Fluorescent prey traps in carnivorous plants
Abstract
Carnivorous plants acquire most of their nutrients by capturing ants, insects and other arthropods through their leaf-evolved biological traps. So far, the best-known attractants in carnivorous prey traps are nectar, colour and olfactory cues. Here, fresh prey traps of 14 Nepenthes, five Sarracenia, five Drosera, two Pinguicula species/hybrids, Dionaea muscipula and Utricularia stellaris were scanned at UV 366 nm. Fluorescence emissions of major isolates of fresh Nepenthes khasiana pitcher peristomes were recorded at an excitation wavelength of 366 nm. N. khasiana field pitcher peristomes were masked by its slippery zone extract, and prey capture rates were compared with control pitchers. We found the existence of distinct blue fluorescence emissions at the capture spots of Nepenthes, Sarracenia and Dionaea prey traps at UV 366 nm. These alluring blue emissions gradually developed with the growth of the prey traps and diminished towards their death. On excitation at 366 nm, N. khasiana peristome 3:1 CHCl3–MeOH extract and its two major blue bands showed strong fluorescence emissions at 430–480 nm. Masking of blue emissions on peristomes drastically reduced prey capture in N. khasiana pitchers. We propose these molecular emissions as a critical factor attracting arthropods and other visitors to these carnivorous traps. Drosera, Pinguicula and Utricularia prey traps showed only red chlorophyll emissions at 366 nm.
Introduction
Carnivorous plants are adapted to grow in low-nutrient habitats, and they compensate for this deficiency by attracting, trapping and digesting ants, insects and other arthropods through leaf-evolved traps (Merbach et al. 2002; Bohn & Federle 2004; Forterre et al. 2005; Raj et al. 2011). The major prey trapping devices in carnivorous plants are pitfall traps (Nepenthes, Sarracenia), flypaper traps (Drosera, Pinguicula), snap traps (Dionaea) and bladder traps (Utricularia). The best-known attractants in carnivorous prey traps are nectar, colour and olfactory cues (Bennet & Ellison 2009; Di Giusto et al. 2010; Raj et al. 2011). Various physical phenomena (Bohn & Federle 2004; Forterre et al. 2005), nectar, waxes, toxic secondary metabolites at capture spots (Bennet & Ellison 2009; Raj et al. 2011) and digestive enzymes in the trap fluids (Raj et al. 2011) play crucial roles in prey capture, digestion and absorption of nutrients. Moran and co-workers studied the roles of UV absorption patterns and colour in prey attraction in Bornean Nepenthes species (Moran 1996; Moran et al. 1999, 2012a,b). Recent studies also reported mutualistic interactions between small mammals (bats, rats, tree shrews) and prey traps of Nepenthes spp. native to Borneo (Clarke et al. 2009; Grafe et al. 2011; Wells et al. 2011). Here we report the (i) discovery of distinct blue fluorescence emissions from prey-capturing regions of Nepenthes, Sarracenia and Dionaea traps at UV 366 nm, (ii) chemical tests, DART-MS and LC/MS/MS analyses on Nepenthes peristomes and pitcher fluids, (iii) fluorescence emission spectra of Nepenthes peristome extractives on excitation at 366 nm and (iv) field data on prey capture in Nepenthes.
