Cytokinin- and auxin-induced stomatal opening is related to the change of nitric oxide levels in guard cells in broad bean
Abstract
The relationship between cytokinin- and auxin-induced stomatal opening and nitric oxide (NO) levels in guard cells in broad bean was studied. Results indicate that cytokinins and auxins reduced the levels of NO in guard cells and induced stomatal opening in darkness. In addition, cytokinins not only reduced NO levels in guard cells caused by sodium nitroprusside (SNP) in light but also abolished NO that had been generated by dark, and then promoted the closed stomata reopening, as did NO scavenger 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide. However, unlike cytokinins, auxins not only had incapability to reduce NO levels by SNP but also could not abolish NO having been generated by dark, so auxins could not promote the closed stomata to reopen. The above-mentioned effects of auxins were similar to that of nitric oxide synthase (enzyme commission 1.14.13.39) inhibitor NG-nitro-l-Arg-methyl ester. Hence, it is concluded that cytokinins reduced probably the levels of NO in guard cells via scavenging, and auxins reduced NO levels through restraining NO generation in all probability, and then induced stomatal opening in darkness.
Abbreviations –
-
- 6-BA
-
- 6-benzyl amino purine
-
- ABA
-
- abscisic acid
-
- cPTIO
-
- 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide
-
- DAF-2 DA
-
- 4,5-diaminofluorescein diacetate
-
- DMSO
-
- dimethyl sulfoxide
-
- H2O2
-
- hydrogen peroxide
-
- IAA
-
- indole-3-acetic acid
-
- KT
-
- 6-furfuryl amino purine
-
- l-NAME
-
- N G-nitro-l-Arg-methyl ester
-
- MES
-
- 2-(N-morpholino) ethanesulfonic acid
-
- NAA
-
- naphthaleneacetic acid
-
- NO
-
- nitric oxide
-
- NOS
-
- nitric oxide synthase
-
- SNP
-
- sodium nitroprusside
Introduction
The plant hormone cytokinins and auxins play a wide range of important regulatory role in plant growth and development (Brault and Maldiney 1999, MacDonald 1997). In plant, guard cells open and close the stomata to control transpiration and regulate gas exchange in leaves of higher plant. Both cytokinins and auxins have been demonstrated to regulate stomatal behavior (Jewer and Incoll 1980, Pemadasa 1982). Up to now, the mechanism of cytokinin- and auxin-induced stomatal behavior remains still unclear. Irving et al. (1992) reported that guard cell [Ca2+]cyt increased, whereas pHcyt decreased after treatment of closed stomata with 6-furfuryl amino purine (KT) and indole-3-acetic acid (IAA), and then resulted in stomatal opening. The pattern of cytokinin action on guard cells might involve induction of membrane hyperpolarization by stimulation of an electrogenic H+-pump, and stimulation of adenylate cyclase activity which might lead to an increase in intracellular adenosine 3′,5′-cyclic monophosphate content, and stimulation of guanylate cyclase activity or interaction with a calcium-calmodulin system (Morsucci et al. 1991, Pharmawati et al. 1998). Auxins (IAA and naphthaleneacetic acid, NAA) can affect inwardly and outwardly rectifying K+ currents in a dose-dependent and pH-dependent manner (Bauly et al. 2000, Blatt and Thiel 1994, Grabov and Blatt 1998), and induce a shift of the voltage dependence of anion channels (Lohse and Hedrich 1995). Additionally, Schroeder et al. (2001) reported that auxins-induced stomatal opening is probably related to H+ extrusion through plasma membrane H+-adenosine triphosphatase.
Stomatal movement is also regulated by nitric oxide (NO). Garcia-Mata and Lamattina (2001) reported that sodium nitroprusside (SNP, applied as NO donors) induced stomatal closure and reduced transpiration in Vicia faba, Salpichroa organifolia and Tradescantia sp., and the process is time and dose dependent, and fully reversible (Neill et al. 2002). There is now compelling evidence that NO is involved in darkness-, ultraviolet-B-, salicylic acid-, jasmonic acid- and abscisic acid (ABA)-induced stomatal closure (Desikan et al. 2002, Garcia-Mata and Lamattina 2002, He et al. 2005, Liu et al. 2003, 2005, Neill et al. 2002, She et al. 2004). However, it is unknown whether cytokinin- and auxin-induced stomatal opening is related to the change of NO levels in guard cells, and the mechanism of NO change caused by cytokinins and auxins needs to be proved. In the present studies, we attempt to provide evidences that cytokinin- and auxin-induced stomatal opening is related to the change of NO in broad bean guard cells by means of stomatal bioassay and using laser-scanning confocal microscopy, and the pattern of NO reduction caused by cytokinins and auxins is also studied.
