Peroxisomal disorders I: biochemistry and genetics of peroxisome biogenesis disorders
Abstract
The peroxisomal disorders represent a group of genetic diseases in humans in which there is an impairment in one or more peroxisomal functions. The peroxisomal disorders are usually subdivided into two subgroups including (i) the peroxisome biogenesis disorders (PBDs) and (ii) the single peroxisomal (enzyme-) protein deficiencies. The PBD group is comprised of four different disorders including Zellweger syndrome (ZS), neonatal adrenoleukodystrophy (NALD), infantile Refsum's disease (IRD), and rhizomelic chondrodysplasia punctata (RCDP). ZS, NALD, and IRD are clearly distinct from RCDP and are usually referred to as the Zellweger spectrum with ZS being the most severe and NALD and IRD the less severe disorders. Studies in the late 1980s had already shown that the PBD group is genetically heterogeneous with at least 12 distinct genetic groups as concluded from complementation studies. Thanks to the much improved knowledge about peroxisome biogenesis notably in yeasts and the successful extrapolation of this knowledge to humans, the genes responsible for all these complementation groups have been identified making molecular diagnosis of PBD patients feasible now. It is the purpose of this review to describe the current stage of knowledge about the clinical, biochemical, cellular, and molecular aspects of PBDs, and to provide guidelines for the post- and prenatal diagnosis of PBDs. Less progress has been made with respect to the pathophysiology and therapy of PBDs. The increasing availability of mouse models for these disorders is a major step forward in this respect.
Zellweger syndrome (ZS) is the prototype of the group of peroxisomal disorders and was first described in the 1960s in two pairs of sibs, showing a series of abnormalities including craniofacial, hepatological, ocular, and skeletal aberrations. At about the same time, De Duve and coworkers performed systematic studies in which rat liver homogenates were subjected to differential and density gradient centrifugation. These studies led to the identification of a new organelle containing a number of H2O2-generating oxidases and catalase which decomposes H2O2 to O2 and H2O. The connection between ZS and peroxisomes first became apparent in 1973 when Goldfischer et al. (1) reported the absence of morphologically identifiable peroxisomes in hepatocytes and kidney tubule cells of Zellweger patients. At that time, however, virtually nothing was known about peroxisomes and it took another 10 years before the true significance of peroxisomes for human physiology started to become clear, thanks to two key observations in Zellweger patients. First, Brown et al. (2) discovered distinct abnormalities in the fatty acid profile of plasma from Zellweger patients with markedly elevated levels of the very-long-chain fatty acids (VLCFAs) C24:0 and C26:0, whereas normal levels were found for the other fatty acids including long-chain fatty acids like palmitic, oleic, and linoleic acid. At that time, peroxisomes were already known to contain a fatty acid beta-oxidation system, just like mitochondria, but the function of this system had remained obscure. The findings by Brown et al. (2) suggested that peroxisomes are the site of beta-oxidation of VLCFAs, which was soon established experimentally (3). The second major discovery demonstrating the crucial role of peroxisomes in humans appeared 1 year later when Heymans et al. (4) reported the deficiency of plasmalogens, a special type of phospholipids belonging to the group of ether-linked phospholipids, in tissues from Zellweger patients. Since then, much has been learned about the metabolic role of peroxisomes and many different functions of peroxisomes have been identified. In addition, many of the enzymes involved in the different metabolic pathways within peroxisomes have been characterized, purified, and their respective cDNAs and genes cloned. Parallel to this work, the essential details of peroxisome biogenesis have been worked out and many of the genes, coding for proteins essential for peroxisome biogenesis, have been identified. Thanks to this explosion of new information, enormous progress has been made with respect to the identification of new peroxisomal disorders followed by resolution of the underlying defects. At present, the group of peroxisomal disorders comprises 17 well-defined disorders, which are subdivided into two groups including (i) the peroxisome biogenesis disorders (PBDs) and (ii) the single peroxisomal (enzyme-) protein deficiencies. This review is focused on the first group of disorders, the PBDs (Table 1), and we will begin by discussing what is known about the different PBDs.