Materials and methods
Prey traps at UV 366 nm
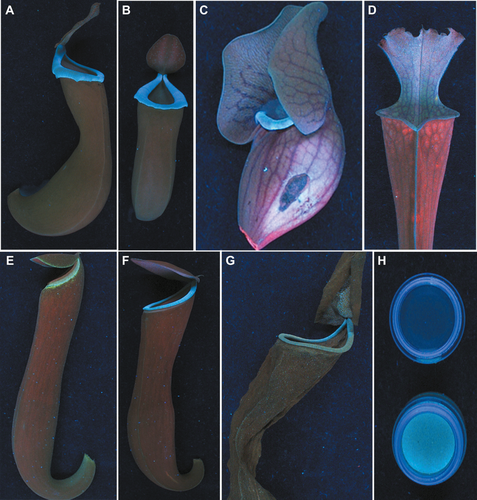
Fresh pitchers of Nepenthes albomarginata, N. ampullaria, N. chaniana, N. glandulifera, N. gracilis, N. gracillima, N. khasiana, N. mirabilis, N. rafflesiana, N. stenophylla, N. truncata, N. ventricosa, N. maxima × N. boschiana, N. hamata × N. platychila, Sarracenia flava, S. oreophila, S. psittacina, S. purpurea and S. rubra were collected (January–March 2012) from the Conservatory of Jawaharlal Nehru Tropical Botanic Garden and Research Institute, Kerala, India. Small cuts were made at the bottom portions and pitcher fluids were emptied. Fluid-free pitchers were scanned at 366 nm (Fig. 1) on a Reprostar 3 with cabinet cover (CAMAG, Muttenz, Switzerland). Pitcher tubes (cut open) and pitcher fluids of both unopened and opened pitchers of Nepenthes and Sarracenia species/hybrids were also viewed at 366 nm (Fig. 1). N. khasiana pitchers in early growth stages, opened pitchers with prey capture, near-dead and dead pitchers were also scanned (Fig. 1E–G). Prey traps of Dionaea muscipula, Drosera adelae, D. capensis, D. prolifera, D. madagascariensis, D. regia, Utricularia stellaris, Pinguicula cyclosecta and Pinguicula sp. collected (January–March 2012) from our Institute Conservatory were also scanned at 366 nm.
Chemicals tests on Nepenthes prey traps
Fresh peristomes of N. ampullaria, N. gracilis, N. khasiana, N. mirabilis and N. ventricosa were cut from their pitchers and separately (sequentially) rinsed with water (40 min), n-hexane (40 min), dichloromethane (DCM; overnight) and methanol (MeOH; 40 min). After each washing step, fluorescence emissions on the cut peristomes were tracked at UV 366 nm. Water, n-hexane, DCM and MeOH rinses of Nepenthes peristomes were also scanned at 366 nm. Sugars (Molisch test), quinones (alcoholic KOH test) and phenolics (Folin-Ciocalteu test) in peristome rinses were tested. As in Nepenthes, S. oreophila pitcher top was cut and sequentially rinsed with water, n-hexane, DCM and MeOH. These four S. oreophila rinses were also tested for sugars, quinones and phenolics.
Extraction of blue metabolites, fluorescence emissions
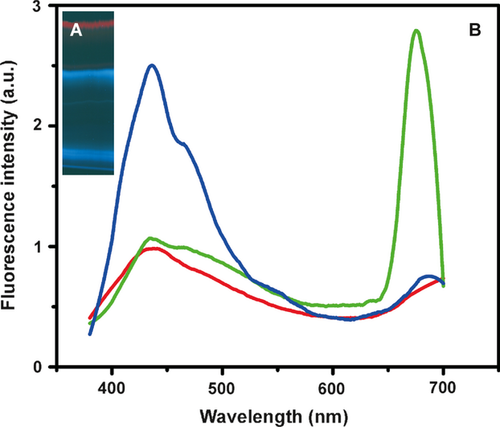
Peristomes of N. khasiana fresh pitchers were cut into small pieces, sequentially rinsed with water (40 min), n-hexane (40 min), DCM (40 min), chloroform (CHCl3; overnight), 3:1 CHCl3–MeOH (40 min), 1:1 CHCl3–MeOH (40 min), MeOH (40 min), and these rinses were concentrated on a rotary evaporator (Büchi Labortechnik AG, Flawil, Switzerland) under vacuum. High-performance thin layer chromatography (HPTLC; Camag, Muttenz, Switzerland) profiles of these N. khasiana peristome rinses were obtained on silica gel 60F254 aluminium plates (E. Merck, Darmstadt, Germany) in 9:1 acetonitrile-water at 366 nm. 3:1 CHCl3–MeOH fraction showed three bands (Fig. 2A). The bottom blue band and dissociated blue (top) band were isolated from the N. khasiana 3:1 CHCl3–MeOH fraction by preparative TLC under similar conditions (Fig. 2A). UV absorption and fluorescence emission (excitation λ 366 nm) spectra of the parent 3:1 CHCl3–MeOH fraction and the two isolated blue bands were recorded on a UV-1650PC UV-visible spectrophotometer (Shimadzu, Kyoto, Japan) and a SPEX Fluorolog F112X spectrofluorimeter (Horiba Jobin Yvon Inc., Edison, NJ, USA), respectively (Fig. 2B).
