Placental immunology and maternal alloimmune responses
Abstract
During pregnancy, women are tolerant of their semi-allogeneic fetus whilst not being immunosuppressed and indeed readily form alloantibodies. This ‘Immunological Paradox of Pregnancy’ may be explained by an understanding of placental anatomy and immunology. Trophoblast cells form the interface between the fetus and maternal tissues and blood and escape allorecognition because they lack classical human leucocyte antigen (HLA) class I and II molecules. Local immunoregulation, or tolerance, in the decidua is mediated partly by HLA-G+ extravillous trophoblasts (EVT) that invade the tissue and prevent killing by maternal natural killer cells, cytotoxic T cells and macrophages. Placental hormones orchestrate the composition and regulatory function of maternal immune cells. In contrast, syncytiotrophoblast cells at the surface of chorionic villi, in contact with maternal blood, maintain a state of mild maternal systemic immunity via activation of innate immunity and skewing towards humoral immunity. This enables maintenance of a healthy immune system in pregnant women and robust protective antibody responses to pathogens whilst enabling survival of the fetus. However, this has the unfortunate consequence that pregnant women readily form alloantibodies to incompatible alloantigens on fetal red cells, platelets and leucocytes if fetomaternal haemorrhage (FMH) occurs. The antibodies are initially low affinity but after re-immunization with further FMH become functionally effective, high-titre IgG.
Introduction
Fetal and neonatal alloimmune thrombocytopenia (FNAIT) is the platelet ‘equivalent’ of haemolytic disease of the fetus and newborn (HDFN). Differences in the development of alloantibodies to platelets or red cells may indicate dissimilar aetiologies. Approximately 80% of cases of FNAIT are caused by antibodies to the human platelet antigen (HPA)-1a [1], but this alloantibody is rarely formed after transfusion [2, 3]. Anti-HPA-5b and anti-HPA-1b are by far the most common HPA specificities induced by transfusion [2, 3] and also the second and third most frequent alloantibodies causing FNAIT [1]. In contrast, anti-D is the most frequent red cell antibody formed after both pregnancy and transfusion. Therefore, it would appear that the anti-HPA-1a response is unique in that pregnancy is almost essential for its induction.
In fact, pregnancy appears to be a physiological condition that is conducive to alloantibody responses. Most red cell blood groups, and platelet and neutrophil alloantigens, were discovered by serological investigations of fetal alloimmune cytopenias resulting from immunization of pregnant or post-partum women by alloantigens on fetal blood. Anti-D (IgM) was the first to be recognized 70 years ago [4].
The human leucocyte antigen (HLA) system was also elucidated mainly by the use of sera from multigravidae, after studies demonstrating leucocyte antibodies were often induced by pregnancy [5, 6]. These sera were the mainstay of HLA-typing reagents before the molecular era. HLA antibodies are not, however, usually deleterious during pregnancy, and curiously, HLA allorecognition by pregnant women of their semi-allogeneic offspring actually appears to be important for successful pregnancy [7]. Fertility is reduced among consanguineous couples, spontaneous abortions being more likely with HLA concordance of mother and fetus [8]. Vertical transmission (mother to fetus) of HIV was found greater (31%) with maximum HLA matching of mother and fetus compared with the protection afforded by no HLA class I match, with only 3% transmission [9]. HLA-C-incompatible pregnancies were associated with induction of T-regulatory lymphocytes (Treg) in maternal decidua [10]. Thus, rather surprisingly, materno-fetal mismatch of HLA class I enhances placental function and is beneficial to both mother and baby.
Sir Peter Medawar studied transplantation for which he received the Nobel Prize for Medicine in 1960. He proposed the concept of the fetal allograft and hypothesized that survival of the semi-allogeneic fetus occurs either through anatomical separation of mother and fetus, lack of fetal antigen expression or its exposure to the maternal immune system, or from a functional suppression of maternal lymphocytes [11]. However, pregnant women are not immunosuppressed and readily make alloantibodies to blood cells. The ‘Immunological Paradox of Pregnancy’ is not yet completely understood.
The placenta and amniotic sac form a physical barrier between the mother and fetus. Knowledge of placental anatomy and recent information from immunological studies at various interfaces may help explain the paradox.
