Xylem vulnerability to cavitation can be accurately characterised in species with long vessels using a centrifuge method
Editor: J. Sparks
Abstract
Vulnerability to cavitation curves describe the decrease in xylem hydraulic conductivity as xylem pressure declines. Several techniques for constructing vulnerability curves use centrifugal force to induce negative xylem pressure in stem or root segments. Centrifuge vulnerability curves constructed for long-vesselled species have been hypothesised to overestimate xylem vulnerability to cavitation due to increased vulnerability of vessels cut open at stem ends that extend to the middle or entirely through segments. We tested two key predictions of this hypothesis: (i) centrifugation induces greater embolism than dehydration in long-vesselled species, and (ii) the proportion of open vessels changes centrifuge vulnerability curves. Centrifuge and dehydration vulnerability curves were compared for a long- and short-vesselled species. The effect of open vessels was tested in four species by comparing centrifuge vulnerability curves for stems of two lengths. Centrifuge and dehydration vulnerability curves agreed well for the long- and short-vesselled species. Centrifuge vulnerability curves constructed using two stem lengths were similar. Also, the distribution of embolism along the length of centrifuged stems matched the theoretical pressure profile induced by centrifugation. We conclude that vulnerability to cavitation can be accurately characterised with vulnerability curves constructed using a centrifuge technique, even in long-vesselled species.
Introduction
Plants transport water from the soil to leaves along a negative pressure gradient according to the cohesion–tension theory (Dixon & Joly 1895). While energetically efficient, transport of water under negative pressure is susceptible to cavitation, and subsequent embolism formation can block water flow through xylem conduits, thereby reducing hydraulic efficiency (Sperry & Tyree 1988; Tyree & Sperry 1989). Species differ broadly in their vulnerability to cavitation (Pockman & Sperry 2000; Jacobsen et al. 2007b), as do plant communities (Jacobsen et al. 2007a). Moreover, vulnerability to cavitation has been linked to drought resistance and survival (Pratt et al. 2008; Kursar et al. 2009), life-history characteristics (Pratt et al. 2007) and stomatal behaviour (Brodribb & Holbrook 2004), and it is evolutionarily correlated with many other functional traits (Maherali et al. 2004; Jacobsen et al. 2007b). The broad significance of the vulnerability of plants to cavitation has made it the focus of intense study.
The vulnerability of plants to cavitation is often characterised by constructing vulnerability curves, which describe the decline in xylem hydraulic conductivity (Kh) due to embolism formation in response to increasingly negative xylem pressure. Declines in Kh are typically expressed relative to the maximum sample Kh as the percentage loss of conductivity (PLC), and comparisons of the vulnerability to cavitation among species are made using the xylem pressure at 50% loss of conductivity (P50). An early method for constructing vulnerability curves involved collecting large branches and equilibrating them to a range of xylem pressures by repeated cycles of drying and equilibration while measuring PLC of stems on those branches at each equilibration xylem pressure (Sperry et al. 1988b). Although labour intensive, this method has the advantage that negative xylem pressure is generated in a similar manner to an intact plant, and it remains a standard to which other methods are compared.
Several techniques for constructing vulnerability curves rely on centrifugal force to induce a range of negative xylem pressures in stem or root segments (Pockman et al. 1995; Alder et al. 1997; Cochard et al. 2005; Li et al. 2008). The negative xylem pressure induced by centrifugation depends on segment length and angular velocity. The pressure profile along the segment is most negative at its centre and becomes less negative towards both ends. All centrifuge techniques include these features, but techniques differ in other attributes.
In the technique used in this study, described by Alder et al. (1997), segments are mounted into a custom centrifuge rotor configured so that when it is spinning, segment ends are submerged in solution maintained at equal levels. Because solution levels are equal, no flow through the segment occurs during centrifugation. In this technique, segments are removed from the centrifuge to measure Kh under a low positive pressure (Sperry et al. 1988a). Through repeatedly centrifuging segments to more negative xylem pressures and measuring Kh between spins, a complete vulnerability curve can be constructed.
An alternate centrifuge technique relying on the ‘Cavitron’ rotor design (Cochard 2002; Cochard et al. 2005) enables the Kh of a segment to be measured while it is spinning in the centrifuge. This is accomplished by increasing the solution level at one end of the segment to create a pressure gradient across the segment that drives flow during centrifugation. This technique has the advantage of measuring Kh while the xylem is under negative pressure, as occurs in vivo. Although similar in concept to the Cavitron technique, the centrifuge technique described by Li et al. (2008) used a different rotor design. These distinctions are important, since differences in rotor design may make some rotors more prone to artefacts than others (Sperry et al. 2012).
