A novel microdeletion syndrome with loss of the MSH2 locus and hereditary non-polyposis colorectal cancer
Abstract
Constitutional chromosome deletions can predispose to the development of cancer with the phenotypic characteristics of inherited cancer syndromes, when the deleted region encompasses a tumour suppressor gene. Examples of such conditions are represented by the cytogenetic deletions associated with retinoblastoma, Wilms tumour and familial adenomatous polyposis. So far, no constitutional deletions involving the genes implicated in hereditary non-polyposis colorectal cancer (HNPCC) have been identified. This may be at least partially because of the lack of distinctive phenotypic manifestations in HNPCC. We describe the first case of a constitutional microdeletion associated with HNPCC. Suspicion of a microdeletion was prompted by the association of mental retardation, postnatal growth deficiency, minor congenital anomalies and early onset (37 years) sporadic colon cancer. The patient was found to harbour a microdeletion within chromosome 2p16-p21, including the MSH2 gene. Since there are very few reports of deletions of the 2p16-p21 region, our observation sets the grounds for the definition of a novel multiple congenital anomaly/mental retardation/cancer microdeletion syndrome.
Some constitutional chromosome deletions are associated with an increased risk of development of specific tumours. Loss of tumour suppressor genes mapping within the deleted regions is functionally equivalent to heterozygosity for inactivating gene mutations. The clinical picture resulting from deletions spanning cancer predisposing genes is characterized by the association of mental retardation, major and/or minor congenital anomalies, and phenotypic manifestations of an inherited cancer syndrome. Such deletions have been instrumental for the localization and subsequently for cloning of the relevant cancer genes (1, 2).
Hereditary non-polyposis colorectal cancer (HNPCC) is a highly penetrant autosomal dominant condition characterized by an increased risk of early-onset colorectal cancer (CRC) and a broad spectrum of extracolonic tumours, particularly cancer of the endometrium, ovaries, small intestine, stomach and upper urinary tract (3). HNPCC is genetically heterogeneous, with at least four genes, all belonging to the mismatch repair (MMR) system, involved in its pathogenesis. These are MSH2, MLH1, MSH6 and PMS2, which map to 2p22-p21, 3p21.3, 2p16 and 7p22, respectively. Unlike other cancer syndromes, HNPCC does not present with pathognomonic clinical manifestations, such as the presence of numerous gastrointestinal polyps. The diagnosis is, therefore, based on familial aggregation of HNPCC cancers, with early onset representing a major distinctive criterion (4).
In this study, we report on the first patient with a documented chromosome deletion spanning one the MMR genes, which was suspected because of the presence of mental retardation and of the occurrence of CRC at an early age. The patient was found to harbour a microdeletion of the 2p16-p21 region, including the MSH2 gene, which was not visible with conventional cytogenetic analysis. Since there are very few reports of deletions affecting this tract, our findings can contribute to the delineation of an as yet poorly recognized chromosome anomaly.
Clinical report
The proposita was the third child of healthy non-consanguineous parents. She was born at term by normal delivery after an uncomplicated pregnancy, and had a birth weight of 3600 g (75th percentile). Her development was reportedly delayed; she started to walk at 20 months and to speak after 3 years. She attended special schools up to the age of 11. She had a premature pubarche at 6 years. A brain CT scan performed at 20 years was normal.
She was admitted to hospital for investigation of iron-deficiency anaemia at the age of 37. Investigations included colonoscopy, which showed the presence of a single large (approximately 5 cm) polypoid mass in the transverse colon proximal to the splenic flexure, for which she underwent partial colectomy. Histological examination showed that the polyp was a mucinous adenocarcinoma. Endoscopy of the upper gastrointestinal tract, chest radiograms and ultrasonography of the abdomen were normal. She made a good postoperative recovery.
Upon physical examination (Fig. 1), her weight was 90 kg (>97th percentile), height 145 cm (<3rd percentile) and occipitofrontal head circumference 53 cm (<25th percentile). She had a brachicephalic skull with a narrow forehead, large and horizontal eyes with thick eyebrows, prominent columella with hypoplastic nostrils, short philtrum, simplified ears, hirsutism and generalized obesity. The hands showed brachydactily, normal creases and an accessory palmar crease bilaterally. Dermatoglyphics were abcd, LuAAAA on right; and abcd, LuAAALu with a fourth IDW on left. Feet were small and broad with broad first toes and cutaneous syndactily between the second and third toes bilaterally. She had moderate mental retardation, with good interaction with the family and social environment, and a sociable attitude.

The proposita at age 37.
Family history was unremarkable. Her mother was alive and well at the age of 72. Her father died at 51 years because of an accident. Both the proposita's parents and siblings were of normal intelligence and presented none of the clinical features described in her.
Cytogenetic and molecular methods
Chromosome analysis (550 band level) was performed on peripheral blood lymphocytes by means of RBG banding.
