Reactive oxygen species in abiotic stress signaling
Abstract
Reactive oxygen species (ROS) are known to accumulate during abiotic stresses, and different cellular compartments respond to them by distinctive profiles of ROS formation. In contrast to earlier views, it is becoming increasingly evident that even during stress, ROS production is not necessarily a symptom of cellular dysfunction but might represent a necessary signal in adjusting the cellular machinery to the altered conditions. ROS can modulate many signal transduction pathways, such as mitogen-activated protein kinase cascades, and ultimately influence the activity of transcription factors. However, the picture of ROS-mediated signaling is still fragmentary and the issues of ROS perception as well as the signaling specificity remain open. Here, we review some of the recent advances in plant abiotic stress signaling with emphasis on processes known to be affected heavily by ROS.
Abbreviations-
-
- ABA
-
- abscisic acid
-
- AOX
-
- alternative oxidase, DREB, drought-responsive element binding
-
- ER
-
- endoplasmic reticulum
-
- ETC
-
- electron transport chain
-
- H2O2
-
- hydrogen peroxide
-
- MAPK
-
- mitogen-activated protein kinase
-
- MAPKK
-
- MAP kinase kinase
-
- MAPKKK
-
- MAP kinase kinase kinase
-
- NO
-
- nitric oxide
-
- 1O2
-
- singlet oxygen
-
- O
 superoxide; OH•
superoxide; OH• - O
-
- hydroxyl radical
-
- Rboh
-
- respiratory burst oxidase homolog
-
- RCD1
-
- radical-induced cell death1
-
- ROS
-
- reactive oxygen species
-
- TF
-
- transcription factor
-
- UPS
-
- ubiquitin-26S proteasome system
Introduction
Abiotic stress is thought to arise when the environment deviates from the optimum conditions enough to disturb cellular functions. Because abiotic stress by definition is a stress caused by any other factor than direct interaction with another organism, the list of conditions under this label is long. Most commonly mentioned ones are drought, salinity (in the form of NaCl), extreme temperatures and excess light. In addition, heavy metals, xenobiotics, ultra-violet radiation, ozone, hypoxia and nutrient deficiency are regularly included (Apel and Hirt 2004, Mittler 2006, Møller et al. 2007). As the variability of stresses suggests, the mechanisms of damage and, consequently, the plant signaling and metabolic responses differ from each other. However, plants respond to all these stresses with the increased production of reactive oxygen species (ROS), although their identity and compartment of origin certainly differ (Li et al. 2009, Navrot et al. 2007)
The focus of this review is the progress made in studying ROS-mediated signaling under abiotic stress during the last few years. For general reviews on ROS signaling, the reader is referred to the recent reviews written, for example, by Foyer and Noctor (2009) and Møller et al. (2007) and, for reviews on aspects of abiotic stress and ROS not discussed here, to Miller et al. (2008) and Shao et al. (2008).
ROS generation
ROS are continuously produced unintentionally through cellular metabolism (Fig. 1) and plant cells are well equipped with antioxidants and scavenging enzymes to keep their levels low under normal growth conditions. Stresses can increase the rate of ROS production and this, together with the compartment-specific (down)regulation of the cells' antioxidant capacity, can lead to significant ROS accumulation. Although the harmful effects of ROS on cellular components are well documented (Møller et al. 2007), a signaling function for them is also firmly established (Foyer and Noctor 2009).
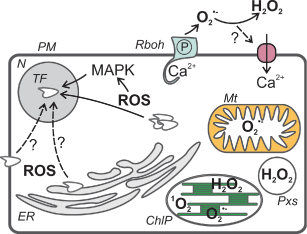
Summary of subcellular sites of ROS generation and the signaling pathways discussed in the text. ChlP, chloroplast; ER, endoplasmic reticulum; Mt, mitochondrion, N, nucleus; P, phosphorylation; PM, plasma membrane; Pxs, peroxisome.
In photosynthetic tissues, the chloroplast is the prime source of ROS having the capacity to produce high amounts of superoxide (O ) and hydrogen peroxide (H2O2), especially during reduced rate of photosynthetic carbon fixation—a typical situation during abiotic stresses (Takahashi and Murata 2008). In addition, chloroplasts can produce singlet oxygen (1O2) through excited chlorophyll molecules. 1O2-mediated signaling has acquired much attention after the isolation of the flu and gun mutants and progress made in chloroplast-nucleus cell death signaling and 1O2-mediated gene expression (reviewed by Galvez-Valdivieso and Mullineaux, this issue, Triantaphylidès and Havaux 2009).
