Roles of the cyanobacterial isiABC operon in protection from oxidative and heat stresses
Abstract
The expression of the isiAB operon, which encodes CP43′ and flavodoxin, is induced under oxidative- or heat-stress conditions as well as iron-limited conditions. The aim of this study is to elucidate their roles for acquisition of tolerance by cells against oxidative or heat stress with using the isiA interruption and the isiB deletion mutants of Synechocystis sp. PCC 6803. In the former mutant, the isiA messenger RNA was truncated and the isiB transcription was totally abolished. Northern blot analysis indicated that an open reading frame sll0249 located immediate downstream of isiB is cotranscribed with isiAB under iron-limited conditions. Thus, we designated the gene as isiC and constructed an isiC deletion mutant to test polar effects of the isiA and isiB disruptions on the isiC expression. All the mutants were highly sensitive to oxidative stress caused by the presence of methyl viologen, indicating an essential role of the operon, especially isiC, under oxidative stress. The isiA mutant was much more susceptible to the sublethal heat stress than the other mutants. Determination of the survival rate after a lethal temperature treatment confirmed the essential role of the isiA gene for the thermal stress management. We found that light conditions immediately after the lethal temperature treatment have a great effect on the survival rate of the wild-type and the isiA mutant strains. We suggest that IsiA and IsiB may play a role in optimizing the light energy supply to avoid cellular damage and utilizing the light energy in the post-heat-stress recovery process.
Abbreviations –
-
- LDS-PAGE
-
- lithium dodecyl sulfate-polyacrylamide gel electrophoresis
-
- mRNA
-
- messenger RNA
-
- WT
-
- wild-type
Introduction
In a cell, iron is essential for redox reactions of important metabolic pathways such as photosynthetic and respiratory electron transports (Straus 1994). However, the low solubility of Fe3+ above neutral pH limits the biological availability of iron in oxygenic, aquatic ecosystems (Straus 1994). As a result, it is important for aquatic microorganisms to cope with iron-limited conditions. It is well established that cyanobacteria respond to iron limitation by inducing the expression of the isi (iron stress inducible) genes, isiA (Laudenbach and Straus 1988) and isiB (Laudenbach et al. 1988) in response to the iron starvation. In some cyanobacteria such as Synechocystis sp. strain PCC 6803, Synechococcus sp. strain PCC 7942 and Synechococcus sp. strain PCC 7002, the isiA and isiB genes constitute an operon (Burnap et al. 1993, Laudenbach and Straus 1988, Leonhardt and Straus 1992, Straus 1994), while they are dispersed on the Anabaena sp. strain PCC 7120 genome (Leonhardt and Straus 1994). In some cyanobacteria such as the thermophilic cyanobacterium Thermosynechococcus elongatus BP-1, only the isiA homolog is conserved, while neither isiA nor isiB is found in the Synechococcus sp. strain WH 8102 genome (Cyanobase, http://www.kazusa.or.jp/cyanobase/). Expression of the isiAB operon in Synechococcus sp. strain PCC 7942 and Synechocystis sp. strain PCC 6803 has been suggested to be regulated by the Fur repressor (Ghassemian and Straus 1996, Kunert et al. 2003). However, the Fur repressor is not the only regulator for expression of the genes (Kunert et al. 2003).
The isiA gene encodes IsiA or CP43′, a homolog of the CP43 chlorophyll-binding protein (Burnap et al. 1993). IsiA proteins are arranged in ring structures in the periphery of photosystem I (Bibby et al. 2001, Boekema et al. 2001). Proposed functions for IsiA are antennas for photosystems and light energy dissipation in photosystem II (Cadoret et al. 2004, Havaux et al. 2005, Ihalainen et al. 2005, Park et al. 1999, Sandström et al. 2001).
The isiB gene encodes a flavodoxin, which acts as an electron acceptor from the photosystem I complex and may substitute for ferredoxin under iron-limited conditions (Straus 1994). In Escherichia coli, flavodoxins encoded by the fldA and fldB genes are members of the soxRS (superoxide response) regulon that is involved in the oxidative stress as well as the iron-limited stress (Gaudu and Weiss 2000, Zheng et al. 1999). Flavodoxin I encoded by fldA is an essential gene under both aerobic and anaerobic conditions in E. coli (Gaudu and Weiss 2000). This is in contrast with the fact that the isiB mutants of Synechocystis and Synechococcus are viable under normal growth conditions (Burnap et al. 1993, Hagemann et al. 1999, Kutzki et al. 1998), although there is no additional isiB homolog gene in these cyanobacteria (Kaneko et al. 1996). As described above, T. elongatus BP-1 does not have a flavodoxin homolog. Thus, it appears that flavodoxin in cyanobacteria plays a physiological role that is different from that in E. coli.
sll0249, designed as isiC in this study, is an open reading frame with unknown function which is located immediate downstream of isiB (Fig. 1A). The sll0249 homologous gene is conserved in Anabaena sp. and Synechococcus sp. (Cyanobase, http://www.kazusa.or.jp/cyanobase/, CYORF, http://cyano.genome.jp). These open reading frames are also located immediate downstream of isiB. Although sll0249 has motifs typical of a thioesterase (Singh et al. 2004), no study has been done on this gene.