Multiple ligation-dependent probe amplification (MLPA) analysis was employed in order to look for copy number alterations of the MSH2 and MLH1 genes in DNA isolated from peripheral leucocytes. MLPA allows the detection of gross genomic rearrangements (deletions/duplications) involving single or multiple exons within specific genes (5). The presence of MLH1 or MSH2 deletions was investigated by using an MPLA kit obtained from MRC Holland (Amsterdam, the Netherlands). The MLPA mix contains 16 exon probes for MSH2, 19 exon probes for MLH1 and 7 control probes specific for DNA sequences unrelated to MSH2 and MLH1. MLPA analysis was performed according to the manufacturer's instructions. Fragment analysis was performed on an ABI 310 capillary sequencer (Applied Biosystems, Warrington, Cheshire, UK) by using TAMRA-500 as size standard. A pattern of 42 peaks ranging in size from 130 to 472 nt was obtained. A deletion of one copy of a probe target sequence will usually be apparent by a 35–55% reduction of relative peak area of that probe amplification product. A gain in copy number from two to three copies will usually be apparent by a 30–55% increase in relative area.
Microsatellite instability (MSI) was assayed on DNA extracted from peripheral lymphocytes and paraffin-embedded tumour tissue by investigating a standard battery of microsatellite loci, including BAT25, BAT26, D2S123, D5S346 and D17S250 (6). For each locus tested, one of the amplification primers was fluorescently labelled. PCR products were run onto the 310 Applied Biosystems sequencer.
Fluorescent in situ hybridization (FISH) analysis was performed on standard chromosome preparations by using probes RP11-761B3, RPC11-295P2 and RP11-417P1 that span the chromosome region from 2p21 to 2p16. The RPC11-295P2 probe contains the MSH2 gene. RP11-761B3 and RP11-417P11 map proximally and distally to RPC11-295P2, respectively. Probes were labelled with SpectrumGreen-dUTP (Vysis, Inc., Downers Grove, IL), according to the manufacturer's protocol with minor modifications.
Results
The patient karyotype was found to be normal (data not shown). In order to identify the potential region of a submicroscopic rearrangement involving an HNPCC gene, we first employed MLPA, which allows rapid screening of the whole MSH2 and MLH1 genes. MLPA results were compatible with the presence of a single copy of all MSH2 exons, thus pointing to chromosome 2p as the site of the chromosome loss in the patient (Fig. 2).
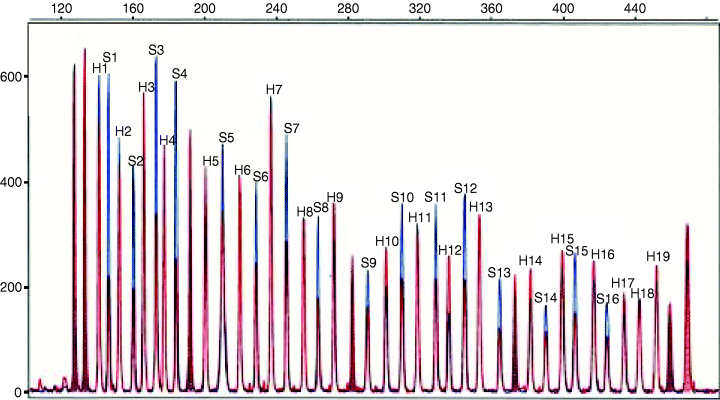
Deletion of the entire MSH2 gene detected by means of multiple ligation-dependent probe amplification. Normalized peak patterns from the index patient (red) and from control DNA (blue) have been plotted for comparison. Peaks are labelled with numbers corresponding to single exons (H indicates MLH1 exons, from 1 to 19, whereas S indicates MSH2 exons, from 1 to 16). Unlabelled peaks represent internal control fragments.
The MLPA findings were then confirmed with the help of FISH by using probes from the region, one of which contains MSH2. For all the three probes that we used, a specific signal was detected on a single chromosome 2 (Fig. 3).
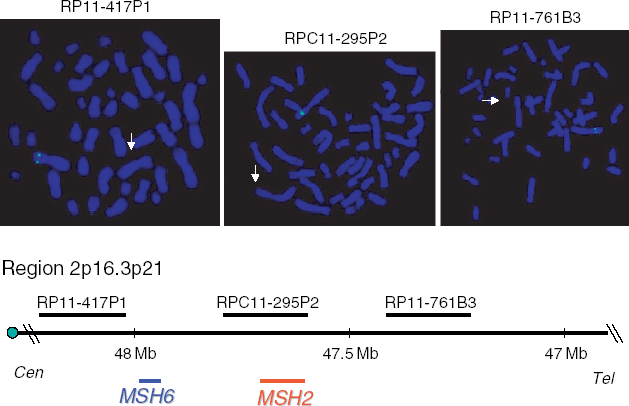
Fluorescent in situ hybridization results (top) and physical mapping (bottom) of the region containing the chromosome 2p probes RP11-761B3, RPC11-295P2 and RP11-417P1. White arrows indicate the deleted chromosome 2 homologues, showing the absence of specific signals for each of the three probes. The map was established according to Ensembl Human map (http://www.ensembl.org).