) and hydrogen peroxide (H2O2), especially during reduced rate of photosynthetic carbon fixation—a typical situation during abiotic stresses (Takahashi and Murata 2008). In addition, chloroplasts can produce singlet oxygen (1O2) through excited chlorophyll molecules. 1O2-mediated signaling has acquired much attention after the isolation of the flu and gun mutants and progress made in chloroplast-nucleus cell death signaling and 1O2-mediated gene expression (reviewed by Galvez-Valdivieso and Mullineaux, this issue, Triantaphylidès and Havaux 2009).
In non-photosynthetic tissues, mitochondria are the biggest source of ROS, but in a green cell of a plant, their contribution is considered small in comparison with chloroplasts (Navrot et al. 2007), although direct inference from non-photosynthetic organisms can underestimate their importance in ROS metabolism (Noctor et al. 2007). The redox status of the mitochondrial electron transport chain (ETC) is an important indicator of the cell energy status and ROS, especially O from complexes I and III, and the reduction status of the ubiquinone pool are integral parts of this monitoring system (Taylor et al. 2009).
from complexes I and III, and the reduction status of the ubiquinone pool are integral parts of this monitoring system (Taylor et al. 2009).
The third intracellular ROS source is the peroxisomes. They contain several oxidases that produce H2O2 and O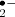 as byproducts of the reactions they catalyze. The photorespiratory glycolate oxidase is located in peroxisomes and its function is especially relevant during abiotic stresses, which are often accompanied by stomatal closure and the resulting decrease in gas exchange leads to reduction in carbon dioxide availability for Rubisco, followed by increased photorespiration and H2O2 production (Foyer and Noctor 2009).
as byproducts of the reactions they catalyze. The photorespiratory glycolate oxidase is located in peroxisomes and its function is especially relevant during abiotic stresses, which are often accompanied by stomatal closure and the resulting decrease in gas exchange leads to reduction in carbon dioxide availability for Rubisco, followed by increased photorespiration and H2O2 production (Foyer and Noctor 2009).
In addition to these metabolic ROS sources, in the presence of redox-active metals, hydroxyl radicals (OH•) can be formed from H2O2 through the Fenton reaction or from H2O2 and O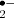 through the Haber–Weiss reaction. The extremely reactive OH• radical can run rampant in the cell causing extensive oxidative damage. It is not considered to have signaling function, although the products of its reactions can elicit signaling responses, and cells sequester the catalytic metals to metallochaperones efficiently avoiding OH• formation (Halliwell 2006, Møller et al. 2007).
through the Haber–Weiss reaction. The extremely reactive OH• radical can run rampant in the cell causing extensive oxidative damage. It is not considered to have signaling function, although the products of its reactions can elicit signaling responses, and cells sequester the catalytic metals to metallochaperones efficiently avoiding OH• formation (Halliwell 2006, Møller et al. 2007).
NADPH oxidases (Rbohs for respiratory burst oxidase homologs) are an important ROS-generating system in plants producing O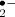 which is usually rapidly dismutated to hydrogen peroxide. There are 10 Rboh-coding genes in Arabidopsis and the isoforms have been shown to participate in different processes: RbohC is necessary for root hair tip growth and mechanosensing (Foreman et al. 2003, Monshausen and Gilroy 2009) and RbohD and F function in pathogen defense and abscisic acid (ABA) signal transduction (Kwak et al. 2003, Torres and Dangl 2005). There is recent evidence to support a role for Rbohs in heavy-metal induced accumulation of ROS (Pourrut et al. 2008, Rodriguez-Serrano et al. 2006) and early response to salt stress (Leshem et al. 2007). In the latter case, the ROS-generating activity was localized to internalized plasma membrane vesicles in contrast to the apoplastic ROS production during biotic interactions, root hair growth and ABA signaling. An additional function for the Arabidopsis RbohD was discovered by (Miller et al. 2009) who demonstrated that it was responsible for the fast-moving ROS signal mediating the systemic acclimation to several abiotic stresses.