Two studies have questioned whether vulnerability curves constructed with a centrifuge method accurately characterise the vulnerability to cavitation for long-vesselled species (Choat et al. 2010; Cochard et al. 2010). Choat et al. (2010) found that centrifuge vulnerability curves constructed for Vitis vinifera (grapevine) using the technique described in Alder et al. (1997) did not agree with vulnerability curves constructed using other methods. In a second study, Cochard et al. (2010) showed that vulnerability curves for a long-vesselled species constructed with the Cavitron centrifuge technique differed markedly from dehydration curves, and were shifted by altering the length of the stem segment (Fig. 1). The authors of both studies hypothesised that vessels cut open at segment ends and extending to the middle or entirely through segments (open vessels) are more vulnerable to cavitation during centrifugation than they would be in situ during dehydration. If supported, this open-vessel artefact hypothesis raises doubts about the interpretation of centrifuge vulnerability curves constructed for species with many open vessels. However, reinterpretation of centrifuge vulnerability curves for long-vesselled species may be unwarranted because of recently reported methodological challenges associated with V. vinifera (Jacobsen & Pratt 2012), and the substantial differences between the Cavitron rotor design and measurement protocol and other centrifuge techniques.
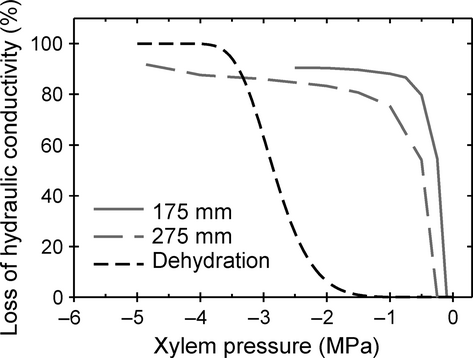
We tested key predictions of the open-vessel artefact hypothesis to determine the reliability of vulnerability curves constructed with the centrifuge technique described in Alder et al. (1997). To test whether open vessels created during stem segment preparation increased segment vulnerability to cavitation, we compared laboratory dehydration vulnerability curves to matched centrifuge curves for a long-vesselled (Quercus wislizeni) and a short-vesselled (Heteromeles arbutifolia) shrub species. The long-vesselled oak was chosen because it has a maximum vessel length of 1.45 m (Jacobsen et al. 2007b), which is comparable to the 1.34 m maximum vessel length of the oak (Quercus robur) that Cochard et al. (2010) found to have widely divergent dehydration and Cavitron vulnerability curves (Fig. 1). We constructed these curves by first determining the PLC of stems sampled from large branches during laboratory dehydration, and then flushing and centrifuging the stems to a xylem pressure similar to that measured for the large branch with stem psychrometers. This allowed us to directly compare the level of stem embolism induced by air-drying large branches or by centrifugation of stem segments. In addition, an acoustic method was used to independently monitor cavitation in branches during dehydration (Tyree & Sperry 1989), and native embolism measurements of plants in the field were compared to vulnerability curves.
We also tested the prediction of the open-vessel artefact hypothesis that a larger proportion of open vessels in a stem segment will increase its vulnerability to cavitation during construction of a centrifuge vulnerability curve. To test this prediction, we compared centrifuge vulnerability curves constructed with stem segments that differed in length and therefore also the proportion of open vessels. Comparisons were made for four species, including Q. robur, the long-vesselled oak tested by Cochard et al. (2010) and reported to have Cavitron vulnerability curves that differed depending on segment length (Fig. 1), and V. vinifera, the species found by Choat et al. (2010) to have anomalous centrifuge vulnerability curves.
As another test of the centrifuge technique used in this study, we characterised the distribution of embolism along the length of centrifuged stem segments of a long-vesselled species to compare it with the theoretical pressure profile induced by centrifugation. The distribution of embolism produced by the Cavitron (Fig. 5 in Cochard et al. 2010) did not match theoretical predictions that the level of embolism should be highest at the centre of the stem; therefore we tested our technique for a similar departure from theoretical predictions.
Material and Methods
Study site and plant material
Chaparral shrub plant material was collected from a site in the San Gabriel Mountains of southern California (1040 m a.s.l.; 34°21.613 N, 118°26.125 W). The site has a mediterranean-type climate with hot, dry summers and cool, wet winters and a mean annual precipitation of 685 mm from 1996 to 2010 (Western Regional Climate Center 2011). Branches of long-vesselled V. vinifera L. cv. Glenora were collected from a mature, irrigated plant growing in Bakersfield, CA, USA. For V. vinifera, all branches were collected from the same individual to control for interplant variability and to reduce variability in the number of open vessels not due to differences in sample length. Branches of long-vesselled Q. robur L. cv. fastigiata were collected from plants grown in a greenhouse in Bakersfield with daily irrigation for 8 months, following their purchase as ~1-m plants from Lawyer Nursery Inc., MT, USA.