Anatomy and development of the placenta
After conception, cellular division of the fertilized egg forms the blastocyst. This is a ball of cells with the entire outer surface comprised of trophoblast cells and with a cluster of cells in the centre, the embryoid body, from which the embryo and then fetus will develop. On contact of the blastocyst with maternal endometrial epithelial cells bordering the decidua (the lining of the uterus), these cytotrophoblast (CT) cells at the contact site fuse to form syncytiotrophoblast (ST; Fig. 1a). The ST acquires an invasive phenotype. Several enzymes are secreted by ST to degrade the extracellular matrix (ECM) and allow invasion of the blastocyst into the maternal tissue (Fig. 1b,c). At this stage, about 1 week after conception, human chorionic gonadotrophin (hCG) is produced by ST to maintain the pregnancy (this hormone is detected by pregnancy tests). Projections of the ST known as villous sprouts erode the decidua basalis and spaces (lacunae) develop in the ST. These gradually fill with interstitial fluid and later with maternal blood (Fig. 1d). CT near the embryoblast proliferates and moves out inside the villi, forming a cell layer underlying the ST. Both the CT (trophoblast stem cells) and ST (terminally differentiated, non-proliferative syncytium) lack classical HLA class I. As the villi enlarge and branch, they develop a mesoderm stromal core of ECM. Blood vessels from the developing circulation of the embryo/fetus grow through the ECM (Fig. 1e). Blood flows to and from the developing fetus and placenta via the umbilical cord. Fetal and maternal blood does not normally mix.
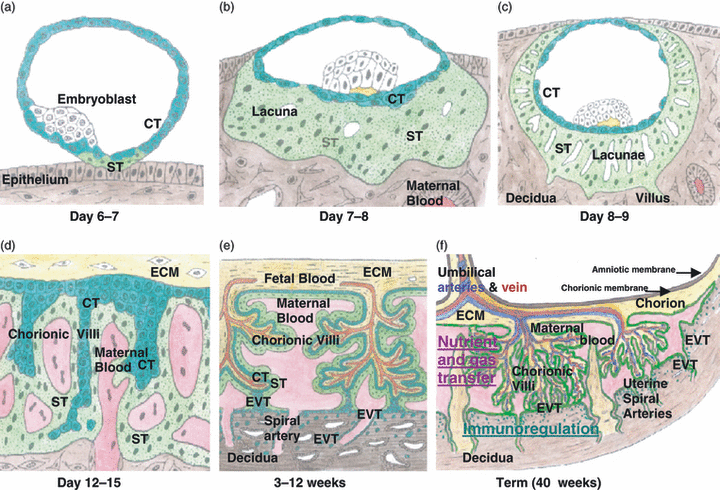
Development of the placenta. (a) The blastocyst contacts uterine epithelium, and then, syncytiotrophoblast (ST) forms by fusion of cytotrophoblast (CT) cells at this site. (b) As the blastocyst embeds into the decidua, ST proliferates and spaces (lacunae) form in the syncytium. (c) Sprouts (villi) of the syncytium invade the decidua. (d) CT cells by the extracellular matrix (ECM) proliferate and migrate down the villi, whilst the lacunae begin to fill with maternal blood from the uterine arteries and veins. Maternal blood contacts the ST. (e) After about a month when the fetal blood and circulatory system forms, blood vessels in the ECM penetrate the villi and begin to take up nourishment and oxygen from the blood in the intervillous spaces. The villi proliferate and branch. They are covered by a double layer of trophoblast, CT cells and ST. At the tips of villi, CT cells proliferate to form cell columns that migrate into the interstitial tissue in the decidua and into the spiral arteries where they replace the endothelium. These are all extravillous trophoblasts. (f) The CT cells and ST not at the contact site mostly degenerate but some remain on the chorionic membrane, which is attached to the amniotic membrane that contains amniotic fluid surrounding the fetus. Placental transfer takes place in the chorionic villi and immunoregulation in the decidua.