Materials and methods
Chemicals
Molecular probes 4,5-diaminofluorescein diacetate (DAF-2 DA, Sigma-Aldrich, St Louis, MO) was dissolved in dimethyl sulfoxide (DMSO) to produce a stock solution, which was aliquoted. Sodium nitroprusside (SNP), 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO), NG-nitro-l-Arg-methyl ester (l-NAME), 2-(N-morpholino) ethanesulfonic acid (MES) and DMSO were also obtained from Sigma. Unless stated otherwise, the remaining chemicals were of the highest analytical grade available from various suppliers of the Chinese companies.
Plant materials
Broad bean (V. faba L.) was grown in controlled-environment plant growth chamber with a humidity of 80%, a photo flux density of 300 μmol m−2 s−1 photosynthetically active radiation generated by cool white fluorescent tubes (Philips, New York, NY) and an ambient temperature 25 ± 2°C with a 14-h light and 10-h dark cycle. The epidermis was peeled carefully from the abaxial surface of the youngest, fully expanded leaves of 4-week-old seedlings, and cut into pieces about 5-mm width and 5-mm lengths.
Stomatal bioassay
Stomatal opening and closing were monitored as the method of McAinsh et al. (1996) with slight modifications. To study the effects of cytokinins and auxins on stomatal aperture in dark, freshly prepared abaxial epidermal strips were incubated in CO2-free MES/KCl buffer (10 mM MES/KOH, 50 mM KCl, 100 μM CaCl2, pH 6.15) with cytokinins, auxins or other compounds [KT, 6-benzyl amino purine (6-BA), IAA, NAA, cPTIO or l-NAME] for 3 h in darkness conditions in the temperature of 25°C. Final stomatal apertures were recorded with a light microscope and an eyepiece graticule previously calibrated with a stage micrometer. To study the effects of cytokinins and auxins on stomatal closing caused by SNP, epidermal strips were incubated in MES/KCl buffer with SNP alone, or containing cytokinins, auxins and other compounds for 3 h under light (300 μmol m−2 s−1) conditions with the temperature of 25°C, and then the apertures were recorded. To study the effects of cytokinins and auxins on closed stomata having been caused by dark, strips were incubated in MES/KCl buffer for 3 h, and then were treated with fresh buffer alone, or containing cytokinins, auxins and other compounds for another 3 h in dark. Final stomatal apertures were recorded.
In all stomatal bioassay, the apertures at starting points were measured. The darkness conditions as described above were taken under identical temperature of 25°C in an incubator (Boxun Industry & Commerce Co., Ltd Medical Equipment Factory, Shanghai, China). To avoid any potential rhythmic effects on stomatal aperture, experiments were always started at the same time of the day. In each treatment, we scored randomly 30 apertures each times, and every treatment was repeated three times. The data presented are the means of 90 measurements ± standard error.
Dye loading of DAF-2 DA
NO measurement was performed as the method of Kojima et al. (1998) with some modifications. To study the effects of cytokinins and auxins on darkness- and SNP-caused NO levels in guard cells, the epidermal strips were treated for 3 h as described in stomatal bioassay section, and they were immediately placed into loading Tris–KCl buffer (Tris 10 mM and KCl 50 mM, pH 7.2) containing 10 μM of DAF-2 DA for 30 min in darkness at 25 ± 2°C. To study the effects of cytokinins and auxins on NO levels having been generated by dark in guard cells, strips were incubated in MES/KCl buffer for 3 h in darkness, and then dye DAF-2 DA was loaded, and then cytokinins, auxins or other reagents were added directly to Tris–KCl buffer.
Laser-scanning confocal microscopy
After washing off excess dye with fresh Tris–KCl loading buffer in darkness, examination of the strips was immediately performed using TCS SP2 laser-scanning confocal microscopy (Leica Lasertechnik Gmbh, Heidelberg, Germany) with the following settings: excitation 488 nm, emission 530 nm, power 10%, zoom about 4, normal scanning speed and frame 512 × 512 pixel. Images acquired from the confocal microscope were analyzed using Leica image software, Time-Course and Photoshop.
In the experiments that determined the effects of cytokinins, auxins or other reagents on NO having been generated by dark in guard cells, strips were incubated in MES/KCl buffer for 3 h in darkness, and then DAF-2 DA was loaded for 30 min. After these steps, cytokinins, auxins or other reagents were added directly to Tris–KCl buffer, respectively. Then the intensity change of the time course plots of DAF-2 DA fluorescence was recorded within 900 s, and the images of guard cells at 0, 100, 300, 500, 700, 900 s and 3 h were also taken.