Two evergreen chaparral shrubs were chosen for the main experiments because of their widely divergent vessel lengths (Table 1): the long-vesselled Q. wislizeni var. frutescens Engelm. (Fagaceae) and the shorter-vesselled Heteromeles arbutifolia (Lindl.) M. Roem. (Rosaceae). Two additional long-vesselled species (mean vessel length >0.10 m), Rhus ovata S. Watson (Anacardiaceae) and Fraxinus dipetala Hook. and Arn. (Oleaceae), and two shorter-vesselled species (mean vessel length <0.06 m), Ceanothus leucodermis Greene (Rhamnaceae) and Ribes malvaceum Sm. (Grossulariaceae), were sampled for some experiments. Of these, F. dipetala and R. malvaceum were deciduous and the rest were evergreen.
| species | mean vessel length (m) | 140-mm stem segments | 271-mm stem segments | ||
|---|---|---|---|---|---|
| open (%) | open to the middle (%) | open (%) | open to the middle (%) | ||
| Quercus wislizeni | 0.124 (0.010) | 10.7 (1.9) | 53.3 (4.1) | 1.5 (0.5) | 21.4 (3.5) |
| Heteromeles arbutifolia | 0.060 (0.004) | 1.1 (0.3) | 18.9 (2.7) | 0.0 (0.0) | 2.5 (0.7) |
| Rhus ovata | 0.143 (0.007) | 14.1 (1.4) | 60.6 (2.3) | 2.4 (0.5) | 27.7 (2.4) |
- Values shown are means and SE in parentheses.
We collected large branches (1.8–3.0 m in length) of Q. wislizeni and H. arbutifolia for laboratory dehydration vulnerability curves from January to June of 2011, before new leaves had flushed. Stem cutting was done under water, branches were enclosed in doubled and humidified plastic bags to reduce transpiration, and the cut end was kept under water during transport to the laboratory. Smaller branches for centrifuge vulnerability curves and a repeated centrifugation experiment (see below) were collected similarly during the same time period, except for V. vinifera and Q. robur, which were sampled in autumn 2011.
Stem hydraulic conductivity, loss of conductivity and centrifugation
We determined Kh from measurements of the net increase in fluid flow rate through a stem after applying a pressure head (Sperry et al. 1988a). We selected stem segments that were ca. 6–8 mm in diameter and prepared them for measurements by cutting segments from sampled stems under water and trimming them to the desired length with a razor blade. To make flow rate measurements, stems were inserted into a tubing apparatus that connected the basal end to an elevated fluid reservoir and the other end to a collection reservoir on an analytical balance (CP124S, Sartorius, Goettingen, Germany) connected to a laptop computer (Inspiron 5000e, Dell Computer Corp., Round Rock, TX, USA) that logged net flow over 10-s intervals. The pressure head was maintained below 2 kPa for stems of V. vinifera and below 4 kPa for all other stems to avoid refilling open vessels of large diameters. Stems were kept submerged in water during and between measurements. The perfusing solution used in the tubing apparatus was 20 mm KCl filtered to 0.1 μm (inline filter; GE Water and Process Technologies, Trevose, PA, USA) and vacuum-degassed for at least 20 min (Melcher et al. 2012). Before and after each measurement of pressure-driven flow, we measured background flow with no pressure head. We calculated Kh as the pressure-driven flow corrected for background flows divided by the pressure gradient (pressure head/stem length). Maximum Kh was measured on stems flushed with the same solution used for Kh measurements at 100 kPa for 1 h to refill embolised vessels. To calculate stem area specific conductivity (Ks), Kh was divided by stem sapwood cross-sectional area, which was calculated from caliper measurements of xylem and pith diameter.
We used centrifugation at a stage in each experiment to induce negative xylem pressure in stem segments (Holbrook et al. 1995). We mounted stems in a custom rotor (Alder et al. 1997) that enabled them to be spun in a centrifuge (Sorvall RC-5C; Thermo Fisher Scientific, Waltham, MA, USA) with stem ends submerged in solution contained in L-shaped reservoirs. The solution level in the reservoirs was kept even at both ends of the stem, so no flow was induced during centrifugation. During some experiments, we added foam pads to the vertical section of reservoirs to keep the solution in contact with stem ends when the rotor was not spinning (see below, Repeated centrifugation tests).
Laboratory dehydration vulnerability curves
Laboratory dehydration vulnerability curves were completed for 11 large branches of Q. wislizeni and three of H. arbutifolia. Both species had substantial native embolism (PLC >50% for many stems) that did not reverse during overnight rehydration of branches. The high native PLC made it impossible to fully characterise the vulnerability to cavitation of stems. Therefore, we refilled native embolism by re-cutting branches under water in the laboratory and attaching them to a tubing manifold pressurised to ~15 kPa for about 18 h with the same solution used for Kh measurements. We confirmed removal of native embolism by measuring the PLC of at least two stems per branch.