By 3 weeks post-conception, columns of proliferative CT protrude from the tips of the chorionic villi, becoming EVT. They migrate into the decidua, forming interstitial trophoblasts, and also into the lumen of spiral arteries (Fig. 1e). Here, these endovascular trophoblasts degrade the endothelium rendering the spiral arteries flaccid, ensuring low resistance to uterine arterial blood flow and enabling maximum oxygenation of the placental villi. These differentiated types of invasive EVT do not express HLA class I A or B antigens but, almost uniquely, express HLA-G (Fig. 2a), a non-classical HLA class I molecule [12].
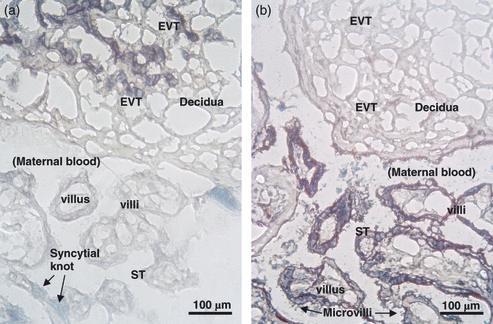
Immunocytochemistry of decidua and placental chorionic villi. Cryostat sections were stained with (a) anti-HLA-G (purple) and (b) anti-(placental alkaline phosphatase) PLAP (purple). The nuclei are counterstained with methyl green. (a) Extravillous trophoblast (EVT) cells in the decidua are HLA-G-positive, whilst syncytiotrophoblast (ST) in the chorionic villi is not stained. A syncytial knot (green) is between two villi. (b) On chorionic villi, ST membranes and microvilli are PLAP positive, as is particulate material in the intervillous space where maternal blood circulates.
As the embryo develops into a fetus and protrudes into the uterine lumen, after 3–4 weeks, it is surrounded by the amniotic membrane that forms as an inner lining to the chorionic membrane (Fig. 1f). The fetus is protected by amniotic fluid. When the fetus grows, the chorionic membrane outside the site of placental attachment retains some residual trophoblast cells that contact the inner lining of the uterus. The chorion is the major part of the placenta, comprising blood vessels, connective tissue and trophoblasts. This type of placentation is known as haemochorial because maternal blood is in direct contact with the chorionic villi (Fig. 1f). The structure of the placenta at the implantation site remains essentially the same for the remainder of pregnancy, and it grows to become a disk about 20 × 2–4 cm at term (40-week gestation).
As pregnancy progresses, the structure of the villi (Fig. 3a,b) adapts to maximize placental transport to supply the needs of the growing fetus. The villi become extremely numerous, highly vascular and increasingly slender with multiple branches and lobes (Fig. 3a,b). The capillaries become situated predominantly at the periphery of villi, underlying the ST (Fig. 3c). Villous CT cells no longer form a complete cell layer under the ST.
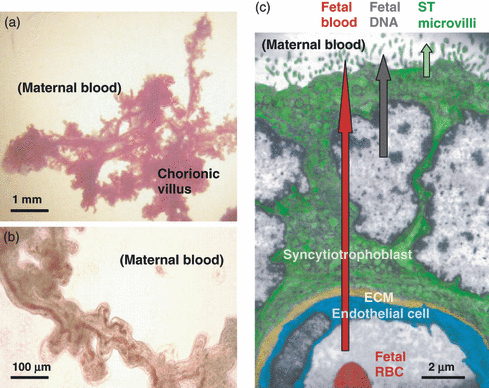
(a,b) Low power photomicrographs of a portion of a villus, dissected from a term placenta, washed free of maternal blood. Fetal blood vessels and bulbous protuberances are evident in (b). (c) Electron micrograph of the outer portion of a villus, showing syncytiotrophoblast (ST) with nuclei and microvilli, extracellular matrix and a fetal capillary. Three types of fetoplacental material are released into maternal blood: fetal blood (only if the integrity of the villus is lost), apoptotic ST nuclei and DNA, and ST microvillous membrane particles.
Placental transport
The chorionic villi transfer nutrients and oxygen to the fetal blood and waste products and carbon dioxide back to the maternal circulation. The ST is an absorptive epithelium and has the major role in transport. It is metabolically very active and has numerous vacuoles evident ultrastructurally (Fig. 3c), many of which are involved in placental transfer of substances including amino acids, low density lipoprotein and IgG. Microvilli (a brush border) on the ST (Fig. 3c) increase the villous surface area tenfold to approximately 100 m2 to maximize absorption [13].