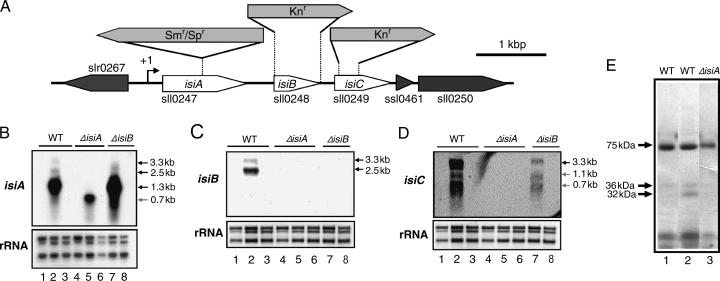
Construction of clones for the interruption and deletion mutants of the isiA, isiB and isiC genes from Synechocystis sp. PCC 6803 (A) and their expression under iron-replete and iron-limited conditions (B, C, D, E). (A) Genetic organization of the isiABC operon of the Synechocystis sp. strain PCC 6803 genome. To interrupt the isiA gene, the 2.0-kb omega fragment (Smr/Spr) was inserted into a site located 494 bp downstream of the isiA initiation codon in the opposite gene orientation as that of isiA. For the construction of the isiB and isiC deletion mutants a 0.54-kb fragment containing the entire coding region of isiB, and a fragment located from 86 to 316 bp downstream of the sll0249 initiation codon, respectively, were replaced with the 1.3-kb kanamycin-resistant gene cassette (Knr). An arrow with +1 indicates the transcriptional initiation site of the isiABC operon. (B–D) Northern blot analysis of the isiA, isiB and isiC genes in the wild-type (WT), the isiA mutant (ΔisiA) and the isiB mutant (ΔisiB). Cells in a 50-ml culture were grown at 30°C under a light intensity of 20 μE m−2 s−1 for 48 h. After a 15-ml aliquot of the culture was collected for RNA preparation (lanes 1 and 4), cells of the remaining culture were precipitated by centrifugation, washed with H2O, transferred to a 50-ml BG-11 medium containing no ferric ammonium citrate and grown at 30°C under a light intensity of 20 μE m−2 s−1 for 48 h (lanes 2, 5, 7). After a 15-ml aliquot of the culture was collected, ferric ammonium citrate (at the final concentration of 6 μg ml−1) was added to the remaining culture. Cells were grown under the same conditions for 48 h and collected for RNA preparation (lanes 3, 6, 8). 2.8 kb, 2.3 kb and 1.3 kb ribosomal RNAs on nylon membrane were detected by the methylene blue staining, and shown as references for the amount of RNA loaded to each lane. The size of the detected messenger RNA shown in the figures was estimated by using an RNA ladder (Gibco-BRL). (E) Lithium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of chlorophyll-binding proteins of thylakoid-enriched fraction from the WT (lanes 1 and 2) and the isiA mutant (ΔisiA, lane 3) cells grown under iron-replete (lane 1) and iron-limited (lanes 2 and 3) conditions.
Recently, it was shown that cyanobacterial isiA and isiB genes are induced in response to high salt, high temperature (Vinnemeier et al. 1998), oxidative (Jeanjean et al. 2003, Yousef et al. 2003) and high light stresses (Havaux et al. 2005), as well as iron-limited stress, suggesting that the isiAB operon plays a role not only under iron stress but also under other stresses. Even under normal conditions in iron-replete media, transcription from the isiAB promoter is induced transiently in the midexponential phase of growth (Durham et al. 2002). Thus, the isiAB operon may play a role during a particular growth phase under normal growth conditions. However, there is no study that evaluates the importance of these genes in vivo for acclimation/adaptation of cells against stresses other than iron and high light stresses. Thus, we compared the phenotype of the wild-type strain with the isiA and isiB mutants of Synechocystis sp. strain PCC 6803 under heat and oxidative stresses as well as iron-limited stress to reveal roles of these genes.
Materials and methods
Organisms and culture conditions
Synechocystis sp. PCC 6803 cells were cultured at 30°C under a light intensity of 20 μE m−2 s−1 in liquid BG-11 inorganic medium or on BG-11 plates containing 1.5% (w/v) agar and 0.3% (w/v) sodium thiosulfate (Rippka et al. 1979). The liquid cultures were continuously aerated. The BG-11 contained ferric ammonium citrate (6 μg ml−1) as the sole iron source. For growth of the isiA mutant, both spectinomycin dihydrochloride and streptomycin sulfate (3 μg ml−1 each) were added, while kanamycin sulfate (20 μg ml−1) was added to the culture media for the isiB and the isiC mutants. To induce iron limitation, exponentially growing cells were harvested by centrifugation and suspended in H2O. The cell suspension was centrifuged and resuspended in a small amount of BG-11 medium containing no ferric ammonium citrate and used to inoculate medium containing no ferric ammonium citrate.
Construction of the isiA-, isiB- and isiC-mutants
The isiA gene was disrupted by inserting an omega fragment into a site located 494-bp downstream of the initiation codon of isiA (Fig. 1A). A 0.6-kb DNA fragment containing either the 5′-half or 3′-half of the isiA gene was amplified from the genomic DNA of Synechocystis sp. PCC 6803 by PCR with a set of sense and antisense primers, either isiA1/isiA2 or isiA3/isiA4 (Table 1). The sequences of the oligonucleotide primers were modified to contain restriction sites as the SalI site in isiA1, the EcoRI sites in isiA2 and isiA3, and XbaI site in isiA4. The PCR products were digested by restriction enzymes at the corresponding restriction sites and cloned into pBluescript II KS (−) (Stratagene, La Jolla, CA, USA). The ligated 1.2-kb DNA fragment contains the coding region, and upstream/downstream regions of the isiA gene. The resulting plasmid was digested by EcoRI and the omega fragment (Prentki and Krisch 1984), bearing a streptomycin and spectinomycin resistance gene, was inserted in the reverse direction (the opposite gene orientation as that of isiA) into the EcoRI site.
| Name | Sequence (5′–3′)a | Strand | Siteb |
|---|---|---|---|
| isiA1 | CGGGTcGACAGAGAACGCTTTA | + | SalI |
| isiA2 | TCGTAgAATtCCCCCCCAATACA | − | EcoRI |
| isiA3 | TTGGGGGGaATTcTACGACG | + | EcoRI |
| isiA4 | GCAGAtCTAGaCAAAGTAAA | − | XbaI |
| isiA5 | CAACGACACCGTTCAGTACG | + | |
| isiA6-T3c | AATTAACCCTCACTAAAGGGCCTGCCAACAGTAGAGCTCC | − | |
| isiB1 | TCACTTCTTCCTGGCCTTTTTC | + | |
| isiB2 | ACACCAACGGAGAGTAGGGA | − | |
| isiB3 | GACAAAAATTGGACTTT | + | |
| isiB4 | GGATTGCAAAATTGGTT | − | |
| isiB5 | GGCAACACTGAAACCATTGC | + | |
| isiB6-T3c | AATTAACCCTCACTAAAGGGAAATCATAGCCAGCGGTGGG | − | |
| isiC1 | GCGGCGTTTTGATCGTCCTC | + | |
| isiC2-T3c | AATTAACCCTCACTAAAGGGAATGGGCTTGCCAGTCGAGG | − | |
| isiC3 | AGCTTTTGCCGGCTGATCAC | + | |
| isiC4_Kn | GATCGTCCTCTACCGAATTCCCCGGATCCGTCG | + | |
| isiC5_Kn | CCGGGGAATTCGGTAGAGGACGATCAAAACGCC | − | |
| isiC6_Kn | GAGTTTTTCTAACTGCCCTCGACTGGCAAGCC | + | |
| isiC7_Kn | CCAGTCGAGGGCAGTTAGAAAAACTCATCGAG | − | |
| isiC8 | AGTTGACAACCGTCGGCAAC | − | |
| slr0267_1 | TCCAATGGTATCCCGGGCAC | + | |
| slr0267_2-T3c | AATTAACCCTCACTAAAGGGGCAACTCCGGCGTGATCATG | − |
For the construction of the isiB deletion mutant (Fig. 1A), a 1.5-kb DNA fragment containing the isiB gene was amplified from the genomic DNA of Synechocystis sp. PCC 6803 by PCR with the two primers, isiB1 and isiB2 (Table 1) and cloned into pGEM-T vector (Promega, Madison, WI, USA). The resulting plasmid was digested by HindIII, which removed a 0.54-kb fragment containing the entire isiB-coding region. The isiB-coding region was replaced by a 1.3-kb kanamycin-resistant gene cassette isolated from plasmid pUC4K (Amersham Pharmacia Biotech, Piscataway, NJ, USA).
