being derived from a haematopoietic cell lineage (Witten and Huysseune, 2009). In contrast to advanced teleosts such as Nile tilapia (Oreochromis niloticus) and haddock (Melanogrammus aeglefinus) less derived teleosts such as zebrafish (Danio rerio) or Atlantic salmon (Salmo salar) have many multinucleated osteoclasts (Witten and Hall, 2002, 2003). Nevertheless, early skeletal development in all teleosts that have been studied so far is dominated by mononucleated resorbing cells (Sire et al., 1990; Witten et al., 2001).
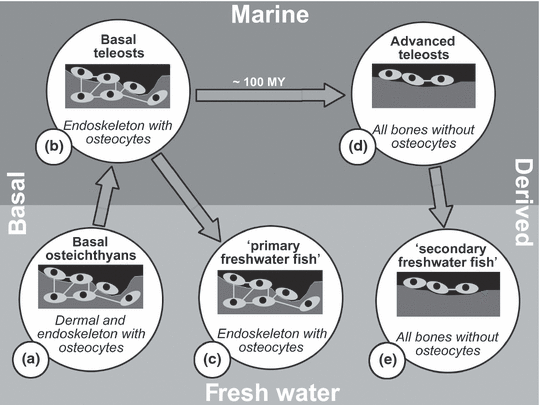
Relationships between phylogeny, environment, the presence of osteocytes and the type of osteoclasts in adult teleost fish, modified after Witten and Huysseune (2009). MY, million years. Basal osteichthyans, that also gave rise to tetrapods, and basal teleosts (Groups a and b) have bone that contains osteocytes, similar to mammalian bone. These bony fish have mononucleated and many ‘mammalian-like’ multinucleated osteoclasts. Osteocyte-containing bone and multinucleated osteoclasts have been preserved in adults during a first wave of freshwater reinvasion by teleost fish (‘primary freshwater fish’; Group c, e.g. zebrafish). During a long evolutionary period in the marine environment osteocytes are no longer incorporated (acellular bone). Acellular bone is found in almost all advanced teleosts (Group d). The predominating osteoclast type of advanced teleosts is mononucleated. This character was maintained when advanced teleosts reinvaded the fresh water (Group e; ‘secondary freshwater fish’, e.g. medaka). Consequently, teleosts that live in fresh water (or in the marine environment) can have very different bone types and different types of bone resorbing cells
In the course of teleost evolution the predominance of bone resorption by mononucleated osteoclasts coincides with the predominance of acellular bone (Fig. 2) (Witten and Huysseune, 2009). Similar observations can be made during teleost ontogeny and in different parts of the teleost skeleton: (i) During zebrafish skeletal development, multinucleated osteoclasts appear when the bone type switches from acellular to cellular (Witten et al., 2001). (ii) Advanced teleosts with acellular bone can develop hyperostotic bones late in life. Hyperostotic bone has been described as cellular and is resorbed by multinucleated osteoclasts (Smith-Vaniz et al., 1995) (iii) Acellular scales of adult salmonids are predominately resorbed by mononucleated osteoclasts, while multinucleated giant osteoclasts are the dominating osteoclast type in the osteocyte-containing endoskeleton (Sire et al., 1990; Persson et al., 1995, 1999; Witten and Hall, 2002, 2003).
Mononucleated bone resorbing cells in teleosts and mammals
Bone resorption in advanced teleosts with small mononucleated osteoclasts, that do not create deep resorption lacunae appears to be very different from bone resorption in mammals. Nevertheless, classic and recent studies on mammalian (human) osteoclasts emphasise the existence of mononucleated osteoclasts with many features in common with mononucleated teleostean osteoclasts. According to Parfitt (1988) a resorption lacuna in human bone is produced for approximately two-thirds of its depth by multinucleated osteoclasts. The remaining third is removed by mononucleated osteoclasts. Chambers (1985) emphasised that mononucleated cells are functional osteoclasts and resorption lacunae are equally common beneath mono- and multinucleated cells. Domon et al. (1994) showed fully functional mononucleated tooth-resorbing cells using transmission electron microscopy. Evans et al. (1979) reported that 34% of osteoclasts have only one nucleus and that 29% did not show nuclei at all when sections were stained for acid phosphatase activity. Based on serial sections and also using acid phosphatase as an osteoclast marker, Kaye (1984) demonstrated that in normal bone at least 32% of all bone resorbing cells were truly mononucleated. Because of the active participation of mononucleated osteoclasts in human bone resorption, Ballanti et al. (1997) cautioned that the identification of osteoclasts should not be based on multinuclearity. Instead, osteoclasts should be identified and counted based on the expression of the osteoclast marker enzyme tartrate-resistant acid phosphatase (TRAP). The situation is similar in teleost fish where TRAP staining reveals the predominance of mononucleated osteoclasts, cells that cannot or cannot easily be recognized as bone resorbing cells based on morphological features (Fig. 1b,c) (Witten and Huysseune, 2009). Recent experimental data provide insights into possible mechanisms that trigger the development of functional mononucleated osteoclasts in mammals. Osteoclasts in transgenic mice that overexpress FGF-23 lack a ruffled border, but the cells secrete cathepsin K and MMP-9 at levels comparable to osteoclasts with ruffled borders, and effectively participate in the degradation of (albeit hypomineralized) bone matrix (Hollberg et al., 2008). The dendritic cell-specific transmembrane protein DC-STAMP is a critical factor for the fusion of mononucleated cells into multinucleated osteoclasts. DC-STAMP-deficient cells fail to fuse, but still function as mononucleated osteoclasts (Bar-Shavit, 2007).