After refilling, 1–3 stem psychrometers (Stem Hygrometer; PWS Instruments Inc., Guelph, OT, Canada) connected to a data logger (CR7; Campbell Scientific, Logan, UT, USA) were installed on each branch to track branch xylem pressure. The bark was sanded away to expose a small patch of xylem where psychrometers were attached, and silicone grease was used to create an airtight seal. An ultrasonic acoustic emissions transducer (Model I15I; Physical Acoustics Corp., Princeton, NJ, USA) was attached to one of the branches to monitor cavitation as the branch dried. Bark was sanded off the branch where it was attached, and the xylem was covered with silicone grease to facilitate contact with the transducer. A spring-loaded attachment held the transducer against the stem with 30 N of force (Jackson & Grace 1996). The transducer was connected to hardware that conditioned the transducer output so that individual acoustic events were sampled only once, and that amplified the signal by 75 dB. A data logger (21×; Campbell Scientific) sampled this output every second and recorded 5-min sums of acoustic emissions.
Branches were equilibrated at increasingly negative xylem pressure through repeated cycles of drying and equilibration. Branches were dried by uncovering them for a short period, then equilibrated by sealing them in double plastic bags, which were externally covered with moist cotton sheets and more plastic bags, and allowing them to equilibrate for 16–23 h. Following equilibration, 1–3 stems were removed from each large branch for PLC measurements, with cuts made under water. At the same time, we collected up to five branchlets for water potential measurements with a pressure chamber (PMS Instrument Co., Albany, OR, USA) to compare with stem psychrometer values. We repeated these steps until the stem loss of hydraulic conductivity was near 100%.
Comparison of dehydration and centrifuge methods
We constructed centrifuge vulnerability curves using stems sampled during laboratory dehydration vulnerability curves for both Q. wislizeni and H. arbutifolia to test if negative xylem pressure induced by either air-drying large branches or centrifugation of stem segments resulted in different curves. After the PLC of stems sampled from large branches during a laboratory dehydration vulnerability curve was determined, we centrifuged the flushed stems for 5 min to a xylem pressure similar to that measured for the large branch with psychrometers. The centrifugation-induced PLC of these stems was then measured, which resulted in a matched pair of centrifuge and dehydration PLC values at similar water potentials for each stem segment.
Comparison of centrifuge vulnerability curves of two stem lengths
We constructed centrifuge vulnerability curves for stems of 140 and 271-mm length for Q. wislizeni, H. arbutifolia, Q. robur and V. vinifera to test the effect of changing the proportion of open vessels. After an initial flush, we measured the Kh of stems and then centrifuged them for 5 min (n = 10 for 140-mm stems and n = 12 for 271-mm stems of V. vinifera, n = 6 for all other species). Stem Kh measurement and centrifugation was repeated, inducing a more negative xylem pressure with each centrifugation, and continued until stem loss of hydraulic conductivity was near 100%.
Distribution of centrifugation-induced embolism
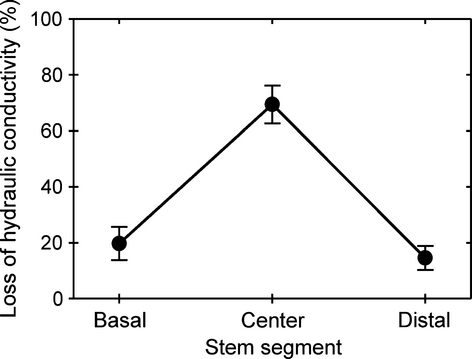
We measured the distribution of embolism along the length of stems to compare it with the theoretical negative pressure profile generated during centrifugation. We centrifuged 271-mm long flushed stems (n = 3) of the long-vesselled R. ovata at an angular velocity that induced -2 MPa of pressure at the rotational centre of the stem and reduced entire stem conductivity by 73–81%. We excised the basal, central and distal 40-mm segments of these stems and measured their PLC.
Repeated centrifugation tests
We repeatedly centrifuged stems of all six study species at the same angular velocity to test for increases in PLC unrelated to inducing more negative xylem pressure. After an initial flush and Kh measurement, stems were either centrifuged at −0.5 MPa for 5 min four times, with Kh measured after each centrifugation, or stems were centrifuged once at −0.5 MPa for 20 min, with Kh measured afterwards. All stems were then flushed, followed by a final Kh measurement. There were three stems per treatment, except R. ovata, for which there were six.