Materno-fetal transport of IgG takes place to confer passive immunity to the fetus, so that after birth, the infant will be protected against infections whilst its own immune system is developing. Active transport of IgG occurs via the neonatal Fc receptor (FcRn) in the ST [14, 15]. Maternal plasma is endocytosed at the apical membrane of the ST, and intracellularly, the endocytotic vacuoles become slightly acidified to approximately pH 6·0 compared with pH 7·4 of plasma. FcRn is present on the ST and endosomal membranes, and then under the acidified conditions, it binds IgG through histidine residues on the CH2 and CH3 domains of IgG. Sorting of the endosomes then occurs, with vacuoles containing IgG–FcRn complexes being transferred across the cell, whilst the contents of those vacuoles lacking these complexes undergo lysosomal degradation [16]. IgM and IgA are not transferred because they have no specific receptors. On reaching the basal (fetal) side of the ST, the endosomes merge with the plasma membrane, and with the rise in pH of the interstitial fluid, IgG dissociates and is released intact, from where it diffuses into the fetal capillaries.
Fetal material released into maternal blood
Three types of fetal or placental material can be detected in the maternal circulation (Fig. 3c) [17]. Fetal blood may enter, by fetomaternal haemorrhage (FMH), after damage to chorionic villi. In contrast, fetal (placental) DNA and ST debris are detectable in maternal blood from about 7 weeks of gestation, in increasing amounts as the placenta grows.
Over half a century ago, it was recognized that fetal red-blood-cells (RBC) could leak into maternal blood through transplacental haemorrhage and, if fetal RBC were D-positive and the mother was D-negative, could stimulate her to produce anti-D, causing HDFN [18]. The ultrastructure of chorionic villi shows that fetal blood must pass through breaks in the capillary endothelial cells, ECM and ST (Fig. 3c) [17]. FMH are usually unpredictable [19]. Because the fetoplacental blood volume is approximately 25, 150 and 400 ml at 20-, 30- and 40-weeks gestation, respectively, the occurrence and volume of FMH increases as pregnancy progresses. FMH are more likely at parturition and often in larger bleeds [20, 21]. Only a minority of susceptible D-negative women with D-positive babies make anti-D, because most are not exposed to enough fetal RBC. Approximately 0·1 ml or less was required to stimulate anti-D formation [22]. The low volume of fetal RBC entering maternal blood during pregnancy also accounts for the fact that the formation of anti-D is rare (<1%) during first pregnancies [23]. The fact that the incidence of anti-HPA-1a alloimmunization in first pregnancies of HPA-1a negative women was found higher (4% [24], 7·6% [25], 24% [26]) when the number of fetal platelets encountered by the maternal immune system from FMH must be very low, suggests this antigen is highly immunogenic during pregnancy.
The ST is a dynamic cellular tissue that is continually remodelled. The nuclei of ST survive for approximately 3 weeks [27] when they then become apoptotic. This is characterized by the appearance of pyknotic (odd shaped) nuclei, condensation of chromatin at the periphery of the nucleus [17], degradation of the nuclear membrane and nuclear shrinkage [28]. DNA fragmentation occurs; endonucleases cleave DNA at internucleosomal linker regions into short (∼200 bp) fragments [29]. The apoptotic nuclei collect into clusters in syncytial knots [30] that are then extruded into maternal blood for disposal (Fig. 2a). Fibrinoid material laid down from maternal blood covers damaged areas of villi [31]. The syncytial layer is rapidly replaced by fusion of underlying CT with ST to maintain the integrity of the villous tissue and prevent haemorrhages. The DNA released from these apoptotic placental ST nuclei is the source of cell-free ‘fetal’ DNA used for non-invasive fetal genotyping [32] of RHD [33], HPA-1A [34, 35], SRY (gender) and other fetal genes.