For the construction of the sll0249 (isiC) deletion mutant (Fig. 1A), a 0.31-kb fragment (from −226 to +85 as numbered from the translation initiation site of sll0249), a 0.53-kb fragment (from +317 to +846 as numbered from the translation initiation site of sll0249) or a 0.97-kb pUC4K fragment (from −149 to +816 as numbered from the translation initiation site of the kanamycin-resistant gene) was amplified from the Synechocystis sp. PCC 6803 genomic DNA or pUC4K by PCR with pairs of sense and antisense primers (Table 1), isiC3/isiC5_Kn, isiC6_Kn/isiC8 and isiC4_Kn/isiC7_Kn, respectively. The three DNA fragments were mixed at equimolecular ratio, heat denatured, annealed and extended by a DNA polymerase, resulting in fusion of the 0.31-kb sll0249 fragment to the 0.97-kb pUC4K fragment, and the 0.53-kb sll0249 fragment to the 0.97-kb pUC4K fragment. Then, the mixture containing the fused fragments was heat denatured, annealed and amplified by a DNA polymerase with a pair of primers, isiC3/isiC8 (Table 1), resulting in an isiC deletion construct. The amplified DNA was cloned into a pT7Blue T-vector (Novagen, Madison, WI, USA).
These constructs were used to transform cells of Synechocystis sp. PCC 6803 through homologous recombination (Golden et al. 1987). Integration and complete segregation of each construct within the mutant genome were tested by Southern blot analysis and/or PCR.
Measurements of whole cell absorption spectra, and the amount of photosynthetic pigments
Whole-cell absorbance was measured at room temperature with a Hitachi 557 double wavelength double beam spectrophotometer (Hitachi Koki, Tokyo, Japan). The amount of chlorophyll, phycocyanin or carotenoid was determined as described previously (Arnon et al. 1974, Hirschberg and Chamovitz 1994).
Preparation of thylakoid-enriched fraction
Cells in 60- or 500-ml culture were harvested by centrifugation at room temperature. The cells were suspended in five volumes of extraction medium (10 mM Tris–HCl, pH 8.0, 1 mM ethylenediaminetetraacetic acid and 1 mM of phenylmethylsulfonyl fluoride). All of the following procedures were carried out at 4°C unless stated otherwise. The cell suspension was mixed with three volumes of glass beads (Sigma, St. Louis, MO, USA) and disrupted by vortexing vigorously for 3 min. This process was repeated three times with a 3-min interval on ice between the vortexing, and the resulting suspension was centrifuged at less than 1000 g for 10 min. The supernatant, which did not contain glass beads, was centrifuged at 16 000 g for 30 min. The pellet (thylakoid-enriched fraction) was washed once with the extraction medium and suspended in a minimum volume (less than 500 μl) of the extraction medium.
Electrophoresis of proteins
Lithium dodecyl sulfate-polyacrylamide gel electrophoresis (LDS-PAGE) was performed as described previously (Delepelaire and Chua 1979). The size of a protein was determined according to molecular weight markers (Amersham Pharmacia Biotech).
Viability assay
Aliquots of a culture were diluted to an optical density of 0.1 at 730 nm, and then serially diluted in fresh, sterile BG-11 medium. A 5-μl aliquot from each dilution was then spotted onto a BG-11 plate, and the plate incubated at 30°C under a light intensity of 20 μE m−2 s−1 for 1 week. Cell survival was determined by counting the number of colonies in the most and second most diluted cultures and multiplying their average by the appropriate dilution factor.
Preparation of total RNAs and Northern blot analysis
Total RNA was prepared as described previously (Nakamoto et al. 2003). One to 2 μg of the total RNA was electrophoresed on a denaturing 1.5% (w/v) agarose gel containing 6.6% (w/v) formaldehyde, blotted to positively charged nylon membrane (Roche, Basel, Switzerland) by the capillary transfer method, and hybridized with a RNA probe at 70°C (Nakamoto et al. 2003). The chemiluminescent signal from each probe was detected by exposing the blot to X-ray film (Fuji-film) or to a chemiluminescence analyzer (BioRad, Hercules, CA, USA).
A gene-specific probe for detection of the corresponding RNA from Synechocystis sp. PCC 6803 was prepared as previously reported (Nakamoto et al. 2003). Digoxigenin-labeled complementary RNA for the gene-specific probe was synthesized in vitro by T3 RNA polymerase with a template DNA fragment amplified with primers (Table 1 and Nakamoto et al. 2003).
Results and discussion
Construction of the isiA insertion mutant and the isiB deletion mutant
To elucidate a role of the isiA gene under various stress conditions, an isiA-interruption was constructed (Fig. 1A). To characterize a role of the isiB gene under stress conditions and also specify the phenotype of the isiA mutant because the insertion inactivation of the isiA gene brought about a pleiotropic effect as described below, we constructed an isiB-deletion mutant (Fig. 1A).
Integration and complete segregation of either the isiA-interruption or the isiB-deletion construct within the mutant genome were confirmed by Southern blot analysis (data not shown). These results indicate that isiA and isiB are dispensable for the cyanobacterial growth under the iron-replete standard growth conditions employed in the present study, although the isiA mutant showed a slightly lower growth rate under iron-replete conditions than the wild-type (see 3, 4).