As a follow up, we tested if several treatments reduced the increase in PLC observed with repeated centrifugation at the same angular velocity. Because the centrifuge reservoir design relies on centrifugal force to maintain solution in contact with stem ends, they are exposed to air when the rotor is not spinning. We added foam pads (CoverGirl Make-Up Masters Sponge Wedges; Procter & Gamble, Hunt Valley, MD, USA) thick enough to be in contact with stem ends to the vertical section of reservoirs. The pads were saturated with solution and thereby kept the solution in contact with stem ends when the rotor was stationary. We tested the effect of foam pads by comparing the PLC increase with and without pads for repeatedly centrifuged stems of R. ovata (n = 12). We also tested the addition of 10 min at 0.1 MPa to the end of centrifugation as a ‘relaxation’ period on Q. wislizeni stems (n = 3). Finally, recent concerns about solution gas content (Espino & Schenk 2011) led us to test if additional degassing of the perfusing solution reduced increases in PLC with repeated centrifugation for R. ovata stems (n = 3). After our standard vacuum degassing of 10 l of continuously stirred solution for at least 20 min at an absolute pressure of ~10 kPa generated with a vacuum pump (model 5KH33DN16X; Millipore, GE, Billerica, MA, USA), we further vacuum degassed the solution using a membrane contactor (Liqui-Cel mini-module 1.7 × 5.5; Membrana, Charlotte, NC, USA).
Native PLC
We measured native PLC in Q. wislizeni at the end of the summer dry period in 2009, and again during the rainy season in early spring of 2010. For summer measurements, we collected large branches (n = 6) by cutting them in air near midday, and brought them to the laboratory sealed in doubled and humidified plastic bags. After an equilibration period, we determined branch water potential from branchlets using the pressure chamber and measured PLC of stems cut under water from the large branches at a distance from the original cut end that exceeded maximum vessel length. Spring measurements of native PLC were done in the field on stems collected pre-dawn, with cuts made under water. Pre-dawn water potential for each sampled shrub (n = 6) was determined from branchlets collected with stems. Laboratory and field-portable conductivity systems differed primarily in the method used to quantify flow, which was done in the field by timing the rate at which solution volume increased in a 0.1 ml pipette.
Vessel length distributions
Vessel length distribution was measured on six stem segments per species for the six chaparral study species using a silicon injection method (Sperry et al. 2005; Wheeler et al. 2011). We used stem segments that were 6–8 mm in diameter and longer than 250 mm. Prior to injection, stems were trimmed and flushed as described above for Kh measurements. The basal end of the segments were then injected with a 10:1 silicone hardener mixture (Rhodorsil RTV 141; Bluestar Silicones, distributed by Skycon, Toronto, OT, Canada) that was mixed with a soluble fluorescent dye (1% w/w; Uvitex OB, Ciba Specialty Chemicals, Tarrytown, NY, USA dissolved in chloroform) at 50 kPa for 24 h. Stem segments were cured at room temperature for at least 72 h before sectioning. Stems were sectioned at several distances from the injection surface, and cross-sections were analysed to determine the percentage of vessels filled. The vessel length distribution was calculated based on the equations described in Wheeler et al. (2011), and this distribution was used to estimate the percentage of vessels open all of the way through segments and percentage of vessels open to the middle of segments from either cut end.
Statistical analysis
Plant material was carefully matched within each experiment, and vulnerability curves were compared within each experiment. We tested if dehydration and matched centrifuge vulnerability curves differed by comparing models that included both curves using nested models constrained to fit a single curve (Crawley 2007). Models were fitted to mean PLC of stems (n = 1–3) at each branch equilibrium or centrifuge xylem pressure. A reparameterised Weibull model (Ogle et al. 2009) was fitted to Q. wislizeni values using generalised nonlinear least squares, and incorporated a variance model to account for non-constant variance (Pinheiro & Bates 2000). Models were fitted with maximum likelihood, and comparison of nested models resulted in a likelihood ratio test statistic (LRT). The shape of the relationship between PLC and xylem pressure for H. arbutifolia was poorly fitted with the Weibull model, so a generalised additive model (GAM) was fitted (Wood 2006). This type of model does not assume a specific functional form for the shape of the relationship between response and explanatory variables.
We tested if centrifuge vulnerability curves constructed with 140 or 271-mm stems differed using two separate analyses. In both analyses, curves were corrected for cavitation fatigue (Hacke et al. 2001) by calculating the PLC using the Kh measured following the initial spin at 0.5 MPa in place of the maximum Kh. This minimised the effect of xylem vessels unlikely to be functional in intact plants. To compare P50 between vulnerability curves, we employed a common approach of fitting separate curves (GAM for H. arbutifolia and Weibull for other species) to data for each stem and then used the P50 estimates from fitted curves in an analysis of variance (anova). We included species, stem length (140 mm or 271 mm) and their interaction in the model and tested for differences by stem length within species using a priori contrasts. A second approach that tested if the entire curves differed by stem length consisted of comparing models fitted to all stems that included two curves or were constrained to fit a single curve. This analysis was analogous to the dehydration and centrifuge curve comparison, but random effects were incorporated to account for the repeated measurements made on individual stems (Pinheiro & Bates 2000; Wood 2006).