Shedding of ST debris in the form of microparticulate material is also a physiological process. Using antibodies to a placental-specific molecule highly expressed on the ST apical membrane, placental alkaline phosphatase (PLAP) [36], it can be seen that this membrane appears ‘rough’ because of staining of the microvilli (Fig. 2b). An ultrahistochemical study localized PLAP to the ST membrane and microvilli [37]. PLAP enzyme activity in maternal plasma rises during the third trimester [38]. PLAP-positive microparticles are present in the intervillous spaces where maternal blood circulates (Fig. 2b). These ST membrane fragments, vesicles or microparticles, here called syncytiotrophoblast microparticles (STMP), are <500 nm [39] and may have an important role in maternal systemic immunity.
Functions of trophoblast
Trophoblast cells form the fetomaternal interface. EVT cells are in contact with maternal tissue and cells in the decidua. ST on the surface of the villi is bathed in maternal blood. All trophoblast cells lack classical HLA class I A and B antigens, suggesting a role in immune evasion, but their physiological and immunological functions are quite dissimilar at these two interfaces.
Immunoregulation by placental cells in the decidua
Several mechanisms involving different cells and molecules in decidual tissue are involved in maintaining placental function and survival of the fetoplacental unit. First, maternal natural killer (NK) cells, CD8+ cytotoxic T cells, macrophages and dendritic cells (DC) are affected by ligation with HLA class I expressed on EVT, both HLA-C (classical) and, importantly, HLA-G (non-classical). Second, the location and function of maternal NK cells, Tregs and DCs are altered by placental hormones. Most interactions downregulate immune responses and promote tolerance by preventing killing of allogeneic cells. In the first trimester, this is essential for placental growth and tissue remodelling.
Uterine NK (uNK) cells have a slightly different phenotype to those in peripheral blood, being CD16dim and CD56bright and they reside in the decidua at high concentration early in pregnancy, their proliferation stimulated by hCG [40]. uNK cells express killer inhibitory receptors (KIRs) specific for HLA class I on EVT. HLA-C on EVT interacts with KIR2DL1,2,3 and KIR2DS1,2 (the sole activating receptor) on uNK. There are two groups of HLA-C alleles: C1 binds KIR2DL2,3 and C2 binds KIR2DL1 [41]. Some KIR/HLA-C combinations lead to ineffective invasion of EVT, associated with the disease of pre-eclampsia [42]. However, in HPA-1a alloimmunized pregnancies, no KIR/HLA-Cw gene combinations were found to have a detrimental or protective effect [43].
Uterine NK cells are also downregulated by interaction with the non-classical HLA class I molecules expressed on EVT. The most important is HLA-G that inhibits uNK through KIR2DL4, NKG2A (CD94), p49 and immunoglobulin-like transcript (ILT) receptor ILT2 (CD85j) on uNK cells [41, 44]. HLA-E is present at low concentration on EVT and binds NKG2A on uNK cells [41].
CD8+ effector memory T cells in the decidua are rendered non-functional because production of cytotoxic molecules is downregulated, thus sparing EVT from cytolysis [45]. Experimentally, activated CD8+ lymphocytes were triggered into apoptosis by soluble rHLA-G1, mediated through the CD95/CD95 ligand (Fas/Fas ligand) pathway, suggesting that this mechanism may explain the paucity of CD8+ cells in the decidua [46].
Maternal macrophages are present in the decidua throughout pregnancy and secrete immunoregulatory molecules, interleukin (IL)-10 [47], IL-1Ra and indoleamine 2,3 dioxygenase (IDO) [48]. (IDO catabolizes tryptophan and suppresses T cells by reducing the concentration of this essential amino acid required for their metabolism.) Decidual macrophages express the inhibitory receptors ILT2 and ILT4 that bind HLA-G dimers, recently identified on the surface of first-trimester EVT [49].
Some DCs reside in the decidua but are rendered immature or tolerant by progesterone (produced by the placenta) and experimentally by interacting with HLA-G through ILT2 (LILRB1) [50]. The DCs secrete immunoregulatory cytokines; IL-10 promotes Tregs, and IL-4, IL-10 and TGFβ drive T-cell differentiation down the T-helper (Th)2/3 pathway, away from cytotoxic T cells [51].
Finally, Tregs (CD4+, CD25++) accumulate in the decidua despite decreasing in the maternal circulation during pregnancy [52, 53], attracted by hCG [54]. Their concentration in the decidua may reach 14% of the T cells, much higher than in peripheral blood [55]. Lack of Tregs leads to rejection of the fetus [56].