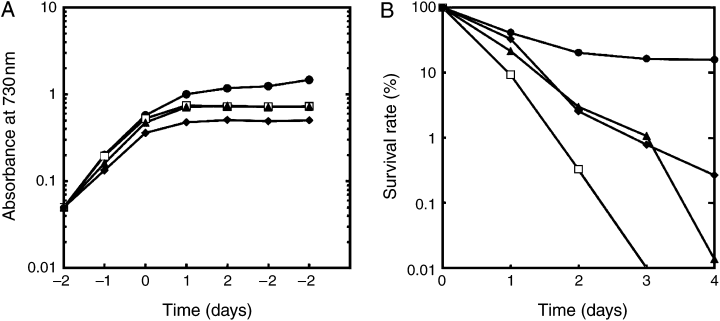
Characterization of phenotype of various strains incubated in the presence of methyl viologen. Cells were grown in the presence of iron at 30°C under a light intensity of 20 μE m−2 s−1. Methyl viologen at the final concentration of 5 μM was added to cells at time “0,” and then growth (A) and survival rate (B) were measured. Each value shows the mean of six independent replicate experiments with all the strains except the isiC mutant. Data with the isiC mutant are presented as the mean of three independent replicate experiments. Symbols of circle, diamond, triangle and open square indicate the wild-type, the isiA mutant, the isiB mutant and the isiC mutant, respectively.
Characterization of phenotype of various strains incubated at 42°C, a sublethal temperature. Cells of a 70-ml culture growing in the presence of iron at 30°C under a light intensity of 20 μE m−2 s−1 were shifted to 42°C at time “0,” and then growth (A), survival rate (B) and cellular contents of pigments (C–E) were measured. Each value in A and B shows the mean of six independent replicate experiments with all the strains except the isiC mutant. Data with the isiC mutant are presented as the mean of four independent replicate experiments. Each value in (C–E) shows the mean of two independent replicate experiments. Symbols of circle, diamond, triangle and open square indicate the wild-type, the isiA mutant, the isiB mutant and the isiC mutant, respectively.
Expression of the isiABC operon and characterization of the isiA and isiB mutants under iron-limited conditions
It was reported that the isiA and isiB genes produce a bicistronic mRNA in Synechocystis sp. PCC 6803 (Vinnemeier et al. 1998) as in the case of Synechococcus sp. PCC 7942 (Laudenbach and Straus 1988) and Synechococcus sp. PCC 7002 (Leonhardt and Straus 1992). The transcription of the isiAB operon is tightly regulated by iron. Northern blot analysis was carried out to confirm that the isiAB operon is indeed expressed under our experimental conditions. For iron limitation, we transferred cells grown in a preculture containing ferric ammonium citrate (6 μg ml−1) as the only iron source (designated as an “iron-replete” medium) to medium containing no ferric ammonium citrate (designated as “iron-limited” medium). We designated the medium as iron-limited medium rather than iron-deficient medium because trace amounts of iron may be present in nutrient components of BG-11 other than ferric ammonium citrate. The transcriptional analysis with the isiA-specific probe revealed one distinct 1.3-kb mRNA in total RNA from wild-type cells grown in iron-limited medium for 48 h (Fig. 1B, lane 2). In addition to the major band, we detected minor bands of 3.3 and 2.5 kb by Northern blot analysis. The isiB-specific probe hybridized with the 2.5-kb mRNA (Fig. 1C, lane 2), indicating that it is the cotranscribed product of the isiA and isiB genes as reported previously (Vinnemeier et al. 1998). The intensity of the 2.5-kb signal was weak, because the filters incubated with the isiB-specific probe were exposed more than twice as long as the filters with the isiA-specific probe. Our results, as well as those obtained previously with cells grown under iron limitation (Laudenbach and Straus 1988, Leonhardt and Straus 1992, Vinnemeier et al. 1998), suggest that the isiA gene may be preferentially transcribed as a monocistronic mRNA of 1.3-kb or the 1.3-kb mRNA may be a stable processed product of the 2.5-kb mRNA. We further examined whether the 3.3-kb mRNA that produced a weak signal in the Northern blot analysis is the cotranscribed product of isiA, isiB and sll0249. Both the isiB-specific probe and the sll0249-specific probe hybridized with the 3.3-kb mRNA (Fig. 1C and D, lane 2). The transcription for sll0249 as well as that for isiB appears to start from the promoter(s) upstream of the isiA gene because the interruption of isiA by an antibiotic resistance gene resulted in total disappearance of the isiB and sll0249 mRNAs (Fig. 1C and D, lane 5). These results strongly suggest that the 3.3-kb mRNA is the cotranscribed product of isiA, isiB and sll0249. This gene is induced by iron limitation as shown below. Thus, we designated the gene as isiC. The sll0249-specific probe also hybridized with the 1.1- and 0.7-kb mRNAs (Fig. 1D, lane 2), which may be degradation/processed products of the 3.3-kb mRNA. Alternatively, these signals could be explained to be single transcripts of sll0249 (0.7-kb signal) or joint transcripts with downstream ssl0461 (1.1-kb signal). However, this possibility is not well supported by the fact that the interruption of isiA by an antibiotic resistance gene resulted in total disappearance of all the signals (Fig. 1D, lane 5), although we cannot exclude indirect effects of the isiA interruption that result in suppression of the expression of the potential sll0249–ssl0461 operon.
The isiA, isiB and isiC transcripts were not detected under iron-replete conditions, or 48 h following a shift to iron-replete medium from iron-limited medium (Fig. 1B–D, lanes 1 and 3), confirming that the isiABC operon is tightly regulated by the iron concentration. These results are consistent with the microarray analysis that showed all these genes are upregulated under iron starvation (Singh et al. 2003).