which is usually rapidly dismutated to hydrogen peroxide. There are 10 Rboh-coding genes in Arabidopsis and the isoforms have been shown to participate in different processes: RbohC is necessary for root hair tip growth and mechanosensing (Foreman et al. 2003, Monshausen and Gilroy 2009) and RbohD and F function in pathogen defense and abscisic acid (ABA) signal transduction (Kwak et al. 2003, Torres and Dangl 2005). There is recent evidence to support a role for Rbohs in heavy-metal induced accumulation of ROS (Pourrut et al. 2008, Rodriguez-Serrano et al. 2006) and early response to salt stress (Leshem et al. 2007). In the latter case, the ROS-generating activity was localized to internalized plasma membrane vesicles in contrast to the apoplastic ROS production during biotic interactions, root hair growth and ABA signaling. An additional function for the Arabidopsis RbohD was discovered by (Miller et al. 2009) who demonstrated that it was responsible for the fast-moving ROS signal mediating the systemic acclimation to several abiotic stresses.
Recently, significant progress has been made in studying regulation of Rbohs and the ubiquitous second messenger calcium (Ca2+) seems to be a crucial component in it. Plant Rbohs have two Ca2+-binding EF hands in their N-terminal tails and Ca2+ has been shown to activate both RbohC and RbohD (Ogasawara et al. 2008, Takeda et al. 2008). Subsequently, ROS produced by Rbohs are thought to activate Ca2+ channels leading to further increases in cytosolic Ca2+ (Foreman et al. 2003) and downstream signaling. Furthermore, RbohD was activated by phospholipase Dα1-generated phosphatidic acid (Zhang et al. 2009) and phosphorylation (Ogasawara et al. 2008). Phosphorylation of the RbohF isoform by the OST1 kinase, discovered by Sirichandra et al. (2009), could be a guard cell-specific mechanism of regulating ROS production during ABA signal transduction. In addition, the small GTPase Rac1 was found to interact with a Rboh in rice (Wong et al. 2007).
In addition to the Rbohs discussed above, the apoplast harbors a number of other ROS-generating systems, such as amine and oxalate oxidases and peroxidases. Their contribution to the ROS generation and signaling—especially during abiotic stress—is not well understood. However, it was recently shown that H2O2 produced by apoplastic polyamine oxidase can influence the salinity stress signaling in tobacco and can play a role in balancing the plant response between stress tolerance and cell death (Moschou et al. 2008).
Although ROS are the most familiar of the redox-active molecules in the cell, nitric oxide (NO) and reactive oxylipins are also commonly formed. Especially NO has a firm role in stress signaling (cf. Moreau et al., this issue) but the diversity of oxylipin signaling is gaining attention as well (reviewed by Mueller and Berger 2009).
Alternative oxidase
An intriguing component of the mitochondrial ETC in plants and several other organisms is the alternative oxidase (AOX). It can oxidize ubiquinol diverting electrons from the primary cytochrome c pathway in an energetically unproductive process. Several functions for AOX have been proposed ranging from protection from ROS production to oxygen scavenging and balancing of carbon metabolism with ETC function (Blokhina and Fagerstedt, this issue, Vanlerberghe et al. 2009). It has been shown that AOX can dissipate excess reducing units not only from mitochondria but also from chloroplasts (Yoshida et al. 2007) and that it is necessary for responses against combined drought and light stress, possibly through the reduction of ROS accumulation (Giraud et al. 2008). In general, work with AOX is revealing a more complicated role for the enzyme than expected. For example, the major AOX isoform, AOX1a, can be knocked out without dramatic stress sensitive phenotypes for single stresses and overexpression of AOX in tobacco rendered the plants sensitive to ozone treatment in stead of protecting them (Pasqualini et al. 2007). The expression of AOX1a transcript is induced by various stresses that inhibit mitochondrial ETC function and it is considered a marker for the activation of mitochondrial retrograde signaling. The AP2/ERF domain transcription factor (TF), ABI4, which is involved in the chloroplast—nucleus signaling, was shown to be responsible also for the transcriptional regulation of AOX1a (Giraud et al. 2009) and thus combining the retrograde signals from both organelles. However, as Rasmusson et al. (2009) point out, posttranslational regulation, including redox regulation, is important for AOX activity. Therefore, transcriptional changes might not reflect its function at a given time but in stead indicate preparation for future stress-derived metabolic fluctuations, against which AOX could function. On the other hand, Vanlerberghe et al. (2009) emphasize the mitochondrial role in stress signaling. In their hypothesis, AOX activity could regulate the strength of this signaling output, which again could be metabolites, ROS or both. These ideas do not have to be mutually exclusive; in stead they demonstrate the complexity of interorganellar signaling involving mitochondria.