The PLC response to repeated centrifugation was analysed as a mixed-model anova. We included centrifugation (repeat number), vessel length (long or short) and centrifugation type (repeated or single centrifugation) as fixed effects, and species and stem as random effects. Initial and final flushed Ks were similarly analysed by substituting flushed (initial or final) for centrifugation, and examined if initial and final Ks differed using a priori contrasts. For the repeated centrifugation experiment that tested the effect of foam pads in reservoirs, the PLC response to repeated centrifugation was analysed as a mixed-model anova that included centrifugation (repeat number) and treatment (with foam or without foam) as fixed effects, and stem as a random effect. The first and the fourth centrifugation PLCs for each treatment were compared using a priori contrasts. Additional treatments tested for their effect on PLC induced by repeated centrifugation were modelled similarly. The analyses were performed using R version 2.8.1 (R Development Core Team 2011) including packages nlme (Pinheiro et al. 2011) and mgcv (Wood 2006).
Results
Comparison of dehydration and centrifuge methods
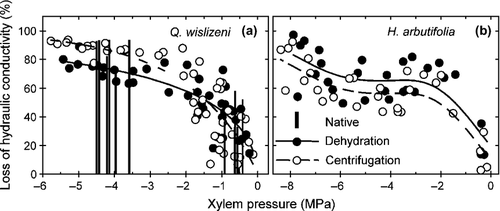
Dehydration and centrifuge methods for inducing negative xylem pressure in stems were compared by constructing matched vulnerability curves for stems that were initially dehydrated and subsequently flushed of emboli and centrifuged. For both long-vesselled Q. wislizeni and short-vesselled H. arbutifolia, the shape of the centrifuge vulnerability curve was similar to the dehydration vulnerability curve (Fig. 2). The P50 for Q. wislizeni did not differ between dehydration and centrifuge curves (Table 2), but centrifugation produced modestly higher PLC than dehydration at a PLC >60% (Fig. 2a), resulting in differences between curves compared along their entire span (LRT2 = 31.85, P < 0.001). Native PLC of Q. wislizeni stems sampled at the time of year when plants had high and low pre-dawn water potential closely matched the vulnerability curves (Fig. 2a). For H. arbutifolia, the dehydration and centrifuge vulnerability curves did not differ (Fig. 2b; F1.8, 52.1 = 2.68, P = 0.083), nor did P50 values (Table 2). Thus, in contrast to results of Cochard et al. (2010) (Fig. 1), the cavitation response of stems did not diverge widely depending on whether negative xylem pressure was generated by either centrifugation or air-drying large branches or natural dehydration in the field.
| vulnerability curve | P50 (MPa) | |
|---|---|---|
| Quercus wislizeni | Heteromeles arbutifolia | |
| Dehydration | −1.35 (−1.71, −0.99) | −1.44 (−1.96, −0.99) |
| Centrifugation | −1.37 (−2.17, −0.59) | −2.09 (−5.22, −1.50) |
- Values shown are means and 95% confidence intervals in parentheses.
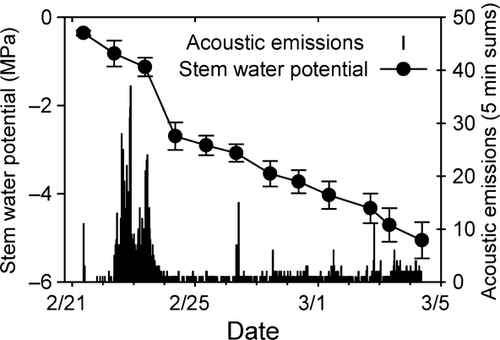
As a second independent measure of cavitation, we measured ultrasonic acoustic emissions emitted by xylem tissue during dehydration vulnerability curves. A high rate of acoustic emissions was observed early in the dehydration of Q. wislizeni branches as xylem pressure began to decrease, but thereafter emissions declined to a low rate, even as xylem pressure continued to drop (Fig. 3). This pattern of acoustic emissions corroborates the shape of the Q. wislizeni vulnerability curves, which predict extensive cavitation at the xylem pressures where the rate of acoustic emissions was high, but less additional cavitation at more negative xylem pressures where the acoustic emissions rate was low.
While constructing dehydration vulnerability curves, we found that xylem pressure potential of branchlets from the equilibrated branches measured with the pressure chamber agreed with stem psychrometer water potential measurements for H. arbutifolia, but were more negative at water potentials below ~−4 MPa for Q. wislizeni (Figure S1).