Chitin induction, DART-MS, LC/MS/MS
Near mature pitchers of N. khasiana were induced with colloidal chitin and pitcher fluids were collected after 5 days from these pitchers before lid opening (Raj et al. 2011). N. khasiana chitin uninduced, induced pitcher tissues and lyophilised pitcher fluids were subjected to direct analysis with real time-mass spectrometry (DART-MS) on an AccuTOF JMS-T100LC mass spectrometer (JEOL, Peabody, MA, USA). Samples were analysed in ESI+ mode directly in front of the DART source. Dry helium was used at a flow rate of 4 l·min−1 for ionisation at 350 °C. Orifice 1 was set at 28 V, spectra were collected and data from six to eight scans were averaged (Figs S1–S3). N. khasiana peristome 3:1 CHCl3–MeOH fraction was subjected to liquid chromatography-tandem mass spectrometry (LC/MS/MS) by direct infusion (5 μl·min−1, declustering potential 20, positive electrospray ionisation mode, amu ≤ 300) onto an API 2000 system (AB Sciex, Framingham, MA, USA).
Field studies on prey capture
N. khasiana peristome was carefully coated with the acetone extract of its pitcher slippery zone (non-fluorescent pitcher zone between pitcher fluid and upper peristome) and viewed at UV 366 nm. Acetone extract masked the blue emissions on N. khasiana peristome. Similarly, peristomes of six near-mature N. khasiana field pitchers were masked with the acetone extract. After 10 days, prey capture in masked (n = 6) and control pitchers (n = 6) was observed by transferring their contents into Petri dishes (Fig. S4). Secondly, N. khasiana pitcher peristomes (n = 6) in near-mature field pitchers were cut circularly with their pitcher lids intact, and prey capture was observed after 10 days, with normal pitchers as controls (n = 6; Fig. S5). Thirdly, on field observations over 18 months, we found trapped insects and other aerial preys in the pitcher fluids of most lid-opened N. khasiana pitchers on a regular basis. Ants were often seen roaming on newly opened pitcher peristomes, presumably in search of nectar. But aerial preys were rarely spotted around N. khasiana pitchers in daylight. Again we found the bottom halves of near-dead or dead Nepenthes pitchers loaded with the digested remains of aerial and non-aerial preys.
Results and discussion
Fluorescent prey traps, pitcher fluids
Here we report the discovery of distinct blue fluorescence emissions at the landing (top) spots and other (inner) capturing regions in prey traps of 12 Nepenthes species, two Nepenthes hybrids, five Sarracenia species and D. muscipula at UV 366 nm. Peristomes of Nepenthes species and hybrids flashed like well-designed, blue fluorescent tracks (Fig. 1A,B and F). Interior pitcher tubes showed blue emissions upto the digestive fluid marks. Nepenthes pitcher tubes between pitcher liquids and peristomes appeared faint red at 366 nm. This is due to the red fluorescence emission of chlorophyll from these tissues. Pitchers in early growth stages progressively developed the blue fluorescence, and its emission intensity sharply increased on prey capture followed by lid opening (Fig. 1E and F). Near dead and dead Nepenthes pitchers with digested arthropod preys showed gradually diminishing blue emissions (Fig. 1G). As in Nepenthes, pitchers of five Sarracenia spp. showed blue fluorescence emissions from their top portions (peristome, lid), inner pitcher tubes and pitcher fluids at UV 366 nm (Fig. 1C and D). Prey capture in D. muscipula (Venus flytrap) is by the rapid closure of its leaf lobes, which is one of the fastest movements in the plant kingdom (Forterre et al. 2005). We found the blue glitter at the inner sides of D. muscipula traps (leaf lobes) at 366 nm. Outer sides of these traps showed only red chlorophyll emissions. But the sticky, glandular tentacle covered leaves (traps) of five Drosera spp., two Pinguicula spp. and the tiny traps of U. stellaris emitted only red chlorophyll fluorescence at UV 366 nm. Joel et al. (1985) reported UV absorption-reflection patterns in leaves and prey traps of S. flava, D. muscipula, D. capensis, Drosophyllum lusitanicum, Pinguicula ionantha, Cephalotus follicularis, Heliamphora nutans and Nepenthes sp. (Joel et al. 1985). Here, we found blue fluorescence emissions only from the prey capture spots of Nepenthes, Sarracenia and D. muscipula. Their leaves showed only red chlorophyll emissions at UV 366 nm. Again Joel et al. (1985) found UV absorption patterns on Drosera and Pinguicula species (Joel et al. 1985), but here no blue emissions were detected on their prey traps at 366 nm. More recently Moran and co-workers reported UV and colour patterns in Bornean Nepenthes species (Moran 1996; Moran et al. 1999, 2012a,b).