MAP kinases
Mitogen-activated protein kinase (MAPK) cascades are commonly employed by eukaryotes and have gained much research attention over the past decade. The minimal signal transduction unit is considered to consist of a stimulus-activatable MAP kinase kinase kinase (MAPKKK), a MAP kinase kinase (MAPKK), a MAP kinase (MAPK) and, finally, their downstream target. A sequential phosphorylation-activation relay transmits and amplifies the signal from the MAPKKK to the target, which can be a TF whose activity, localization or half-life is affected by the phosphorylation (reviewed by Nakagami et al. 2005). Arabidopsis has at least 60 putative MAPKKKs, 10 MAPKKs and 20 MAPKs (MAPK Group 2002). The proportions indicate that MAPKKKs can be activated by specific stimuli and the signaling pathways may converge at the MAPKK level of the cascade. A single MAPKK could then phosphorylate multiple MAPKs. The signaling through MAPKKKs and MAPKKs could also proceed through other mechanisms than phosphorylation of their direct downstream target expanding their function outside the neatly hierarchical MAPK cascade. This is the case with the Arabidopsis MAPKKK, MEKK1, which might phosphorylate the WRKY53 TF directly and in addition bind to its promoter functioning as a transcriptional activator (Miao et al. 2007).
Although there has been considerable interest in elucidating the functions of plant MAPK signaling, examples of fully compiled cascades are not many (Fig. 2). One, however, is the cold and salt responsive MEKK1-MKK1/2-MPK4/6 signaling cascade (Teige et al. 2004), which seems to have a bi-directional interaction with ROS: The MEKK1 protein was found to be activated and stabilized by H2O2 and also the MAPK components MPK4 and MPK6 were activated by ROS and abiotic stresses (Teige et al. 2004). On the other hand, the ROS balance in plants lacking any step of the cascade is severely disturbed and transcriptional profiling revealed a large overlap between the oxidative stress-induced genes identified by Gadjev et al. (2006) in these plants (Pitzschke et al. 2009). When drawing conclusions from the results obtained by Pitzschke et al. (2009), one should, however, remember that the metabolism in these mutants is fundamentally altered and some of the transcriptional responses might represent secondary effects.
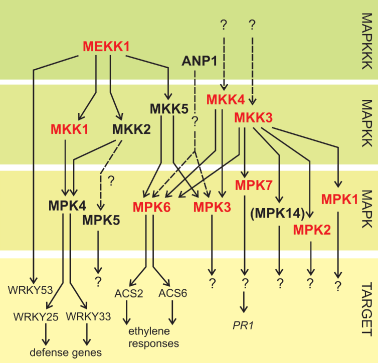
MAPK cascade components involved in abiotic stress responses. Figure is compiled from the references in the text and the induction of individual components by ROS is highlighted with red color. The ethylene-CTR1-MKK9-MPK3/6 pathway has been omitted for clarity.
Out of the 20 Arabidopsis MAPKs, the functions of only the abovementioned MPK4, MPK3 and MPK6 have been thoroughly characterized. They can all be activated by ROS, abiotic stress and pathogen elicitors but different MAPKKs seem to transmit the signal depending on treatment: During salt and cold stresses MPK6 (and MPK4) was activated by MKK2 (Teige et al. 2004) but although MKK3 has also been shown to activate MPK6, it is not required for salt tolerance (Takahashi et al. 2007). In addition, the overexpression of MKK9, another MPK3/6-activating MAPKK, rendered transgenic plants sensitive to salt treatment (Xu et al. 2008, Fig. 2). In addition, the kinase activities of MPK1 and MPK2 have been shown to increase in response to wounding, jasmonic acid, H2O2 and ABA (Ortiz-Masia et al. 2007), and MPK7 is activated by H2O2, as well was by the MAPKK MKK3 in response to jasmonic acid and Pseudomonas syringae challenge (Dóczi et al. 2007). In addition, the stabilization of MKK4 and MKK5 MAPKKs by H2O2 was demonstrated and the authors hypothesized that H2O2 might have a generally stabilizing effect on MAPKKs (Dóczi et al. 2007).