We examined whether the genetic manipulation in the isiA and isiB genes exerts any effect on the induction of isiA, isiB and isiC by iron limitation. In the isiA-interruption mutant, we detected a 0.7-kb mRNA with the isiA-specific probe instead of the 2.5- and 1.3-kb mRNAs (Fig. 1B, lane 5). No RNA hybridizing with either the isiB-specific probe or the isiC-specific probe was detectable in total RNA from the isiA mutant (Fig. 1C and D, lane 5). Thus, the 0.7-kb mRNA corresponds to a truncated isiA mRNA because of the insertion of the antibiotic resistance gene. The predicted size of the truncated mRNA from the transcriptional start site (Vinnemeier et al. 1998) to the interruption site is 705 nucleotides. The 0.7-kb mRNA was undetectable both under iron-replete conditions and after a shift to iron-replete conditions from iron-limited conditions (Fig. 1B, lanes 4 and 6). The results indicate that the interruption of the isiA coding region does not affect its transcriptional activity significantly under iron-replete and -limited conditions. To check whether or not a truncated IsiA protein is made, insoluble fractions of cell extracts were analyzed to detect chlorophyll-binding proteins by LDS-PAGE. Membranes solubilized with 1.0% (w/v) LDS were resolved into several green bands on a non-stained 5–15% gradient acrylamide gel (Fig. 1E). After 45 h of iron limitation, a 32-kDa chlorophyll-binding polypeptide accumulated greatly in the wild-type cells (Fig. 1E, lane 2). It was absent in the isiA mutant (Fig. 1E, lane 3). It was the only chlorophyll-binding protein in the wild-type strain that was induced under iron limitation. The size of the chlorophyll-binding protein corresponds well to the size of IsiA estimated by sodium dodecyl sulfate-PAGE (Bibby et al. 2001). Thus, we concluded that the 32-kDa band corresponds to the IsiA polypeptide. We could not detect any chlorophyll-binding polypeptide, which is present in the mutant, but absent in the wild-type strain. It indicates that a truncated IsiA protein does not accumulate in the isiA mutant. The truncated protein may be unstable in a cell.
No RNA hybridizing with the isiB-specific probe was detectable in total RNA from the isiB mutant grown in iron-replete or iron-limited medium (Fig. 1C, lanes 7 and 8), confirming the deletion of the isiB gene. The mutant did produce the isiA mRNA of 1.3 kb (Fig. 1B, lane 7). Thus, the isiB deletion does not interfere with the transcription of the isiA gene under iron-limited conditions. The isiC mRNAs were detected in the isiB mutant, although their levels were much more reduced than in the wild-type (Fig. 1D, lane 7). We think that some transcription through the kanamycin resistance gene may occur to produce these mRNAs.
We characterized the wild-type, isiA- and isiB-mutant cells grown for 45 h in iron-limited medium. There was no significant difference in both growth rate and the cellular content of chlorophyll among the wild-type and mutant strains (data not shown). One of the characteristics in iron-stressed cyanobacterial cells is a wavelength shift of the chlorophyll a absorption peak from 678 to 670 nm (Öquist 1974). The “blue shift” became evident when the wild-type cells were cultured for 35 h in iron-limited medium. The wavelength shift of chlorophyll absorption was directly related to the expression of the isiA gene, as the isiA-mutant cells failed to induce the shift when grown for 45 h under iron limitation (data not shown) as in the case of Synechococcus sp. PCC 7942 (Burnap et al. 1993). The isiB-mutant cells showed the same shift as the wild-type, excluding the possible pleiotropic effect that the absence of the blue shift in the isiA-mutant cells is caused by the transcriptional inactivation of the isiB gene. The results also support that the isiB deletion does not interfere with the transcription/translation of the isiA gene.
Characterization of the isiA, isiB and isiC mutants under oxidative stress
As described in the introduction, IsiA (CP43′) was proposed to protect the photosystem against oxidative stress caused by excess light under iron-limited conditions (Park et al. 1999, Sandström et al. 2001, Yousef et al. 2003) and against high light stress under iron-replete conditions (Havaux et al. 2005). In E. coli, flavodoxins (encoded by fldA and fldB genes) are members of SoxRS regulon, which is involved in oxidative stress (Gaudu and Weiss 2000, Zheng et al. 1999). To elucidate a role of IsiA and/or IsiB in protection of cells from oxidative stress, we examined the effect of methyl viologen, which is known to produce active oxygen when it is reduced by process photosynthetic electron transport (Fujii et al. 1990).
It has been reported that the isiA mRNA accumulates when cells were cultured in the presence of 100 μM methyl viologen under a light intensity of 60 μE m−2 s−1 (Jeanjean et al. 2003) or in the presence of 50 μM methyl viologen under a light intensity of 120 μE m−2 s−1 (Yousef et al. 2003). On the other hand, DNA microarray analysis did not detect significant induction of the isiAB genes in the presence of 10 μM methyl viologen under light intensities of 50 or 200 μE m−2 s−1, although it revealed that one of the Fur homologs is induced (Kobayashi et al. 2004). In the present study, we analyzed phenotypes at lower concentration of methyl viologen (5 μM) and weaker light intensity (20 μE m−2 s−1) than those used in the previous reports. Methyl viologen whose concentration is higher than 5 μM is lethal to wild-type cells even under weak light intensities less than 50 μE m−2 s−1 (data not shown).
First, we analyzed the expression of the isiA gene in response to oxidative stress in the presence of methyl viologen. The isiA mRNA was detected 4 h after the addition of methyl viologen to the wild-type culture, however, in a much lower level than that detected under iron-limited conditions (Fig. 2). Its level increased slightly after 18 h. Similar to the wild-type isiA mRNA, the truncated isiA mRNA was detected in the isiA mutant 4 h after the addition of methyl viologen, indicating that the isiA mutant also induced the isiA gene, however, to a much higher extent after 18 h than the wild-type (Fig. 2). The isiA induction in the isiB mutant was remarkably strong as compared with the wild-type and isiA mutant strains (Fig. 2), which took place less than 30 min after the methyl viologen addition. We could not detect the blue shift in the whole-cell absorption spectra of the wild-type and the isiB mutant, indicating that induction of CP43′ was not significantly induced under these conditions. Yousef et al. (2003) also reported that they could not detect the shift in the wild-type grown in the presence of 20 mM H2O2, although H2O2 induced expression of the isiA and isiB genes. Similarly, Li et al. (2004) demonstrated high levels of isiA transcription following peroxide treatment, such treatment failed to yield accumulation of IsiA protein to a significant level.
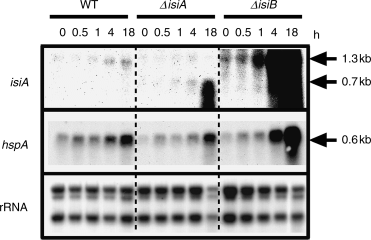
Expression of the isiA and hspA genes in the presence of methyl viologen. Methyl viologen at the final concentration of 5 μM was added to cells of the wild-type (WT), the isiA mutant (ΔisiA) and the isiB mutant (ΔisiB), in a 70-ml iron-replete culture growing at 30°C under a light intensity of 20 μE m−2 s−1. Fifteen-milliliter aliquots of the culture were collected for RNA preparation prior to (0 min) and after incubation with methyl viologen for 0.5, 1, 4 and 18 h. One microgram of total RNA isolated from cells was used for Northern blotting. The gene whose messenger RNA was detected was indicated at the left of each panel. 2.8-kb, 2.3-kb and 1.3-kb ribosomal RNAs on nylon membrane were detected by the methylene blue staining, and shown as references for the amount of RNA loaded to each lane.
