A male linkage map constructed for QTL mapping in Spanish Churra sheep
Summary
A male ovine linkage map has been constructed on the basis of 11 half-sib families of a commercial population of Spanish Churra sheep as part of a genome scan for quantitative trait loci mapping. A total of 1421 daughters and their sires were genotyped for 182 microsatellite markers evenly distributed along the ovine autosomes. A total of 259 192 genotypes were obtained, generating an average of 669 informative meioses per marker. An autosomal genome length of 3262 cM was estimated for the Churra population with a mean marker interval of 17.86 cM. Our map represents an approximate 90% coverage of the autosomal ovine genome and constitutes a useful tool for the genetic dissection of complex traits in this breed. General agreement was found between the Churra map and other published maps for sheep, despite certain length discrepancies.
Introduction
Since the discovery of DNA microsatellites, the development of linkage maps for livestock species has become a relatively standard process for locating genes influencing economically interesting traits. In sheep, the appearance of maps with an increased marker density (Crawford et al. 1995; De Gortari et al. 1998; Maddox et al. 2001) has been of great value in this regard. However, despite the more comprehensive coverage of published maps, their use in breeds other than those used for their construction has certain limitations. The development of special mapping populations may generate genetic variation that may not occur in local populations. Moreover, a genetic basis has been detected for differences in recombination rate with different estimates reported for distinct cattle breeds (Thomsen et al. 2001; Weimann et al. 2003). Spanish Churra sheep are one of the most important dairy breeds in Spain and a daughter design involving a commercial population was developed to detect quantitative trait loci (QTL) influencing economically important traits. For the application of this whole genome scan a low-density marker map was developed. We present here the comparison of the linkage map obtained for 182 microsatellite markers (dispersed along the 26 ovine autosomes) with published maps.
Materials and methods
A total of 1421 daughters belonging to 11 paternal half-sib families of the Selection Nucleus of the Spanish Association of Churra breeders (ANCHE) were included in the present study, as part of a project for QTL detection. Unrelated sires were studied and daughters (obtained through artificial insemination) belonged to 17 different flocks in north-central Spain. The average family size was 130, ranging from 60 to 261 daughters per sire.
Firstly, 194 microsatellite markers were selected from the International Mapping Flock map (Maddox et al. 2001) to give marker coverage of approximately every 15–20 cM in the autosomal sheep genome. The multiplex technique was used and electrophoretic separation was performed through multiloading runs using the ‘four colours/one lane’ technology (details can be found in Gutiérrez-Gil et al. submitted). Genotyping records were stored on a home-made database, which allowed the identification of Mendelian inheritance problems due to paternity assignation or genotyping problems. Twelve markers were ruled out because of genotyping difficulties, insufficiently informative families or linkage inconsistencies, so that 182 microsatellites were included in the final linkage analysis. Linkage maps were generated by multipoint linkage analysis using the BUILD option of CRIMAP, version 2.4 (Green et al. 1990), with a threshold of 3 LOD established to support the most likely map. Morton’s likelihood ratio test for heterogeneity of the recombination fraction among different groups of families was applied to the two-point LOD scores to detect residual genotyping anomalies. The FLIPS option of CRIMAP was used (FLIPS = 3) to confirm the marker order previously obtained. Detection of multiple recombinants was performed using the CHROMPIC option of the package. A second confirmation of genotypes was performed based on the CHROMPIC results and markers were reanalysed when considered necessary. Recombination fraction estimates between markers were converted to genetic distances by means of the Kosambi map function. The resulting linkage maps were drawn using MapChart software (Voorrips 2002).
Results and discussion
The linkage map built for the Churra sheep has a mean marker interval of 17.86 cM and a total length estimated at 3262 cM, equivalent to ∼3400 cM on the updated version of the domestic International Mapping Flock (IMF) male sheep map (version 4.7) and corresponding to ∼90% of the autosomal sheep genome (compared to best positions map). Marker order was in agreement with the IMF map (Australian Sheep Gene Mapping at http://rubens.its.unimelb.edu.au/~jillm/jill.htm) and a comparison of the chromosomal lengths for regions flanked by common markers between the IMF and the Churra maps is graphed in Figure 1.