Chemical tests on prey traps
Water and n-hexane rinsing of N. ampullaria, N. gracilis, N. khasiana, N. mirabilis and N. ventricosa peristomes showed no substantial reduction in their blue fluorescence emissions at 366 nm. But the blue emissions on the cut Nepenthes peristomes diminished drastically after DCM washing. On MeOH washing, the blue rings were entirely washed off from all five Nepenthes peristomes, and the red chlorophyll fluorescence emerged. Water, n-hexane and DCM washes showed blue fluorescence emissions at 366 nm with highest emission in the DCM washes. MeOH rinses of Nepenthes peristomes emitted the red fluorescence of chlorophyll at 366 nm. Water washes of the five Nepenthes peristomes tested strongly positive for sugars (Molisch's test, purple ring). Water washes also gave faint positive tests for quinones (alcoholic KOH, brownish yellow) and phenolics (Folin-Ciocalteu test, blue). n-Hexane washes were waxy on GC-MS (Raj et al. 2011) and gave weak positive tests for sugars, quinones and phenolics. Traces of phenolic metabolites washed down caused the faint blue fluorescence in water and n-hexane washes. Nepenthes DCM washes tested strongly positive for quinones (magenta pink) in alcoholic KOH whereas they tested negative for sugars. DCM washes also showed a light blue colour when treated with Folin-Ciocalteu reagent indicating phenolic metabolites. MeOH washes of Nepenthes peristomes tested positive for phenolics (blue) and strongly positive for sugars (purple ring). These sugars are polar glycosides or sugar esters of phenolics washed into MeOH from Nepenthes peristome tissues. Nepenthes MeOH washes gave only a brownish yellow colour on addition of alcoholic KOH (quinone test). S. oreophila pitcher top rinses were also subjected to sugar (Molisch test), quinone (alcoholic KOH) and phenolic (Folin–Ciocalteu reagent) tests. Water wash tested sharply positive for sugars (purple ring), water and DCM washes gave positive tests for quinones (light brownish yellow), and MeOH wash tested positive for phenolics (blue) and sugars (purple ring).
Blue fluorescent metabolites, fluorescence emissions
Blue fluorescent metabolites were rinsed off N. khasiana peristomes, and on HPTLC profiling their highest intensities were detected in the 3:1 CHCl3–MeOH fraction at 366 nm. N. khasiana liquid zone tissues also showed similar extraction patterns. 3:1 CHCl3–MeOH extract showed two strong, broad UV absorptions at 240–285 and 340–420 nm. Again, the 3:1 CHCl3–MeOH extract on HPTLC profiling in 9:1 acetonitrile-water showed a parent blue (bottom) band, a dissociated blue (top) band and a red band at 366 nm (Fig. 2A). These two blue bands were isolated by preparative TLC. Both isolated bands showed major UV absorption maxima at 240 nm and broad UV absorptions at 240–400 nm. Fluorescence spectra of both these blue bands on excitation at λ 366 nm showed emission maxima at 430–480 nm (Fig. 2B). The top blue spot showed relatively high blue emissions. These fluorescence emissions are mostly in the blue region (450–495 nm) of the visible spectrum. Parent 3:1 CHCl3–MeOH fraction showed an emission at 430–480 nm, and a second major emission at 650–700 nm, corresponding to chlorophyll (Fig. 2B).