Maternal cells in the decidua are not completely suppressed, however. Macrophages actively recognize and phagocytose pathogens, which is important in protection against intrauterine infections [57].
Systemic immunomodulation
A state of mild systemic activation of the innate immune system exists during pregnancy, especially in the third trimester, with increased numbers of monocytes and granulocytes found in maternal blood [58]. Monocytes from pregnant women had enhanced production of IL-12 in vitro compared with monocytes from non-pregnant women [59]. STMP shed into maternal blood may be responsible for this. In vitro, STMP were found to stimulate the release by monocytes of the inflammatory cytokines IL-1β, IL-6 and IL-8 [60] and TNF-α, IL-12p70 and IL-18 [61] and by DC of IL-6 and IL-8 [62]. Innate immunity and inflammation are necessary for priming antigen-presenting cells (macrophages, DC) to initiate T-helper (Th) and B-cell immune responses (Fig. 4).
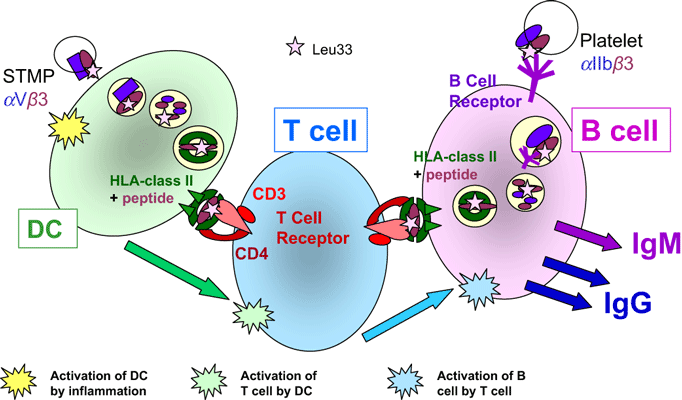
The antibody response (summarized). Dendritic cells (DC) endocytose plasma and particulate matter non-specifically; the contents of the endocytotic vacuoles are digested; peptides are bound to HLA class II molecules and presented on the cell surface for recognition by T-cell receptors (TCR) on CD4+ T-helper (Th) lymphocytes. If the conformation of the TCR enables it to bind the peptide–HLA complex with high affinity and the Th cell receives stimulation from the DC in the form of cytokines and cell-surface costimulatory molecules, the Th cell becomes activated. It then proliferates, and the clonal progeny migrates to B cell-rich areas of lymph nodes and spleen. B cells recognize their cognate antigen through conformational binding of the B-cell receptor (BCR; surface IgM) to the antigen. This stimulates endocytosis and then antigen processing and presentation in a similar way to that of the DC. If the B cells are contacted by Th cells with the correct TCR to bind the peptide–HLA complex, the B cell will be activated by the Th cell, proliferate and secrete antibody (IgM, IgG) of the same specificity as the BCR.
Villous trophoblasts may also affect the systemic immune system via production of cytokines. Isolated villous trophoblast cells (ST and CT) in culture were found to secrete IL-10 [63–65], to express mRNA for IL-10, IL-4 and IL-13 [65] and to produce IL-10 receptor mRNA and protein [66] suggesting a strong Th2 bias of these cells. Progesterone and hCG, hormones synthesized by ST, caused shifts of immunity from Th1 (IL-2, IL-12, IL-15, interferon-γ; cell-mediated) to Th2 (IL-4, IL-5, IL-6, IL-10, IL-13; humoral) [67]. NK cells were reduced in the blood of pregnant women [58]. The number of Tregs in peripheral blood of pregnant women was markedly elevated during early pregnancy, then after mid-gestation returned to normal levels at term [68], indicating different mechanisms operate at the beginning of pregnancy, when the fetal–placental unit is being established, and later, when fetal growth is of major importance.
Therefore, a complex mix of systemic immune activation and a cytokine bias towards Th2, first proposed by Wegman in 1993 [69], appears to be induced in pregnant women. The low-level inflammatory state combined with skewing towards humoral immunity may explain why antibody responses towards small volumes of allogeneic fetal blood cells are robust. Th1 cell-mediated immunity is, however, somewhat compromised, evidenced by increased susceptibility of pregnant women to viral (e.g. swine flu, H1N1) and intracellular bacterial (e.g. listeria monocytogenes) infections, with the latter carrying a high risk of fetal loss if the placenta is colonized.