To enable the comparison of changes in signal intensity, confocal images were taken under identical conditions (in manual setup) for all samples, and in each treatment we measured three epidermal strips, and the treatment was repeated at least three times. The selected confocal images represented the same results from three times repeat.
Results
Both cytokinins and auxins induce stomatal opening in darkness
Previous studies suggested that both cytokinins and auxins regulate the stomatal behavior (Blackman and Davies 1983, Pemadasa 1982). The responses of stomata to cytokinins depend on the cytokinins variety and concentration (Incoll and Jewer 1987). Similarly, auxins have been reported to induce not only stomatal opening but also stomatal closure (Mansfield and Atkinson 1990). To gain insights into the effects of cytokinins and auxins on stomatal pore changes, isolated epidermal strips of V. faba were incubated with different concentrations of KT, 6-BA, IAA or NAA, respectively, for 3 h in darkness conditions. As shown in Fig. 1, both cytokinins and auxins induced stomatal opening in darkness (Fig. 1), which is the same as the previous results (Morsucci et al. 1992). Cytokinins at the concentration of ≥0.05 μM induced significantly stomatal opening in darkness (Fig. 1A), so did auxins at the concentration of ≥0.05 μM (Fig. 1B). The optimum concentrations of cytokinins and auxins were 0.2 and 10 μM, respectively.
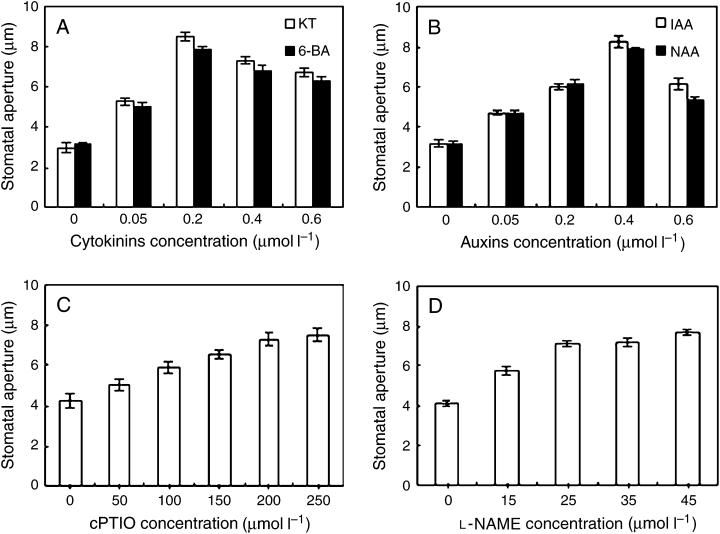
Both cytokinins and auxins induce stomatal opening in darkness. After the primal values of stomatal aperture were measured, isolated epidermal strips of Vicia faba were incubated in CO2-free 2-(N-morpholino)-ethanesulfonic acid/KCl containing different concentrations of 6-furfuryl amino purine, 6-benzyl amino purine (0, 0.05, 0.2, 0.4, 0.6 μM) (A), indole-3-acetic acid, naphthaleneacetic acid (0, 0.1, 1, 10, 100 μM) (B), 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (0, 50, 100, 150, 200, 250 μM) (C) and NG-nitro-l-Arg-methyl ester (0, 15, 25, 35, 45 μM) (D) for 3 h in darkness with the same temperature of 25°C. Stomatal apertures were determined. Values are the means of 90 measurements ± standard error of three independent experiments. Error bars are indicated. The primal values of A, B, C and D are 8.1034 ± 0.2354, 8.2409 ± 0.4312, 9.1256 ± 0.1268 and 8.9546 ± 0.5416, respectively.
Darkness-induced stomatal closure is related to the production of endogenous NO, and the levels of NO are higher in darkness than in light (She et al. 2004). To know if there is a relationship between cytokinins/auxins-induced stomatal opening and the levels of NO in guard cells, the strips were treated by cPTIO and l-NAME, which is the specific NO scavenger (Garcia-Mata and Lamattina 2001) and an inhibitor of NO synthase (NOS) (Neill et al. 2003, Wendehenne et al. 2001), respectively. The results showed that both cPTIO and l-NAME induced stomatal opening in darkness and the effects were in a dose-dependent manner (Fig. 1C, D). The effects of cPTIO and l-NAME on stomatal aperture were highly significant (P < 0.01) at 200 and 25 μM, respectively. These results presume that, probably like cPTIO and l-NAME, cytokinin- and auxin-induced stomatal opening in darkness is associated with decrease of NO levels in guard cells.