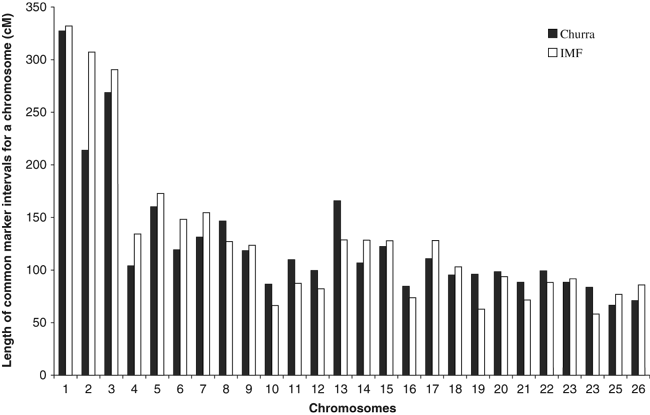
Comparison of common interval lengths between the Churra and IMF male (best positions) maps. Autosomal chromosome number is shown on the x-axis, while chromosome lengths in cM are indicated on the y-axis.
Figure S1 shows the detailed Churra linkage map, with marker order and positions for each of the 26 ovine autosomes. Table S1 shows the following values for each marker: alleles identified, allele range, informative meioses, heterozygous rams, families showing null alleles, polymorphism information content (PIC) and the two-point LOD score obtained for linkage between adjacent markers.
The reliability of the Churra map is substantiated by the high number of informative meioses analysed (669 meioses per marker on average), a mean value which greatly exceeds the maximum number of informative meioses in the IMF population, estimated at 222 (Maddox et al. 2001).
The mean number of alleles per locus obtained in Churra sheep was 13.8, while for the same markers the mean recorded for the IMF population was 8.5 (Australian Sheep Gene Mapping at http://rubens.its.unimelb.edu.au/~jillm/jill.htm), despite the latter having several breeds in the founding generation. This finding might be at least partially explained by the fact that more sheep were genotyped in the Churra population than in the IMF, making it more likely for rare alleles to be detected. Moreover, previous genetic studies had already reported this increased genetic variability in Churra sheep (Arranz et al. 2001).
As can be seen in Figure 1, a high concordance was found at most chromosomes in the length of common marker intervals between the Churra and the IMF maps. Significant differences were detected in a few cases, with one of the most outstanding ones being for chromosome OAR2, where the length estimated in the Churra population was ∼30% lower than the common interval in the IMF map. An analysis of marker intervals at OAR2 indicated that the differences were not equally distributed along this chromosome but were centred on two parts: the telomeric end (interval BMS356-OARFCB11) and the central region (interval TGLA10-RM321-ILSTS030-RM356). On the other hand, chromosomes OAR13 and OAR19 showed the opposite pattern, common intervals calculated by Maddox (http://rubens.its.unimelb.edu.au/~jillm/jill.htm) being ∼20–30% lower than our estimates.
The lower number of markers in our study may explain, at least partially, the differences between the Churra and IMF maps, but as this cause would be expected to affect the whole genome and not simply individual chromosomes, we cannot rule out an alternative explanation, specially for discrepancies such as those observed on the cited chromosomes. Moreover, the reliability of our estimate in Churra sheep is substantiated by the markedly greater number of informative meioses involved (see Table S1) in comparison with the IMF population (Maddox et al. 2001).
Variability in the recombination rate between Churra sheep and other populations might partially explain these results, for differences in recombination have been described for certain chromosomes among livestock breeds (Thomsen et al. 2001; Weimann et al. 2003), as well as between families and individuals (Simianer et al. 1997; Lien et al. 1999) within a species. In contrast to initial hypotheses, increasing importance is attributed to inheritance in the control of recombination (Kong et al. 2004).
Moreover, in the particular case of sheep, three different marker orders have been recently identified by McRae & Beraldi (2006) on a region at OAR1, across the following ovine populations: IMF, Charollais, Scottish Blackface and Soay sheep. Moreover, in the latter population a distinct marker order to that of the IMF map was also found for an interval at OAR12 (Beraldi et al. 2006).
A comparison of the Churra linkage map with that obtained by Beraldi et al. (2006) in this free-living ovine population showed that 40% of markers included in the Churra map were common to the two studies. A comparison of common intervals revealed a concordance in marker order with one exception regarding the particular region cited at chromosome OAR12, where the order of markers BM4025 and TGLA53 in the Churra population was inverted with respect to Soay sheep, and similar to that reported for the IMF flock.
In conclusion we would point out that results for the Churra map support the suggestions of other authors (McRae & Beraldi 2006) that, although there may be general agreement with published maps, the possible existence of certain differences makes it more appropriate to construct a linkage map specifically for the population under study, as a suitable tool for use in the genetic dissection of economically important traits in livestock. Moreover, it would be interesting to include a greater number of markers in certain chromosomes in order to ascertain whether the distinct recombination values obtained here were caused by experimental factors or partially derived from differences between Churra sheep and other ovine populations.
Acknowledgements
This work was supported by Spanish Ministry of Education (Project 1FD97-0225) and the European Union through the project genesheepsafety (QLK5-2000-00656).