Could STMP stimulate the maternal anti-HPA-1a immune response?
The glycoprotein (GP) that carries the HPA-1 polymorphism (GPIIIa, CD61 or β3 integrin) was found biochemically to be expressed on the ST ‘brush border’ (i.e. STMP) prepared from term placentas [70]. It was later shown to be present on first-trimester ST as well as term ST by electron microscopy [17]. On ST, GPIIIa is associated with CD51 (αV integrin) and not with GPIIb (CD41, αIIb integrin) as the latter is expressed on platelets but was not detected on ST by immunocytochemistry [71]. Binding of anti-HPA-1a to these two complexes may differ.
STMP in maternal blood are likely to gain access to lymph nodes and the spleen, where they could be endocytosed by macrophages or DC. This endocytosis is non-specific; DCs are continually sampling their environment. After antigen processing, peptides are presented on HLA class II on the DC surface for presentation to CD4+ Th cells. In FNAIT, there is a very strong HLA DRB3*0101 (DRw52a) restriction of the HPA-1a antibody response [72]. Therefore, peptides encompassing the polymorphic residues from GPIIIa will be bound to DRB3*0101 molecules [73–75]. On the DC surface, this HPA-1a-peptide complexed to HLA would select HPA-1a-specific Th (CD4+) cells (Fig. 4). These cells must have T-cell receptors (TCR) that recognize a complex of HPA-1a peptide bound to DRB3*0101, the restricting HLA molecule. If the HPA-1a-specific Th cells receive stimulation from the DC (cytokines and interaction with membrane costimulatory receptors), the Th cells become activated, proliferate and migrate to B-cell areas. HPA-1a-specific T cells were identified in HPA-1a-immunized women by culturing peripheral blood mononuclear cells with HPA-1a peptides and measuring T-cell proliferation; most of the pregnant and post-partum women studied were found to have HPA-1a-specific T cells, whereas non-HPA-1a-immunized individuals did not [76]. These T-cell responses were generally stronger during pregnancy [76] than from non-pregnant women [77]. This illustrates that exposure to HPA-1a during pregnancy stimulates specific immune responses in susceptible women.
Antibody response
B-lymphocyte responses are initiated by recognition of the conformational shape of antigens by B-cell receptors (BCRs). The BCR (surface IgM) binds antigen that mediates endocytosis of the BCR–antigen complex followed by antigen processing and presentation of peptides on HLA class II for recognition by antigen-specific Th cells. Thus, to receive help (activation) from Th cells, the B cells must present the same peptide–HLA class II complex as the DC that initially activated the Th cells (Fig. 4). Following help in the form of cytokines and costimulatory molecules from these Th cells, the antigen-specific B cells become activated, proliferate and produce antibodies of the same specificity as their BCR. Therefore, to produce an antibody that binds to HPA-1a on platelets, the maternal B cells must have endocytosed HPA-1a-positive platelets. These can only be encountered by the maternal immune system by FMH. As mentioned earlier, FMH are generally unpredictable, infrequent and small, probably containing usually fewer than 25 × 106 fetal platelets – a very small number to cause a primary immune response in an adult. However, if antigen-specific Th cells are already present in high numbers and ready to activate the antigen-specific B cells, this low dose may be sufficient.
Pregnant women appear to make alloantibodies more readily than transfused patients, considering the great difference in volumes of blood in FMH (rarely over 20 ml) compared with transfusions. Interestingly, without prophylaxis, approximately 10–20% of susceptible (antigen mismatched) gravid women develop alloantibodies, 16% with anti-D [78], 12% and 10% with anti-HPA-1a [26, 79] and 15% with anti-HLA [80], indicating a similar immunization by FMH.
Maternal alloantibodies
Immunizations
The initial antibody response to an antigen is predominantly IgM, of high avidity and agglutinating activity but relatively low specificity and affinity. Re-immunization with antigen is required to mature the antibody response. (This is the purpose of booster injections after vaccination.) Thus, in order that high-titre maternal alloantibodies to fetal blood alloantigens may develop, further doses of antigen (i.e. via FMH) are required.