Effects of cytokinins and auxins on the darkness-induced NO levels in guard cells
Having been established that both cytokinins and auxins induced stomatal opening in darkness (Fig. 1A, B), to determine further whether cytokinin- and auxin-induced stomatal opening for a certainty is accompanied by decrease of NO levels in darkness, epidermal strips were loaded with the NO-fluorescent dye DAF-2 DA, which allows the detection of NO presence in both animal and plant cells (Foissner et al. 2000, Kojima et al. 1998). As shown in Fig. 2A, darkness could induce an intense DAF-2 DA fluorescence in guard cells, which is consistent with the previous reports (She et al. 2004). However, darkness-induced DAF-2 DA fluorescence in guard cells was largely prevented by cytokinins and auxins (Fig. 2D–G). Similarly, treatment with cPTIO and l-NAME also substantially suppressed dark-induced DAF-2 DA fluorescence (Fig. 2B, C). These results provide evidence that both cytokinins and auxins lessen assuredly NO levels in darkness like cPTIO and l-NAME.
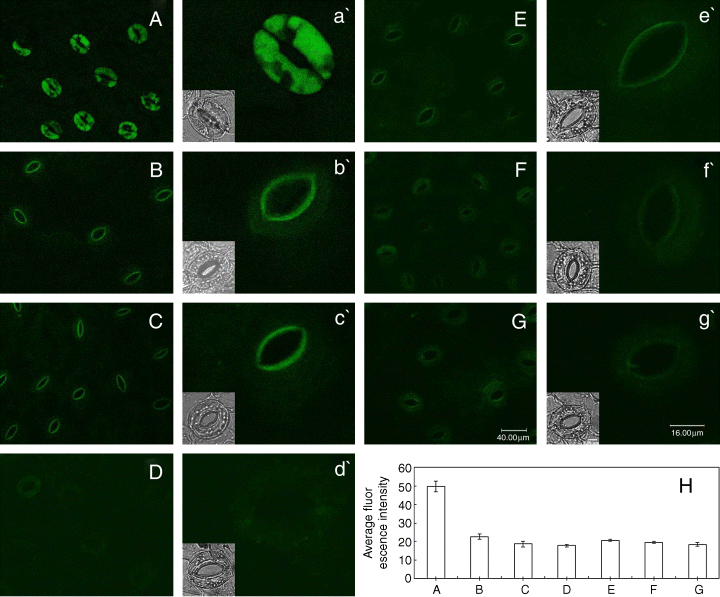
Effects of cytokinins and auxins on the darkness-induced nitric oxide levels in guard cells. Guard cells of Vicia faba shown in image (A) treated in darkness alone for 3 h, and in image (B) with 200 μM 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide, (C) 25 μM NG-nitro-l-Arg-methyl ester, (D) 0.2 μM 6-furfuryl amino purine, (E) 0.2 μM 6-benzyl amino purine, (F) 10 μM indole-3-acetic acid and (G) 10 μM naphthaleneacetic acid, respectively, in the darkness for 3 h. Above-treated epidermal strips were immediately loaded with 4,5-diaminofluorescein diacetate for 30 min in darkness, then excess dye was removed and the strips were examined by using laser-scanning confocal microscopy. (H) The average fluorescent intensity of guard cells in images from (A) to (G), data are the means ± standard error. The guard cells shown in image (a′) to (g′) are the representative of guard cells shown in images (A–G). The insets show the bright-field images corresponding to the fluorescence images (a′) through (g′). The length of scale bar in image (G) and (g′) represents 40 and 16 μm for image (A–G) and (a′–g′), respectively. The bar in inset of image (g′) represents 8 μm for all the insets. Each experiment was repeated at least three times, and the selected confocal image represented the same results from about nine time measurements.
Cytokinins can, but auxins not, reverse SNP-induced stomatal closure
The fact, cytokinin- and auxin-induced stomatal opening is associated with decrease of NO levels of guard cells in dark, attracts us to gain the pattern of cytokinins- and auxins-reduced NO levels. To address further this question, strips were incubated in MES/KCl with SNP alone or containing cytokinins, auxins and other compounds for 3 h in light conditions. As shown in Fig. 3, cytokinins, auxins, cPTIO and l-NAME did not cause any changes of stomatal aperture in light. However, similarly to cPTIO, the specific NO scavenger, 6-BA or KT could prevent SNP-induced stomatal closure. The effects were significant (P < 0.01) (Fig. 3A). Contrarily, both IAA and NAA had no effect on SNP-induced stomatal closure, which is similar to the effect of l-NAME that is an inhibitor of NOS (Fig. 3B). The results imply that cytokinins prevent stomatal closing caused by SNP via scavenging NO, but auxins do not.