Osteocytic osteolysis
Bone resorption by osteocytes (osteocytic osteolysis) provides another challenge for the idea that only multinucleated osteoclasts are capable of bone resorption. Osteocytes, embedded in the bone matrix and connected by an elaborate network of cell processes, deposit and resorb bone around the osteocyte lacuna in which they are housed (Fig. 1d) (Cullinane, 2002; Franz-Odendaal et al., 2006; Bonucci, 2009). The capacity to remove bone (osteocytic osteolysis) is often not regarded as characteristic of human osteocytes (see Bonucci, 2009; for the debate about osteocytic osteolysis in humans), but evidence for its occurrence derives from studies on many vertebrate species, such as bats (Kwiecinski, 1985; Kwiecinski et al., 1987), hamsters (Steinberg et al., 1981), squirrels (Haller and Zimny, 1978), rats (Bélanger, 1977; Tazawa et al., 2004), rabbits (Zhang et al., 2000), snakes (Alcobendas et al., 1991), eels (Lopez et al., 1980), salmon (Hughes et al., 1994) and carp (Witten et al., 2000). In favour of osteocytic osteolysis, Neuman and Ramp (1971) argue that osteoclastic resorption has little, if any, role in the daily metabolism-related calcium release from the skeleton. In humans, osteocytes comprise 95% of all bone cells with 20 000–80 000 cells per mm3 of bone tissue and cover about 94% of all bone surfaces (Frost, 1960; Marotti, 1996). The osteocyte surface is approximately 100 times larger than the trabecular bone surface (Teti and Zallone, 2009). Likely, the enormous osteocyte surface and the very small amount of resorption per cell makes it so difficult to detect osteocytic osteolysis in human bone (Baylink and Wergedal, 1971). Studies on the fragile skeleton of pregnant and lactating bats, however, clearly demonstrate that the females’ enhanced calcium demand is met by osteocytic osteolysis and not by osteoclastic resorption (Kwiecinski, 1985; Kwiecinski et al., 1987). Studies on rat bone revealed similar findings. Tazawa et al. (2004) showed that the administration of PTH increases the volume of osteocyte lacunae by 45% together with expression of acid phosphatase by osteocytes.
Concluding remarks
The concept of multinucleated bone resorbing cells as the only cellular units that can resorb bone has become increasingly popular over the last two decades despite established knowledge about bone resorption by mononucleated osteoclasts and by osteocytes (Cullinane, 2002; Tazawa et al., 2004; Teti and Zallone, 2009; Witten and Huysseune, 2009). Focussing exclusively on multinucleated osteoclasts and their traces provides several practical advantages. Often, only one of the three key features of these cells (multinuclearity, ruffled border, resorption lacuna) is sufficient to establish the cells’ presence or the occurrence of bone resorption. Palaeontologists and neontologists use the presence of Howship’s lacunae as evidence for the existence of bone resorption. In vitro, an increase of numbers of multinucleated cells is a sign of the activation of osteoclasts. The creation of pits on dentin or on hydroxyapatite slices strongly suggests osteoclast activity. Furthermore, the upregulation of specific genes and enzymes can easily be related to an identifiable cell type, the multinucleated osteoclast. Although widely accepted for mammals, such a view on bone resorbing cells has been shown to be insufficient to explain bone resorption in advanced teleosts (Witten and Huysseune, 2009). A critical analysis of the literature suggests that the same holds for mammals. Given the evidence for active bone resorption by mononucleated cells and given the fact that osteocytes also resorb bone, focussing on multinucleated bone resorbing cells alone is not sufficient to fully explain bone resorption in mammals or to understand all types of bone loss in humans, under both physiological and pathological conditions (Fig. 1). Understanding bone resorption in teleosts requires to move away from the paradigm ‘only multinucleated osteoclasts resorb bone’; this helps to acknowledge the role of mononucleated osteoclasts in mammals.
Acknowledgements
We thank our colleagues Leonor Cancela, Brian K. Hall, Santosh Lall and Jean-Yves Sire for inspiring discussions. A.H. acknowledges a grant from the FWO-Vlaanderen no. 3G0040.08, P.E.W. and A.H. acknowledge funding from the Norwegian Research Council (Project No. 172483/s40) and from COST Action B23, ‘Oral facial development and regeneration’.