Class switching and affinity maturation
With further antigen encounter, the B cells switch antibody class from IgM to IgG by selection of Cγ genes for IgG, to replace Cμ (IgM) genes. The Cγ3 gene (coding for IgG3) is upstream of Cγ1 (coding for IgG1); therefore, IgG3 may convert to IgG1 but not the reverse. By a process of somatic mutation (point mutations of the variable regions of Ig), affinity for antigen increases. The cytokine environment of the B cells affects the class and subclass of antibody. Because additional FMH may or may not occur, maturation of the antibody response may be halted during this process (Fig. 5), resulting in heterogeneity of alloantibody isotype, titre and affinity among different antisera [81].
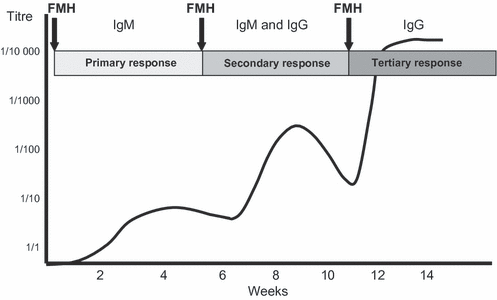
Antibody development. The diagram represents the titre and Ig class of an alloantibody formed in response to one or more immunizations in the form of fetomaternal haemorrhage (FMHs). If there are no secondary or tertiary FMH, the maternal antibody will remain of low titre (weak) and predominantly IgM. (The scale is arbitrary.)
Isotype and functional activity
Most alloantibodies to red cells and platelets are IgG1, sometimes IgG3 or a mixture [82, 83]. IgG is the immunoglobulin class that has most effector functions, mediating adherence, phagocytosis and lysis of IgG-coated cells by IgG Fc receptors (FcγR) on macrophages. Several FcγR are present on macrophages and granulocytes (neutrophils, eosinophils, basophils, mast cells, NK cells) [84]. Complement fixation on the opsonized target cell may also occur. IgG enhances and accelerates the innate immune mechanisms of destruction of target cells by these effector cells. IgG is the only antibody class to cross the placenta to the fetus.
Glycosylation
The glycosylation of IgG also modulates its effector function [85]. The branched core oligosaccharide structure attached to Asn297 on the CH2 domain of IgG has several sugars that may or may not be present [86] (Fig. 6). Terminal galactose is elevated on IgG of pregnant women [87] and is even higher on umbilical cord (fetal) IgG [88]. Galactose on IgG enhances effector cell activity by macrophages [89]. In contrast, fucose reduces effector activity [90]. Affinity-purified IgG1 anti-HPA alloantibodies were found to have less fucose and slightly higher galactose, compared with normal serum IgG1 from the donors, thus enhancing their pathogenicity [91]. This physiological elevation of antibody function may have evolved to help protect pregnant women from pathogens whilst their immune systems are in a state of mild systemic immunoregulation.
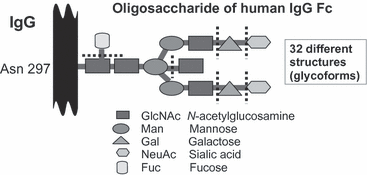
Diagram of the composition of sugars of the oligosaccharide chain attached to Asparagine 297 in the CH2 (constant) domains of IgG. The core structure terminates in GlcNAc, at the dotted lines, and complexity arises because additional sugars (galactose, sialic acid, fucose and bisecting sialic acid) may or may not be present.
Concluding remarks
Human pregnancy is unique in that it allows the growth of a fetus across highly polymorphic HLA mismatches whilst the maternal and fetal immune systems are not compromised. The structure of the placenta and its effect on maternal immune cells drives this change. Skewing of maternal systemic immunity towards humoral responses ensures development of protective antibodies. Materno-fetal–placental transfer of IgG confers passive immunity to the infant. Formation of alloantibodies to alloantigens on fetal blood cells acquired by FMH which then result in alloimmune cytopenias is, unfortunately, a consequence of physiological rather than pathological processes.
Acknowledgement
This research was supported by National Blood Authority.