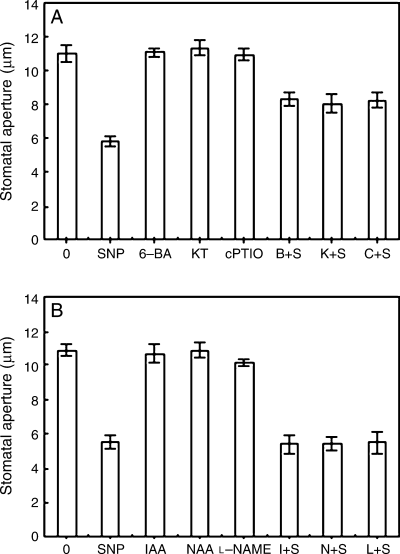
Cytokinins can, but auxins not, reverse sodium nitroprusside (SNP)-induced stomatal closure. After the primal values of stomatal aperture were measured, isolated epidermal strips of Vicia faba were incubated in CO2-free 2-(N-morpholino)-ethanesulfonic acid/KCl alone, or containing 100 μM SNP, 0.2 μM 6-benzyl amino purine (6-BA), 0.2 μM 6-furfuryl amino purine (KT), 200 μM 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO), 0.2 μM 6-BA + 100 μM SNP (B + S), 0.2 μM KT + 100 μM SNP (K + S), 200 μM cPTIO + 100 μM SNP (C + S) (A), or containing 100 μM SNP, 10 μM indole-3-acetic acid (IAA), 10 μM naphthaleneacetic acid (NAA), 25 μM NG-nitro-l-Arg-methyl ester (l-NAME), 10 μM IAA + 100 μM SNP (I + S), 10 μM NAA + 100 μM SNP (N + S), 25 μMl-NAME + 100 μM SNP (L + S) (B) for 3 h under light (300 μmol m−2 s−1) conditions with the same temperature of 25°C. Stomatal apertures were determined after 3 h of incubation. Values are the means of 90 measurements ± standard error of three independent experiments. Error bars are indicated. The primal values of A and B are 8.5419 ± 0.1908 and 8.6553 ± 0.3842, respectively.
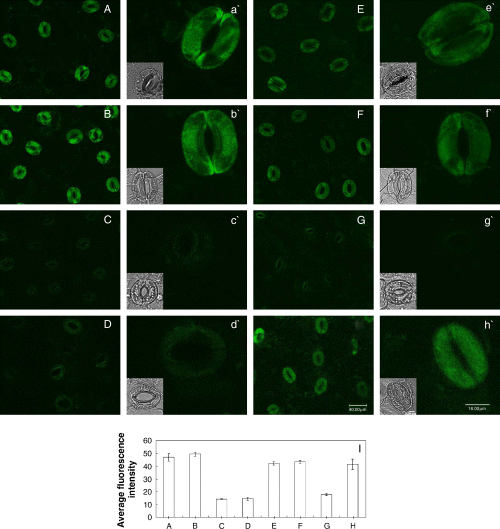
Effects of cytokinins and auxins on the SNP-induced DAF-2 DA fluorescence levels in guard cells
Cytokinins can prevent stomatal closing caused by exogenous NO, but auxins cannot (Fig. 3A, B). To further clarify whether cytokinins and auxins can affect SNP-induced DAF-2 DA fluorescence, the epidermal strips were treated with SNP in the presence of either cytokinins, auxins, cPTIO or l-NAME for 3 h in light, and then NO levels were measured. As shown in Fig. 4, a striking DAF-2 DA fluorescence in guard cells was observed after the treatment of 100 μM SNP in light (Fig. 4B). Compared with the control (Fig. 4A), there was no change of DAF-2 DA fluorescence in guard cells treated by cytokinins or auxins as well as cPTIO or l-NAME in light (Fig. 4C–H). However, SNP-induced DAF-2 DA fluorescence in guard cells was largely prevented by cytokinins (Fig. 4I, J) and cPTIO (Fig. 4M). Dissimilar to cytokinins, IAA and NAA, as well as l-NAME, could not suppress SNP-induced DAF-2 DA fluorescence (Fig. 4K, L, N). These results indicate that cytokinins can scavenge NO, and then prevent stomatal closure induced by SNP. Auxins cannot prevent SNP-induced stomatal closure for incapability to scavenge NO.