All in all, ROS seem to play a central role in regulating MAPK cascades. However, because these pathways show an equally strong connection to pathogen signaling as to abiotic stress responses, further work will be required in elucidating how ROS regulate the abiotic stress branch of MAPK signaling. Currently there is no mechanistic explanation on how ROS signals are translated to the activation of these kinases. Furthermore, the direct phosphorylation targets of MAPKs are mostly unknown, although ethylene synthesis is known to be positively regulated by the MPK6-mediated phosphorylation of key biosyntehsis intermediates ACS2 and ACS6 (Liu and Zhang 2004) and two WRKY family TFs are phosphorylated by MPK4 (Andreasson et al. 2005).
TFs
Extensive transcript profiling experiments have demonstrated the regulation of hundreds of genes in response to abiotic stress and there are generally large similarities to ROS-generating treatments (Gadjev et al. 2006, Vanderauwera et al. 2005). In a comprehensive oxidative stress microarray study, Gadjev et al. (2006) found 32 TFs that were at least five-fold upregulated and common to different oxidative stress treatments. Of these, eight belonged to the AP2/ERF family, seven to NAM/NAC and six to WRKY families clearly exceeding their representation within Arabidopsis TFs in general. A handful of key stress-induced TFs have been studied in more detail. These include, for example, the ZAT proteins (reviewed by Miller et al. 2008) and the drought—stress response coordinating drought-responsive element binding (DREB) TFs.
DREB proteins belong to the large AP2/ERF superfamily of TFs (Nakano et al. 2004) and DREB2A is one of the best-studied TFs in abiotic stress signaling. It has been shown to mediate ABA-independent transcriptional responses to drought, salt and heat (Sakuma et al. 2006a, b) and the 35S-driven expression of a constitutively active form of DREB2A resulted in the upregulation of nearly 500 genes (Sakuma et al. 2006b). DREB2A expression is induced in response to dehydration, salinity, heat and different oxidative stress treatments (Gadjev et al. 2006, Sakuma et al. 2006a) and the second messenger inositol-1,4,5-trisphosphate, better known for its role in ABA signal transduction, is implicated in its negative regulation (Perera et al. 2008) conferring potential crosstalk between the ABA-dependent and -independent stress signaling. DREB2A is apparently also regulated on the posttranslational level, because the protein contains a negative regulatory domain and is the target of two ubiquitin ligases indicative of proteasomal degradation (Qin et al. 2008). In support of the proteasomal regulation of the TF activity, heat and proteasome inhibitor treatments stabilize the protein (Qin et al. 2008, Sakuma et al. 2006b). Interestingly, DREB2A in addition interacts with the Radical-Induced Cell Death1 (RCD1) protein, an important regulator of oxidative stress and programmed cell death signaling (Belles-Boix et al. 2000, Fujibe et al. 2004, Overmyer et al. 2000). However, different motifs in DREB2A seem to be mediating the interaction with ubiquitin ligases and RCD1. The first requires the N-terminal 205 amino acids of DREB2A, which contain the DNA binding and negative regulatory domains (Qin et al. 2008), but in contrast, different amino acids were necessary for the DREB2A-RCD1 interaction (Jaspers, P and Kangasjärvi J, unpublished results).
The activity of TFs can be directly regulated by oxidative modifications as exemplified by the yeast oxidative stress TF Yap1 (reviewed in Herrero et al. 2008). This TF contains two cysteine-rich regulatory domains that can be affected by ROS or thiol-active electrophiles resulting in intramolecular disulfide bond formation and nuclear localization. The ROS modification of Yap1 requires a physical interaction with glutathione peroxidase3 which functions as the primary ROS sensor.
In plants, an important activator of defense gene expression, NPR1, is subject to redox regulation (Mou et al. 2003): In unchallenged cells, oligomerization through intermolecular disulfide bridges sequesters the protein to the cytoplasm but as a consequence of the more reductive state of the cellular environment that follows the oxidative phase of salicylic acid or pathogen treatment, the protein is reduced, monomerized and translocated to the nucleus. This results in upregulation of a set of disease resistance genes. The NPR1 protein contains several cysteine residues that can be the targets of redox regulation. Accordingly, it was found that, in addition to the multiple cysteine-mediated disulfide bridging between the molecules, oligomerization is promoted by nitrosylation of Cys156 by S-nitrosoglutathione. Two NPR1-interacting thioredoxins were found to work in the opposite direction in catalyzing the release of NPR1 monomers thus allowing the defense responses to be activated (Tada et al. 2008). An AP2/ERF family TF, Rap2.4a, was recently demonstrated to be another target of oxidative modulation in plants. It regulates the nuclear expression of a chloroplast peroxiredoxin and is required for plant survival under natural light conditions (Shaikhali et al. 2008).