Chitin induction, DART-MS, LC/MS/MS
DART-MS profiles of N. khasiana chitin uninduced, induced pitcher tissues and pitcher fluids showed M+H+/M+. signals corresponding to various phenolic metabolites (Figs S1–S3). M+H+/M+. signals of p-coumaric acid (M+H+ 163.06), caffeic acid (M+. 180.09), ferulic acid (M+. 194.09), syringic acid (M+H+ 198.10), ellagic acid (M+H+ 303.06) and quercetin (M+H+ 303.06) were found in uninduced pitcher tissues and fluids (Fig. S1). Higher levels of these phenolic metabolites were detected in chitin induced pitchers. M+H+ signals corresponding to scopoletin (M+H+ 193.10) and chlorogenic acid (M+H+ 355.31) were also found at high intensities on chitin induced pitcher tissues and fluids. Moreover M+H+/M+. signals at 390–400 and 550–600 emerged in the DART-MS patterns of N. khasiana chitin-induced pitcher tissues (Fig. S2). Similarly, new M+H+/M+. signals at 420–460 mass units emerged in N. khasiana chitin-induced pitcher fluids (Fig. S3). These M+H+/M+. signals are mostly due to the phenolic derivatives released into N. khasiana pitcher fluid on chitin induction. LC/MS/MS of N. khasiana 3:1 CHCl3–MeOH peristome fraction at DP 20 showed major signals at m/z 134.2, 134.5, 154.8, 161.0, 168.9, 179.8 and 197.7 corresponding to parent or fragmentation peaks of phenolics and their ester derivatives. These phenolics and their esters are the most probable metabolites causing the blue fluorescence emissions from these carnivorous traps. Further characterisation of these fluorescent metabolites is in progress.
Field observations on prey capture
Masking of the fluorescence emissions (366 nm) on N. khasiana peristomes showed drastic reduction in total prey capture in pitchers compared to unmasked field pitchers (Fig. S4). This indicated the blue fluorescence emissions as a critical factor attracting aerial preys. Few aerial preys found in masked pitchers were possibly attracted by the colour of the prey trap, volatile emissions or trapped by chance encounters (Fig. S4). Cutting off the peristomes from field pitchers (with their lids intact) drastically hampered the prey capture mechanism in Nepenthes pitchers (Fig. S5). Control pitchers in both these cases were proved to be very efficient traps of aerial and non-aerial preys (Figs S4 and S5). Moreover our long term field observations inferred to higher rates of nocturnal visits by aerial preys to carnivorous traps. The water washes (outer layers) of Nepenthes peristomes tested strongly positive for free sugars. These sugars contribute to the nectar and act as baits to the landing visitors. Our field data (Figs S4 and S5) indicate that the fluorescence emissions from carnivorous traps act together with other known attraction strategies, such as nectar, colour and olfactory cues (Bennet & Ellison 2009; Di Giusto et al. 2010; Raj et al. 2011).
Conclusions
Based on our findings, we propose that, under UV light pitcher peristomes and other capture spots in Nepenthes, Sarracenia and Dionaea glow in alluring blue emissions, guiding arthropod preys and other visitors towards them (Fig. 1). These blue emissions are only found at the interior capture spots of their prey traps. Arthropod visitors (ants, insects, moths, spiders) and small mammals involved in mutualistic interactions (bats, rats, tree shrews) with Nepenthes pitchers have their vision maxima in UV and visible (blue, green) regions (Peitsch et al. 1992; Briscoe & Chittka 2001; Jacobs et al. 2001; Winter et al. 2003; Zhaoa et al. 2009). These visitors could perceive the blue fluorescent rings on Nepenthes peristomes as attractive landing pads (Fig. 1A,B and F). Similar fluorescence on capture spots of Sarracenia and Dionaea could also have a strong attracting influence to visitors. We propose these blue emissions as a critical factor attracting arthropod preys and other visitors to these carnivorous traps.
Acknowledgements
This work was supported by Plan Project Funds of the Government of Kerala, India. We also acknowledge the Sophisticated Analytical Instrument Facility (SAIF), Central Drug Research Institute (CDRI), India, for DART-MS analyses.
Author contributions
RK, AJJ, SS performed UV scans and chemical analyses; AAH provided N. khasiana specimens; CSK provided Nepenthes, Sarracenia, Drosera, Pinguicula, Utricularia and Dionaea specimens; SB developed the concept, carried out chemical analyses, field studies and wrote the paper with inputs from CSK.