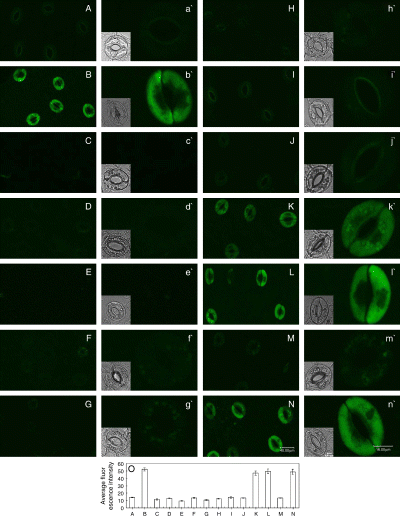
Effects of cytokinins and auxins on the sodium nitroprusside (SNP)-induced 4,5-diaminofluorescein diacetate (DAF-2 DA) fluorescence in guard cells. Guard cells shown in image (A) treated in light for 3 h with buffer only, and (B) with 100 μM SNP, (C) 0.2 μM 6-benzyl amino purine (6-BA), (D) 0.2 μM 6-furfuryl amino purine (KT), (E) 10 μM indole-3-acetic acid (IAA), (F) 10 μM naphthaleneacetic acid (NAA), (G) 200 μM 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO), (H) 25 μM NG-nitro-l-Arg-methyl ester (l-NAME), (I) SNP + 6-BA, (J) SNP + KT, (K) SNP + IAA, (L) SNP + NAA, (M) SNP + cPTIO and (N) SNP + l-NAME, respectively, in light for 3 h. Above-treated strips were loaded with DAF-2 DA for 30 min in darkness, then excess dye was removed and the strips were examined by laser-scanning confocal microscopy. (O) Shows the mean (±se) fluorescent intensity of guard cells in images from (A) to (N). Other explanations are the same as in Fig. 2.
Cytokinins can, but auxins not, reopen the closed stomata caused by darkness
As shown in Fig. 5, the strips were incubated in MES–KCl for 3 h in the darkness, and then were treated with fresh buffer alone, or containing various reagents for another 3 h, cytokinins and cPTIO could promote the closed stomata caused by dark to reopen, but auxins and l-NAME could not. These results suggest that, like cPTIO, cytokinins can scavenge NO having been generated by dark, thus resulting in stomatal reopening. However, like l-NAME, auxins cannot scavenge NO having been generated by dark, so they cannot induce the closed stomata to reopen.
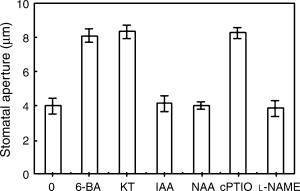
Cytokinins can, but auxins not, reopen the closed stomata caused by darkness. After the primal values of stomatal aperture were measured, strips of Vicia faba, incubated in CO2-free 2-(N-morpholino)-ethanesulfonic acid/KCl for 3 h under darkness conditions, were treated with fresh buffer alone, or containing 0.2 μM 6-benzyl amino purine, 0.2 μM 6-furfuryl amino purine, 10 μM indole-3-acetic acid, 10 μM naphthaleneacetic acid, 200 μM 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide and 25 μM NG-nitro-l-Arg-methyl ester, respectively, for another 3 h, and then stomatal apertures were determined. Values are the means of 90 measurements ± standard error of three independent experiments. Error bars are indicated. The primal values are 9.3212 ± 0.4238.
Effects of cytokinins and auxins on NO levels having been generated by darkness
The effects of cytokinins and auxins on the levels of NO that had been generated by dark in guard cells were also measured in the present studies. After the incubation of 3 h in darkness, epidermis was loaded with DAF-2 DA, washed and examined by using laser-scanning confocal microscopy. During examination of DAF-2 DA fluorescence, cytokinins, auxins, cPTIO and l-NAME were added to the buffer. As shown in Fig. 6A, the fluorescence intensity of control had no change within 900 s. KT and 6-BA reduced strikingly the DAF-2 DA fluorescence intensity compared with the control (Fig. 6B, C), so did cPTIO (Fig. 6F). However, like l-NAME, IAA and NAA could not reduce the DAF-2 DA fluorescence intensity (Fig. 6D, E, G). Additionally, the images of guard cells treated by cytokinins, auxins, cPTIO and l-NAME at 0 and 3 h were also obtained. As shown in Fig. 7, the DAF-2 DA fluorescence of guard cells treated by cytokinins and cPTIO for 3 h (Fig. 7C, D, G) was lower than that of the control (Fig. 7B) and 0-h treatment (Fig. 7A), but the DAF-2 DA fluorescence of guard cells treated by auxins and l-NAME for 3 h (Fig. 7E, F, H) was unchanged. These results indicate intensively that cytokinins initiate probably NO scavenging mechanisms, but auxins do not.