The proposita's mother, sister and brother showed staining of both chromosome 2 homologues upon FISH analysis with the MSH2-containing probe (data not shown). The rearrangement is likely to have occurred de novo in the proposita, because the deceased father was not reported to have any of her phenotypic manifestations.
Compared analysis of tumour and lymphocyte DNA revealed instability at two of the five microsatellite loci investigated (data not shown). The tumour sample could, therefore, be classified as MSI-H (high degree of MSI), as expected for HNPCC-associated cancers.
Discussion
The association of mental retardation, postnatal growth deficiency, minor dysmorphic features and excess of fingertip arches in the patient reported was suggestive of a constitutional chromosome anomaly. In addition, the occurrence of early-onset CRC not associated with intestinal polyposis, along with the mucinous histology and the location of the tumour in the right colon, which are frequent in HNPCC-related CRC (7), prompted us to search for a deletion spanning the regions containing the known HNPCC genes. Hence, molecular investigations were undertaken in order to identify a rearrangement involving MLH1 or MSH2, which are the genes most frequently involved in HNPCC. We first employed MLPA, which allows rapid simultaneous investigation of the major HNPCC genes. FISH analysis provided further evidence for the presence of a submicroscopic deletion on the short arm of chromosome 2, in the region where the MSH2 gene is located. The presence of MSI in the tumour sample from the proband is indicative of inactivation of the MMR system, which is the molecular hallmark of HNPCC.
Because the FISH probes that we used span approximately 1 Mb of chromosome 2, this is the minimum estimate of the deletion size in the patient. Another HNPCC gene, MSH6, is located at <0.5 Mb from MSH2, and it is included in the deletion interval between RP11-417P1 and RP11-295P2 (Fig. 3). Although the simultaneous deletion of MSH2 and MSH6 could underlie a more aggressive phenotype, no indicators of a very severe form, such as very early development of cancer or occurrence of multiple tumours, were present in our patient. This is not surprising, because it has been demonstrated that the MSH6 protein is secondarily degraded when MSH2 is absent, due to the inability to form MSH2/MSH6 heterodimers, which are essential for mismatch recognition by the MMR system (8).
The 13q14 deletion has been the first constitutional chromosome rearrangement reported to be associated with an increased cancer risk, and its description has played a pivotal role for localizing and cloning the RB1 gene implicated in retinoblastoma (1). Subsequently, the APC gene, responsible for familial adenomatous polyposis (FAP), was mapped and cloned following the report of a patient with multiple congenital anomalies, mental retardation, FAP and an interstitial deletion of chromosome 5q (2). The phenotype of the 5q21–q22 deletion associated with FAP has subsequently been well defined by the observation of further cases (9). Other cancer-prone chromosome deletions and/or microdeletions are those associated with Wilms tumour, Cowden syndrome and type-1 neurofibromatosis, which involve 11p13, 10q23.31 and 17q11.2, respectively (10–12).
Our findings add a novel condition, involving chromosome 2p16–21 and characterized by the association with HNPCC-related tumours, to the group of cancer-prone chromosome deletion syndromes. There are very few reports in the literature of cytogenetic deletions involving this region, and indeed, to the best of our knowledge, this is the first time that this chromosome segment is implicated in a microdeletion identifiable only by molecular cytogenetic analysis. Petit and Fryns (13) described a moderately mentally retarded patient with mild dysmorphisms who had a de novo 47,XY,del(2)(p11p21),+acefr. Recently, a child with a cytogenetically detectable deletion of 2p16–2p21 has been described (14). The proposita was reviewed at the age of 17 months; her weight and height were above the 95th percentile and her head circumference was below the 2nd percentile. She had a mild clinical phenotype characterized by microcephaly, mild hypotelorism, a prominent nasal bridge with a long philtrum and mild developmental delay. The authors wondered about the risk of developing HNPCC in this patient. The characteristics of our patient cannot be easily compared to those of the last two cases. Although the extension of the deletion was apparently much larger in the case with the 47,XY,del(2)(p11p21),+acefr karyotype, was found to be derived from chromosome 2p (13), the supernumerary marker chromosome. As to the case reported by Sanders et al. (14), the comparison is hampered by the difference in age at observation with our patient, because of the age-related phenotypic evolution, which is well documented for a number of multiple congenital anomalies/mental retardation syndromes.
In conclusion, we have described for the first time a cytogenetic condition associated with HNPCC, and our findings provide the initial grounds for the definition of a novel microdeletion syndrome. The identification of patients harbouring this condition is especially important for the prevention and early detection of HNPCC-related cancers, which include frequent colonoscopies and pelvic ultrasound, as well as prophylactic colectomy and/or hysterectomy.
Acknowledgements
The authors thank the family of the proposita for their co-operation, and Prof Mariano Rocchi, University of Bari, for providing some of the probes used for FISH. This work was supported by Telethon contract C50 (M. Rocchi), and by a grant of the Italian Ministry of Education, University and Research (M. Genuardi).