Small heat shock proteins have been shown to increase resistance to oxidative stress at least in eukaryotic cells (Arrigo et al. 2001), indicating that they play an important role for the cell’s tolerance to oxidative stress. Thus, we measured hspA expression to confirm that cells are experiencing the oxidative stress under the present experimental conditions, and to examine whether there is any difference in the expression of this stress gene among the different strains. The wild-type and the isiA mutant accumulated a significant level of the hspA mRNA 18 h after the methyl viologen addition (Fig. 2), while the isiB mutant accumulated at much higher level after 18 h (Fig. 2). These results suggest that the isiB mutant experienced a higher level of oxidative stress than the wild and isiA mutant strains. Perhaps under these conditions, the presence of IsiA alone may yield increased HspA synthesis/production.
To evaluate the importance of the isiA and isiB genes in the cell’s adaptation to methyl viologen stress, we compared the phenotype of the wild-type strain with that of the isiA or the isiB mutant. By the addition of methyl viologen, both mutants ceased growing after 1 day, whereas the wild-type continued growth at decreased rates for 4 days (Fig. 3A). The viability of all the mutants decreased to less than 10% within 2 days, while that of the wild-type decreased by only 50% (Fig. 3B).
Under oxidative conditions, photosynthesis pigment quantities of these mutant strains were greatly decreased (data not shown). Both carotenoid and chlorophyll contents started decreasing without a lag period in both mutants, while there was a lag of 2 days before the significant reduction of phycocyanin. Thus, the cell color of the mutants became blue 3 days after the methyl viologen addition. In contrast to the mutants, the time course of the reduction of all the pigments in the wild-type showed a longer lag period. After 4 days, all the pigments in the isiA and isiB mutants diminished totally, resulting in transparent cultures, while the wild-type strain retained 40–50% of chlorophyll and phycocyanin contents of the level prior to the methyl viologen addition.
We performed the same set of experiments as those for which the results are described above with cells grown under iron-limited conditions (data not shown). The iron-starved cells of all the strains were much more sensitive to methyl viologen stress than the iron-replete cells. The wild-type itself under iron-limited conditions was as sensitive as the isiA and isiB mutants grown under iron-replete conditions in the presence of methyl viologen. The presence of methyl viologen in the iron-limited medium was highly lethal to the isiA and isiB mutants. Thus, we found that the combination of oxidative and iron-limited stresses has a strong synergistic and damaging effect on Synechocystis cells.
Our results seem to be contrary to earlier findings (Havaux et al. 2005) that only the isiA mutant was sensitive to high light, while the isiB mutant behaved like the wild-type cells. They showed that genetic deletion of isiAB resulted in a photosensitive phenotype with loss of culture coloration under high light conditions, while the isiB null mutant expressing isiA was phototolerant. Cell survival was not measured in their experiments. It is generally accepted that both high light and methyl viologen produce reactive oxygens. However, cells may not respond to methyl viologen stress in the same way as they do to high light stress. DNA microarray analysis showed that a number of genes induced by high light were not induced by the treatment of methyl viologen under conditions of low or high light (Kobayashi et al. 2004). It is also possible that levels of reactive oxygens generated might differ significantly for our and their experiments. They showed that the growth rate of the wild-type and the isiA mutant after transfer to high light was not greatly different, while growth of both mutants was inhibited in our experiments. Finally, small changes in experimental conditions/procedures also affect the mutant’s sensitivity to stress. As shown in Fig. 6B and C, the isiA and isiB mutant’s high-temperature sensitivity is greatly affected by a small change in light intensity during incubation after heat treatment of cells.
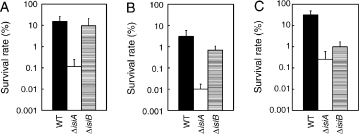
Survival rates after a lethal heat shock treatment. Cells of the wild-type (black column, indicated by WT), the isiA mutant (open column, ΔisiA) and the isiB mutant (striped column, ΔisiA) grown at 30°C under iron-limited conditions (A) or iron-replete conditions (B, C) were directly shifted to 48°C for 15 min under the same light intensity as prior to the shift (20 μE m−2 s−1). After the lethal temperature treatment, cells were spotted onto a BG-11 plate, and the plate was incubated at 30°C under a light intensity of either 20 μE m−2 s−1 (A, B) or 5 μE m−2 s−1 (C) for 1 week. For all treatments, the number of cells present before the shift was taken as 100%. Each value shows the mean and standard deviation of three independent replicate experiments.
To examine the possibility of a polar effect of the isiA and isiB disruptions on the immediate downstream gene isiC, we made a knockout mutation in isiC (Fig. 1A). Northern blot analysis showed that the mutation did not have any effect on the isiA and isiB expressions under iron-limited conditions (data not shown). No RNA hybridizing with the isiC-specific probe was detectable in total RNA from the isiC mutant grown in iron-limited medium, consistent with the deletion of the isiC gene.
The isiC mutant ceased growing 1 day after the addition of methyl viologen as other mutants did (Fig. 3A). Surprisingly, the mutant lost viability more rapidly than the isiA and isiB mutants (Fig. 3B). Comparing Fig. 3A with Fig. 3B, one might ask why 1 day after methyl viologen addition at least twice as many cells of the isiC mutant are already dead, while the isiC culture shows the same growth rate as that with the isiB-mutant cells. We think that methyl viologen-treated cells may divide several times to increase apparent absorbance of liquid culture at 730 nm, however, they stop dividing before forming a visible colony on an agar plate.
The isiC transcription is inactivated, depressed and interrupted in the isiA, isiB and isiC mutants, respectively. Thus, IsiC appears to play a particularly important role among the operon products. Expression of the isiA and/or isiB genes in the absence of IsiC may not be beneficial to cells may be rather toxic because the isiC mutant showed higher sensitivity to methyl viologen stress than the isiA mutant. This is the first report to show an essential role of IsiC under oxidative stress, although the function of IsiC remains to be elucidated.