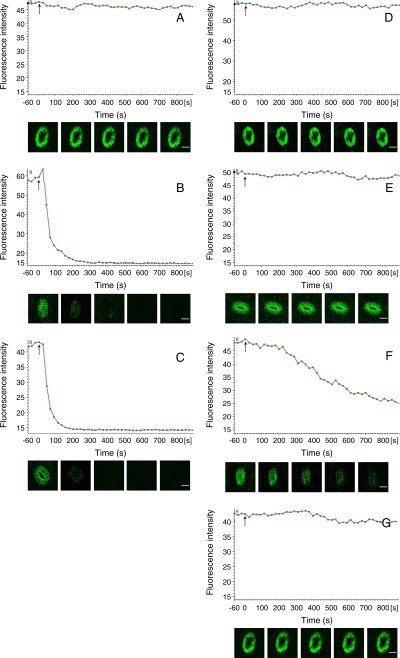
The intensity changes of the time course plots of 4,5-diaminofluorescein diacetate (DAF-2 DA) fluorescence. Strips of Vicia faba, incubated in CO2-free 2-(N-morpholino)-ethanesulfonic acid/KCl for 3 h under darkness conditions, were loaded with DAF-2 DA for 30 min apart from light, washed, and examined by laser-scanning confocal microscopy. During image acquisition, Tris–KCl buffer (A, control), 0.2 μM 6-furfuryl amino purine (B), 0.2 μM 6-benzyl amino purine (C), 10 μM indole-3-acetic acid (D), 10 μM naphthaleneacetic acid (E), 200 μM 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (F) and 25 μM NG-nitro-l-Arg-methyl ester (G) were added directly to the buffer, respectively. (A–G) Time plots course changes in the DAF-2 DA fluorescence intensity of guard cells, higher intensity stands for higher nitric oxide concentration. (A–G) Arrow indicates the addition of reagents. Fluorescence images of stomata were taken at 0, 100, 300, 500 and 700 s after the addition of reagents. The scale bar in images of the stoma is 20 μm for all the images.
Effects of cytokinins and auxins on nitric oxide levels having been generated by darkness. Strips of Vicia faba, incubated in CO2-free 2-(N-morpholino)-ethanesulfonic acid/KCl buffer for 3 h under darkness conditions, were loaded with 4,5-diaminofluorescein diacetate for 30 min in darkness, washed and then the strips were incubated in Tris–KCl buffer alone or containing other reagents in dark for another 3 h. At 0 h (A) and 3 h, the strips were examined by laser-scanning confocal microscopy. Image of guard cells in dark for 3 h in the presence of (B) buffer only, and (C) 0.2 μM 6-furfuryl amino purine, (D) 0.2 μM 6-benzyl amino purine, (E) 10 μM indole-3-acetic acid, (F) 10 μM naphthaleneacetic acid, (G) 200 μM 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide, (H) 25 μM NG-nitro-l-Arg-methyl ester, respectively. (I) Shows the mean (±se) fluorescent intensity of guard cells in images from (A) to (H). Other explanations are the same as in Fig. 2.
Discussion
Stomatal closure is induced by many abiotic and biotic factors, such as osmotic stress, darkness, high CO2 concentrations, decreased humidity, ABA and some mechanical stresses (Kearns and Assmann 1993, Schroeder et al. 2001). Stomatal opening can be induced by light, low CO2 levels and humidity etc. (Mansfield et al. 1990). Previous studies have shown that cytokinins and auxins can affect stomatal behavior (Jewer and Incoll 1980, Pemadasa 1982). NO also plays an important role in the guard cell signaling pathways (Desikan et al. 2002, Garcia-Mata and Lamattina 2002, He et al. 2005, Liu et al. 2003, 2005, Neill et al. 2002, She et al. 2004). However, until recently, little was known whether cytokinin- and auxin-induced stomatal movement is related to NO levels in guard cells. The results of the present studies showed that, like cPTIO, which is an NO scavenger, and l-NAME, which is an inhibitor of NOS, both cytokinins and auxins induced significantly stomatal opening (Fig. 1), and abolished largely the DAF-2 DA fluorescence of guard cells in darkness (Fig. 2). The results indicate that cytokinin- and auxin-induced opening of stomata is associated with a decrease of NO levels of guard cells in darkness.