Comparison of centrifuge vulnerability curves of two stem lengths
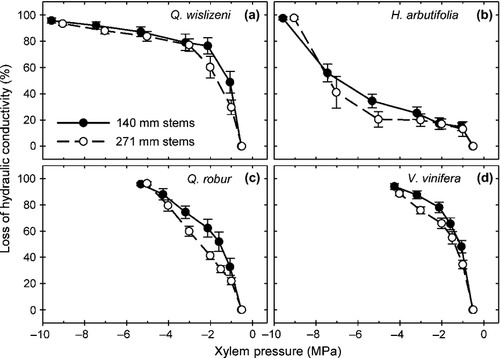
To test if the proportion of open vessels affected vulnerability curves measured with the centrifuge method, we compared curves for stems of 140 and 271-mm lengths. For H. arbutifolia, shortening the length of stem segments from 271 to 140 mm only substantially changed the percentage of vessels open to the middle, but both categories of open vessels changed substantially for Q. wislizeni (Table 1). Changes in percentage of open vessels between stem lengths for V. vinifera were similar to Q. wislizeni (e.g. entirely open changed from 1.5% to 9.4%; Jacobsen & Pratt 2012) and may also be similar for Q. robur, which has a similar maximum vessel length to Q. wislizeni. In spite of these changes, P50 did not differ by segment length for any of the four species examined (Table 3; F1,50 < 2.28, P > 0.137 for all segment length contrasts within species). Note that the P50 values in Tables 2 and 3 cannot be compared directly, because Table 3 values were calculated from vulnerability curves corrected for cavitation fatigue (Hacke et al. 2001), a correction not possible for dehydration vulnerability curves. In a separate test for differences along the entire span of vulnerability curves, the curves for the two stem lengths did not differ for Q. wislizeni or H. arbutifolia (Fig. 4; LRT2 = 3.36, P = 0.187 and LRT3 = 0.02, P = 0.999, respectively), but did for Q. robur and V. vinifera (LRT2 = 8.99, P = 0.011, LRT3 = 7.48, P = 0.024, respectively). The magnitude of the difference between vulnerability curves of the two stem lengths for Q. robur and V. vinifera were small compared to differences in vulnerability curves related to stem length observed by Cochard et al. (2010) for Prunus persica and Q. robur (e.g. Q. robur, Fig. 1).
| stem length (mm) | P50 (MPa) | |||
|---|---|---|---|---|
| Quercus wislizeni | Heteromeles arbutifolia | Quercus robur | Vitis vinifera | |
| 140 | −1.39 (−1.96, −0.82) | −6.78 (−7.98, −5.59) | −1.67 (−2.36, −0.97) | −1.22 (−1.48, −0.96) |
| 271 | −1.72 (−2.42, −1.01) | −7.00 (−8.23, −5.78) | −2.26 (−2.54, −1.97) | −1.45 (−1.73, −1.17) |
- Values shown are means and 95% confidence intervals in parentheses.
Distribution of centrifugation-induced embolism
We compared the distribution of embolism along stems with the theoretical negative pressure profile induced by centrifugation for a long-vesselled species. The PLC of stem segments excised from the centre of a centrifuged stem was higher than the PLC of segments excised from the ends (Fig. 5). This distribution of PLC matches the expected effect of the lower negative pressure generated near the axis of rotation at the centre of the stem during centrifugation (Alder et al. 1997).
Repeated centrifugation tests
Repeated centrifugation of stems at the same angular velocity led to increases in PLC with each additional centrifugation for six chaparral shrub species, but the magnitude of these increases was not related to having long or short vessels (Figure S2). For all species, flushing following repeated centrifugation fully recovered the initial flushed maximum Kh. Centrifugal force maintained solution in contact with stem ends during centrifugation, but stem ends were exposed to air when the rotor was not spinning. We substantially reduced the PLC increases during repeated centrifugation by adding foam pads to the reservoirs to maintain solution contact with stem ends at all times (Figure S3). We also tried adding 10 min at 0.1 MPa to the end of centrifugation as an equilibration period and additional degassing with a membrane contactor, but these treatments had no effect on PLC increases in Q. wislizeni and R. ovata, respectively (data not shown).
Discussion
Centrifuge vulnerability curves agree with dehydration curves
We found no evidence for an artefact associated with open vessels for vulnerability curves constructed using a centrifuge technique. These findings contrast with those of Cochard et al. (2010) and Choat et al. (2010). The vulnerability curves resulting from laboratory dehydration or centrifugation were similar for both a long- and a short-vesselled species, and other studies have found similar agreement between these methods (Alder et al. 1997; Jacobsen et al. 2007a; Jacobsen & Pratt 2012).