Characterization of the isiA, isiB, and isiC mutants under sublethal high-temperature stress
Vinnemeier et al. (1998) reported that the isiAB genes in Synechocystis sp. strain PCC 6803 is induced upon heat shock at 42°C for 3 h, although the induction level is much lower than that under the iron-limited conditions. This suggests that the isiABC operon plays a role under heat stress as well as iron-limited and oxidative stresses. To evaluate the importance of the operon under heat shock conditions, we examined whether the disruption of isiA, isiB and/or isiC gene has any effect on the growth of Synechocystis sp. strain PCC 6803 at a moderately high temperature, 42°C. We used low light (20 μE m−2 s−1) during high-temperature treatment to avoid high light stress because the isiA mutant is sensitive to high light (Havaux et al. 2005).
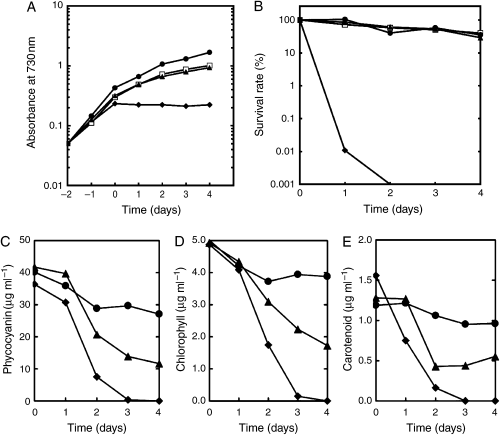
We found that the isiA mutant is highly sensitive to high-temperature stress. It stopped growing at 42°C, while the wild-type could grow (Fig. 4A). The isiB and isiC mutants continued growth, but at a slightly slower growth rate than the wild-type. After 4 days at 42°C, the survival rates of the wild-type, isiB and isiC mutant strains decreased to 35%, 29% and 40% respectively, while that of the isiA mutant was 0.001% after incubation for 2 days (Fig. 4B). We observed dramatic changes in cell pigmentation in the isiA mutant when the mutant was grown at 42°C. In contrast to the color change from blue-green to blue in the presence of methyl viologen, the color of the isiA mutant became chlorotic, distinguishing the effect of high-temperature stress from that of the methyl viologen-inducing oxidative stress on the mutant’s phenotype. To quantify the phenotype, the level of pigments was measured (Fig. 4C–E). As shown in Fig. 4A and B, the isiC mutant showed a similar phenotype to the isiB mutant under the heat stress. Thus, we did not perform further analysis with the isiC mutant. All the pigments in the isiA and the isiB mutants decreased 1 day after the shift to 42°C, while those of the wild-type did not change their levels significantly for 4 days at 42°C. In the case of the isiA mutant, it appeared that carotenoid content decreased first, followed by the reduction of both phycocyanin and chlorophyll contents. After incubation for 3–4 days, the spectrum of whole cell absorbance of the isiA mutant did not show any peaks by those pigments (data not shown), which resulted in totally transparent cultures, and that of the isiB mutant resulted in a yellow green color. The pigment analysis not only confirmed the essential role of IsiA under heat stress, but also revealed significant contribution by IsiB and/or IsiC at least in maintaining the pigment level (Fig. 4C–E). These results support the physiological importance of the isiABC operon, especially the isiA gene, under heat stress. In this respect, it is intriguing that only the isiA homolog is conserved in the thermophilic cyanobacterium T. elongatus BP-1.
As shown in Fig. 5, there was no detectable induction of the isiABC operon in the wild-type and the isiB mutant at 42°C. However, the isiA mutant accumulated the truncated isiA mRNA significantly within 30 min upon a temperature up-shift and further increased the level during the stress (Fig. 5), indicating that the isiABC operon is indeed induced under heat stress. The loss of the isiA translation product may trigger the expression of the isiABC operon by an unknown mechanism, or it may stabilize transcripts of the operon. The physiological interpretation for the results is that the mutant requires enhanced IsiA synthesis under the heat stress, indicating its important role. We think that the level of the isiA mRNA was below a detection limit because of the low light intensity during high-temperature treatment.
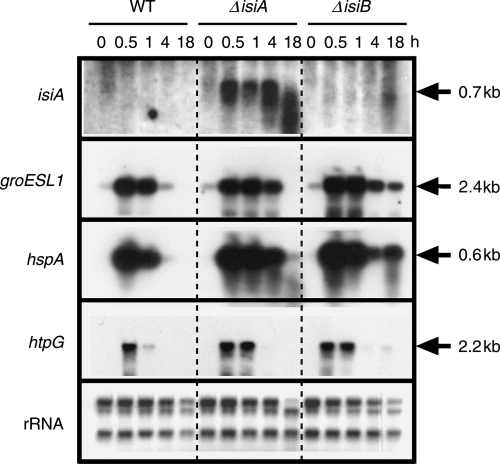
Expression of the isiA, groESL1, hspA and htpG genes upon a shift of cells from 30°C to 42°C. Cells of the wild-type (WT), the isiA mutant (ΔisiA) and the isiB mutant (ΔisiB), in a 70-ml culture growing in the presence of iron at 30°C under a light intensity of 20 μE m−2 s−1 were shifted to 42°C. Fifteen-milliliter aliquots of the culture were collected prior to (0 min) and after incubation at 42°C for 0.5, 1, 4 and 18 h. One microgram of total RNA isolated from cells was used for Northern blotting. The gene whose messenger RNA was detected was indicated at the left of each panel. 2.8-kb, 2.3-kb and 1.3-kb ribosomal RNAs on nylon membrane were detected by the methylene blue staining, and shown as references for the amount of RNA loaded to each lane.
The isiA mutant experienced more stress than the wild-type strain because it maintained high-level transcription of the major heat shock genes such as groESL1, hspA and htpG, for a longer period than the wild-type following temperature up-shift (Fig. 5). We showed that all the molecular chaperones play roles in the acquisition of thermotolerance (Nakamoto et al. 2000, 2003, Tanaka and Nakamoto 1999). Similarly, levels of transcripts of these heat shock genes are enhanced in the isiB mutant as compared with the wild-type (Fig. 5). This suggests that the isiB and/or isiC genes also play a role in protection of cells from heat stress, although it contributes much less than the isiA gene. The high expression of molecular chaperones may complement the loss of the IsiB/IsiC function under the heat stress, but not the loss of the IsiA function.