In addition to direct redox regulation of the TFs, ROS or cellular redox status could be signaled to them by an intermediate redox-regulated proteins. One candidate is the RCD1 protein, which has been shown to interact with a number of TFs, in addition to DREB2A (Jaspers et al. 2009). Miao et al. (2006) indicated a putative glutathione peroxidase3-RCD1 interaction and hypothesized that RCD1 might be something of a plant equivalent of the yeast Yap1.
Although transcriptional activation of a TF can occur within minutes of stress perception, the lag time can still be reduced by keeping the stress-inducible TF in an inactive form and using posttranslational modifications for its activation. One of these is the stimulus-induced cleavage and nuclear transport of an active part of membrane-bound TFs. Because abiotic stresses are likely to have an impact on membrane functions and impair the protein folding machinery within the endoplasmic reticulum (ER), membrane release seems an especially tempting way of responding to this type of stress. Close to 200 TFs in Arabidopsis have been predicted to be membrane associated (reviewed in Seo et al. 2008) but so far only two ER-bound TFs have been shown to mediate abiotic stress responses: The activation of AtbZIP17 upregulates salt stress-inducible genes (Liu et al. 2007) and AtbZIP28 mediates a branch of heat stress response (Gao et al. 2008). However, a number of membrane-bound NAC family TFs as well as other members of the bZIP family are involved in regulating developmental processes (Seo et al. 2008). So far, very little is known about the involvement of ROS in the activation of membrane-bound TFs. However, the oxidation of membrane lipids by ROS can produce reactive oxylipin species (Mueller and Berger 2009) and some of them are known to further react with proteins, thus opening the possibility for functional regulation of membrane-residing TFs.
Conclusions
The biggest gap in our knowledge of ROS signaling at the moment is at the level of perception. As all ROS are more or less ephemeral molecules, individual sensors will be extremely difficult to identify and, at least partly, the perception is likely to occur through the monitoring of the cell redox status by several molecules in different cellular compartments. Currently, several candidates for apoplastic ROS perception are being investigated, among others the STIG1-like protein GRIM REAPER (Wrzaczek et al. 2009) and several ozone-responsive receptor-like kinases (Wrzaczek, M, Karpinski, S, Kangasjärvi, J, unpublished results) and these lines of research can contribute significantly to our knowledge of ROS signaling.
Second, the ubiquitin-26S proteasome system (UPS) is emerging as a fundamental regulator of signal transduction in eukaryotes, and especially in plants. The percentage of UPS components in Arabidopsis genome is several times larger than in non-plant eukaryotes and its diversity is so striking that it has been suggested to equal transcription and phosphorylation in its importance in regulating plant signaling (reviewed by Vierstra 2009). Although most plant hormone responses seem to be mediated by one or more UPS-regulated step, and they certainly intertwine with ROS and abiotic stress, the reports connecting UPS directly to ROS signaling are few.
Third, the specificity of the signaling components identified so far requires further work. As ROS signal down to several different branches of cellular stress response, the results suggesting the involvement of a certain component in a given process require cautious interpretation to dissect between a more general stress response and a signal that is genuinely stress-specific. Gene expression profiling has yielded much information to this direction, but unfortunately RNA levels will not give a whole picture of cellular events in many cases. Protein and metabolite profiling have the promise of being the next steps in filling in the blanks of our map of stress signaling networks.
Taken together, the generation of ROS under stress conditions is reasonably well documented and many ROS-related signaling components have been identified. In contrast to earlier views, it is becoming increasingly evident that even during stress, ROS production is not necessarily a symptom of cellular dysfunction but it could represent a signal for adjusting the machinery to the altered circumstances and the intricacies of this signaling network are only beginning to be elucidated.
Acknowledgements
We acknowledge Dr Mikael Brosché, Dr Michael Wrzaczek and Dr Kirk Overmyer for their invaluable comments on the manuscript. P. J. acknowledges the Viikki Graduate School in Biosciences for funding. Work in the lab of J. K. was supported by the Academy of Finland Centre of Excellence program (2006–2011). Space constraints did not allow us to cover all aspects of ROS and abiotic stress signaling and we would like to apologize to those colleagues whose work was undeservingly left out from this treatment.