Vulnerability curves with a so-called ‘r’ shape, like the Q. wislizeni curve, characterised by large increases in PLC with the initial drops in xylem pressure have been called into question by Cochard et al. (2010). The centrifuge vulnerability curve shape we report for Q. wislizeni was supported by a matching laboratory dehydration curve, the pattern of acoustic emissions of dehydrating branches and seasonal native embolism measurements. When employing the single-vessel air injection method (Melcher et al. 2003), which is a completely different methodology to that used here, Christman et al. (2012) found that another Quercus species had xylem with a similarly high proportion of highly vulnerable vessels. The ‘r’ shape has been previously observed for dehydration vulnerability curves of several other chaparral species (Jacobsen et al. 2007b), suggesting that multiple species have a high proportion of very vulnerable xylem vessels in this plant community. These highly vulnerable vessels may be functional and enhance carbon gain primarily during the wet season, or they may undergo daily cavitation and refilling cycles (Taneda & Sperry 2008; Christman et al. 2012), thereby maximising daily photosynthesis throughout the growing season. Alternatively, highly vulnerable vessels may be an efficient way to construct xylem having the required mechanical support.
The proportion of open vessels does not affect centrifuge vulnerability curves
We found no effect of changing the stem segment length and thereby the proportion of open vessels on the P50 of centrifuge vulnerability curves. The lack of a substantial stem length effect for long-vesselled species, a finding consistent with Sperry et al. (2012) and Jacobsen & Pratt (2012), does not support the hypothesis that open vessels exhibit more vulnerability to cavitation during centrifugation. As described in Sperry et al. (2012), the increased vulnerability to cavitation of shorter stems for medium- to long-vesselled species reported by Cochard et al. (2010) may result from micro-bubbles being carried into the stem by the flow of solution induced as part of the unique Cavitron measurement technique. In contrast to Choat et al. (2010), we saw no evidence for an open-vessel artefact in centrifuge vulnerability curves of V. vinifera. A recent study suggests that V. vinifera branches used in dehydration vulnerability curves can develop vessel blockages, causing dehydration and centrifuge vulnerability curves to differ (Jacobsen & Pratt 2012).
Another result cited in Cochard et al. (2010) as demonstrating increased vulnerability of open vessels was the shift in Cavitron vulnerability curves following injection of air into the ends of stem segments. However, dramatically reducing hydraulic conductivity at the ends of stem segments with air injection (see Fig. 5 in Cochard et al. 2010) will shift vulnerability curves to appear more resistant to cavitation, regardless of which subset of vessels remain conductive. Centrifugation reduces hydraulic conductivity primarily near the middle of the segment length; therefore, a decrease in conductivity due to centrifugation results in a smaller proportional decrease in total stem conductivity when segment ends have been air-injected.
The pattern of cavitation is consistent with theoretical predictions
The differences between the distribution of embolism along the length of centrifuged stems measured in this study and that of Cochard et al. (2010) may partially explain why we do not observe a similar effect of stem length on centrifuge vulnerability curves. The distribution of PLC we measured for a long-vesselled species matches the theoretical expectation that embolism of a stem following centrifugation should be highest at the axis of rotation in the central part of the segment where the induced xylem pressure is most negative (Alder et al. 1997). Similar measurements conducted with the Cavitron found higher PLC at the basal stem end rather than at the centre (Fig. 5 in Cochard et al. 2010). Cochard et al. (2010) suggest this distribution could result from vessel draining, but the match between our results and the predicted pattern of PLC suggest that vessel draining is an unlikely explanation. Sperry et al. (2012) describe the high PLC at the basal stem end as likely arising from micro-bubbles or debris carried into the stem by the flow of solution during Cavitron measurements. The centrifuge vulnerability curve technique we used does not induce flow during centrifugation and therefore would not be vulnerable to this artefact.
Conclusion
We conclude that the vulnerability of plants to cavitation can be accurately characterised with vulnerability curves constructed using a centrifuge technique, even for some long-vesselled species. For long- and short-vesselled species, the centrifuge technique used in this study did not produce the large artefacts reported in Cochard et al. (2010) for medium- and long-vesselled species using the Cavitron technique. Our results are consistent with other recent studies that have investigated the accuracy of centrifuge vulnerability curves (Jacobsen & Pratt 2012; Sperry et al. 2012).
Acknowledgements
We thank Samuel Del Rio and Evan MacKinnon for laboratory assistance. We thank John Sperry and Stephen Davis for valuable discussions relevant to this study, and Katie VinZant of the National Forest Service for help in obtaining research permits. Funding was provided by the National Science Foundation CAREER award (IOS-0845125) and the Andrew W. Mellon Foundation.