Characterization of the isiA and isiB mutants under lethal high-temperature conditions
To evaluate the importance of the isiABC operon under lethal high-temperature conditions, we carried out a viability test after a shift to 48°C for 15 min under weak light. Before the lethal temperature treatment, cells had been cultured under iron-limited conditions for 2 days, which induced the isiABC operon in the wild-type (Fig. 1B–D). Survival rate of the isiA mutant was 0.12%, while those of the wild-type strain and the isiB mutant were 15% and 9.7%, respectively (Fig. 6A). Thus, the isiA mutant was two orders of magnitude more sensitive to this treatment than the wild-type and the isiB mutant. The highest sensitivity of the isiA mutant to the lethal high-temperature treatment is consistent with the phenotype observed at 42°C (Fig. 4). These results suggest that the isiA gene plays an important role for the thermal stress management. As far as we know, this is the first report that shows the involvement of a membrane protein such as IsiA in the acquisition of thermotolerance by cyanobacteria. Previously, we showed that the survival rate of Synechococcus sp. strain PCC 7942 after a lethal temperature treatment in the dark is 10 times higher than that in the light (Nakamoto et al. 2000), indicating a detrimental effect of light during heat stress. As already described in the Introduction, IsiA dissipates the excess light energy to avoid the production of reactive oxygen species by the photosynthetic electron transport. When proteins are denatured at high temperatures, cells cannot efficiently use light energy absorbed by photosynthetic pigments. Thus, cells may be highly susceptible to excess light energy resulting in reactive oxygen species even under weak light, yielding cells greatly dependent on IsiA for the energy dissipation.
We did similar experiments to Fig. 6A except that prior to the lethal temperature treatment cells had been cultured under iron-replete conditions. Survival rate of the wild-type decreased to 3.7% (Fig. 6B), which suggests that the iron-stress induced genes contributed the several fold increase (to 15% as shown in Fig. 6A) in the heat tolerance. Thus, the iron stress induced “cross-tolerance” to heat stress. It is clear that cells acquire thermotolerance by the induction of iron-stress-inducible genes other than the isiABC operon because the survival rates of the isiA mutant increased 10-folds by the iron-stress pretreatments (Fig. 6A and B). However, the isiA gene plays an essential and unique role for the induced thermotolerance because the survival rate of the isiA mutant after the iron-stress pretreatment was still much lower than those of the wild-type and the isiB mutant (Fig. 6A).
Under iron-replete conditions, the survival rates of the isiA and isiB mutants were reduced to two to three orders and one order, respectively, of magnitude lower than that of the wild-type (Fig. 6B). These results suggest that IsiB and/or IsiC as well as IsiA contribute to an increase in basal thermotolerance to some extent. This is unexpected because the transcripts of the isiABC operon were not detected under the iron-replete conditions under which cells were grown until the lethal temperature treatment (Fig. 1B–D). Thus, we expected that the survival rates would be indistinguishable among different strains. Instead, all strains decreased their survival rates to one tenth of those shown in Fig. 6A. We speculate that the lethal temperature treatment might induce the isiABC operon during or after the treatment. However, we could not detect any transcripts of the operon in cells collected during and after the lethal temperature treatment by Northern blot analysis (data not shown). The fact that the wild-type grown under iron-replete conditions showed two to three orders of magnitude higher survival rate than the isiA mutant (Fig. 6B) strongly suggests that the isiABC operon, especially the isiA gene, plays a role under heat stress although its expression is negligible. If IsiA as well as its transcript does not accumulate as a major chlorophyll-binding protein as it does under iron limitation, IsiA may have additional stress-related function independent of the photosynthetic apparatus under heat stress. If this is the case, the novel function may be “catalytic” or “regulatory.”
To characterize a role of the isiABC operon for the recovery from thermal damage, we analyzed the effect of light intensity immediately after a lethal temperature treatment on the survival rates. During the recovery process when most of the cellular processes are still greatly damaged, cells cannot use light energy efficiently. Thus, cells may be highly susceptible to excess light energy. As shown in Fig. 6C, when the light intensity after the lethal temperature treatment at 48°C for 15 min was decreased to 5 μE m−2 s−1 for 3 days, the survival rate of the wild-type cells increased 10-fold, as compared with that of cells incubated at 20 μE m−2 s−1. These results support the importance in restricting the incoming light energy during the recovery process. The survival rate of the isiA mutant increased more than 10-fold, getting close to that of the isiB mutant. Thus, the reduction in light intensity appears to substitute the IsiA function. IsiA may play an important role in reducing the light energy supply to photosynthetic reaction centers when cells are damaged. In contrast to the isiA mutant, the isiB mutant did not show much increase in its survival rate, and given the difference in the survival rate between the wild-type and the isiB mutant, the data suggest that the contribution by flavodoxin and/or IsiC toward cell survival becomes larger after the lethal treatment, and incubation in a very weak light. Under a very weak light where the damage by light is minimized, it is important for cells to optimize the recovery process. Flavodoxin and/or IsiC may play a crucial role in the recovery process.
Previously, we showed that phycocyanin was photobleached and the photosystem II activity was totally diminished when cells of Synechococcus sp. strain PCC 7942 were incubated at a lethal temperature for 15 min (Nakamoto et al. 2000). The photobleaching and the photosystem II inactivation were not restored within 1 day. Thus, it is unlikely that the light harvesting for the photosystem II and the linear electron transport via the photosystem II operate during the early phase of recovery process after the lethal temperature treatment, making the involvement of flavodoxin in the linear electron transport unlikely. Instead, flavodoxin may be involved in cyclic electron transport around photosystem I (Hagemann et al. 1999, Mi et al. 1992) to produce adenosine triphosphate for the recovery.
Concluding remarks
We have shown that the isiABC operon plays an essential role under both oxidative and heat stresses. In addition to these stresses, the isiABC operon is induced upon salt stress (Vinnemeier et al. 1998) and high light stress (Havaux et al. 2005). Thus, the operon encodes stress proteins not only for iron stress, but also for multiple stresses. In the present study, we showed evidence that the isiABC operon plays essential roles under oxidative and heat stresses. To play roles as general stress proteins, the gene products including IsiC may have additional stress-related function independent of the photosynthetic apparatus.
Edited by T. C. Vogelmann
Acknowledgments
Acknowledgements – This work was supported in part by Grant-in-aid for Scientific Research (C) (no. 16570028) and Scientific Research on Priority Area (A) (no. 17053003) from the Ministry of Education, Science, Sports and Culture of Japan to H. Nakamoto. We are grateful to Professor George Bullerjahn for helpful discussions. We are also grateful to Mr T. Watanabe and Mr K. Miyagi for technical assistances.