There have been some reports on the relationship between NO and cytokinins or auxins. Tun et al. (2001) reported that cytokinins led to a rapid release of NO in cell cultures. Additionally, recent research suggested that NO is required for the molecular events involved in auxin-induced adventitious root and lateral root development (Correa-Aragunde et al. 2004, Pagnussat et al. 2002), that NO modulates the expression of auxin-dependent cell cycle regulatory genes (Correa-Aragunde et al. 2006). The results of the present studies proved that cytokinin- and auxin-induced stomatal opening is associated with reduction of NO levels in guard cells, the question arises of how cytokinins and auxins lessen NO levels. Our further experiments exhibited that cytokinins prevented stomatal closing and abolished DAF-2 DA fluorescence caused by SNP in light (3, 4), and also reopened the closed stomata caused by dark and abolished NO that had been generated by darkness (5, 7). The above-mentioned effects of cytokinins are similar to that of cPTIO (4, 7). The intensity changes of the time course plots of DAF-2 DA fluorescence showed that the DAF-2 DA fluorescence that had been caused by darkness was abolished by cytokinins and cPTIO (Fig. 6B, C, F). Although auxins-induced stomatal opening involves a decrease of NO levels of guard cells in darkness, auxins could neither prevent stomatal closing and DAF-2 DA fluorescence caused by SNP in light (3, 4) nor reopen the closed stomata caused by dark and abolish NO that had been generated by darkness (5, 7). The above-mentioned effects of auxins are similar to those of l-NAME (4, 7). The intensity changes of the time course plots of DAF-2 DA fluorescence showed that the DAF-2 DA fluorescence that had been caused by darkness was not abolished by auxins or l-NAME (Fig. 6D, E, G). These results demonstrated that cytokinins can scavenge NO, and thus prevent SNP-induced stomatal closure and promote the reopening of the closed stomata caused by dark, but auxins cannot. Combined with the facts that cytokinins/auxins promote stomata opening and decrease NO levels in guard cells in darkness, we conclude that cytokinins initiate probably NO scavenging mechanisms to reduce the levels of NO in guard cells, and then promote stomatal opening in dark, auxins cannot scavenge NO, but perhaps restrain NO production and then result in opening of stomata in dark.
Guard cells have become a highly developed model system for characterizing early signal transduction mechanisms in plants and for elucidating how individual signaling mechanisms can interact within a network in a single cell. Drought stress leads to increased synthesis and accumulation of ABA, which results in stomatal closure. Darkness can also induce stomata to close (Schroeder et al. 2001, She et al. 2004). It is becoming increasingly apparent that NO is an important component of ABA- and darkness-induced stomatal closure (Garcia-Mata and Lamattina 2002, Neill et al. 2002, She et al. 2004). Additionally, both ABA and darkness can inactivate light-activated H+-ATPase and increase cytosolic pH and K+ efflux from the guard cells (Schroeder et al. 2001). Cytokinins can induce stomatal opening (Jewer and Incoll 1980) and membrane hyperpolarization by stimulation of electrogenic H+-pump in plasma of guard cells (Morsucci et al. 1991, Pharmawati et al. 1998). The results of the previous studies showed that auxins induce stomatal opening (Pemadasa 1982) and extrusion of H+ through activating plasma membrane H+-ATPase (Schroeder et al. 2001), and affect inwardly and outwardly rectifying K+ currents (Bauly et al. 2000, Blatt and Thiel 1994, Grabov and Blatt 1998), and Irving et al. (1992) reported that KT- and IAA-induced stomatal opening involved pHcyt decrease of guard cell. Recent research indicates that inhibition of blue light-dependent H+-ATPase by ABA was because of a decrease in the phosphorylation levels of H+-ATPase and that hydrogen peroxide (H2O2) mediates this response (Zhang et al. 2004), and H2O2 induces a rapid production of NO in Mung bean (Lum et al. 2002) and in V. faba (She et al. 2004). So we presume that ABA inhibits H+-ATPase also through NO-induced dephosphorylation of the plasma membrane H+-ATPase in guard cell. The results of the present studies show that cytokinin- and auxin-induced opening of stomata involves decrease of NO levels in guard cells. Based on these results, we presume that cytokinins and auxins activate probably plasma membrane H+-ATPase through decreasing NO levels, and then promote stomatal opening in darkness. Therefore, NO might act as a pivotal role in cytokinins, auxins, ABA and darkness signaling network of guard cells.
Edited by J. Zuo
Acknowledgments
Acknowledgements – Research is supported by the Natural Science Research Plan of Shaanxi Province of People’s Republic of China (grant no. 2003C101 and 2005C112). We thank Dr. Josephine Richardson for her enthusiastic help in this study.