| Number | Disorder | Abbreviation | Protein involved | Gene | Chromosome | MIM |
|---|---|---|---|---|---|---|
| 1 | Zellweger syndrome | ZS | Peroxins | PEX-genes | Multiple loci | 214100 |
| 2 | Neonatal adrenoleukodystrophy | NALD | Peroxins | PEX-genes | Multiple loci | 214110 |
| 3 | Infantile Refsum's disease | IRD | Peroxins | PEX-genes | Multiple loci | 202370 |
| 4 | Rhizomelic chondrodysplasia punctata type 1 | RCDP Type 1 | Pex7p | PEX7 | 6q21–q22 | 215100 |
The peroxisome biogenesis disorders: a clinically and genetically heterogeneous group of disorders
The PBD group is comprised of four different disorders including ZS, neonatal adrenoleukodystrophy (NALD), infantile Refsum's disease (IRD), and rhizomelic chondrodysplasia punctata (RCDP). ZS, NALD, and IRD are clearly distinct from RCDP and are nowadays usually referred to as ‘the Zellweger spectrum’ with ZS being the most severe and NALD and IRD less severe disorders. ZS is generally considered as the prototype of the PBD group. ZS is dominated by: (i) the typical craniofacial dysmorphism including a high forehead, large anterior fontanel, hypoplastic supraorbital ridges, epicanthal folds, and deformed earlobes, and (ii) profound neurological abnormalities. ZS children show severe psychomotor retardation, profound hypotonia, neonatal seizures, glaucoma, retinal degeneration, and impaired hearing. There is usually calcific stippling of the epiphyses and small renal cysts. Brain abnormalities in ZS include not only cortical dysplasia and neuronal heterotopia but also regressive changes. There is dysmyelination rather than demyelination. Patients with NALD have hypotonia and seizures, may have polymicrogyria, progressive white matter disease, and usually die in late infancy. Patients with IRD may have external features reminiscent of ZS but do not show disordered neuronal migration and no progressive white matter disease. Their cognitive and motor development varies between severe global handicaps and moderate learning disabilities with deafness and visual impairment due to retinopathy. Their survival is variable. Most patients with IRD reach childhood and some even reach adulthood. Clinical distinction between the different PBD phenotypes is not very well defined. Common to all three are liver disease, variable neurodevelopmental delay, retinopathy, and perceptive deafness with onset in the first months of life.
RCDP is clinically quite different from ZS, NALD, and IRD and characterized by a disproportionally short stature primarily affecting the proximal parts of the extremities, typical facial appearance, including a broad nasal bridge, epicanthus, high arched palate, dysplastic external ears, micrognathia, congenital contractures, characteristic ocular involvement, dwarfism, and severe mental retardation with spasticity. Most RCDP patients die in the first decade of life.
ZS, NALD, IRD, and RCDP have been found to be genetically heterogeneous as concluded from complementation studies as discussed later in this review. The molecular defects underlying these different complementation groups (CGs) have been resolved in recent years. Two different strategies have been very rewarding in the identification of these mutant genes, which includes (i) homology probing, making use of the information from different yeast mutants, and (ii) functional complementation analysis based on the generation of peroxisome-deficient Chinese hamster ovary (CHO) cells. We will proceed by describing the current stage of knowledge about peroxisome biogenesis.
Peroxisome biogenisis: general aspects
Peroxisomal proteins are all encoded by nuclear genes and translated on free polyribosomes as first shown for urate oxidase and catalase, two peroxisomal matrix proteins, by Goldmann and Blobel (5), and Robbi and Lazarow (6), respectively. Later studies have shown the same for peroxisomal membrane proteins (PMPs) (7). After synthesis on free polyribosomes, the newly made peroxisomal proteins are targeted to peroxisomes and then imported into pre-existing peroxisomes post-translationally, which implies that synthesis and import are sequential rather than simultaneous processes. In this way, peroxisomes get bigger which requires recruitment of phospholipids most likely from the endoplasmic reticulum (ER) to be incorporated into the peroxisomal membrane. Growth of peroxisomes may continue until a critical size is reached after which peroxisomes divide into two daughter peroxisomes that can then undergo the same cycle of events (Fig. 1a).
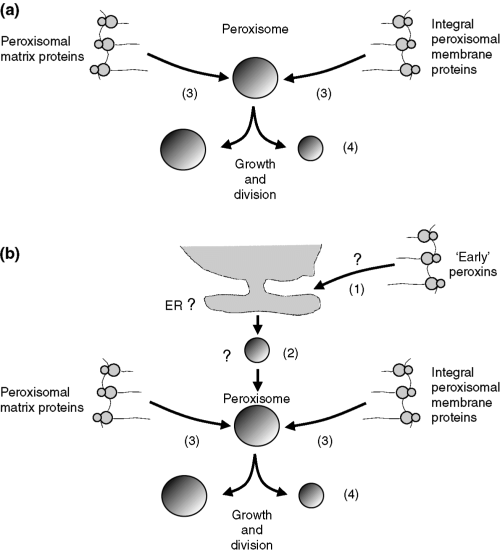
Original (a) and modified (b) model for peroxisome biogenesis. (a) The original growth-and-division model proposed by Lazarow and Fujiki (139) in which peroxisomes were thought to be autonomous organelles, which could not form de novo. (b) The modified model of Lazarow and Fujiki with peroxisomes now envisaged as semiautonomous organelles with the capacity to form de novo.
The import of peroxisomal matrix and membrane proteins into peroxisomes is a multistep process involving recognition of the cargo protein by a receptor in the cytosol, docking of the receptor–cargo complex at the peroxisomal membrane, translocation across the membrane, cargo release into the organelle, and receptor recycling. Correct targeting of peroxisomal matrix proteins is achieved via cis-acting sequences present in the primary peptide sequences, which are called peroxisomal targeting signals (PTSs). Most matrix proteins are equipped with a PTS type 1 (PTS1), which is a C-terminal serine-lysine-leucine-COOH (SKL) tripeptide, or a conservative variant thereof, like SHL in D-aminoacid oxidase, AKL in sterol carrier protein 2 (SCP2), etc (Table 2). A few matrix proteins are targeted via a different signal named PTS2, which is a 9-amino acid sequence located near the N-terminus with the amino acids in positions 1, 2, 8, and 9 being most important. The consensus PTS2 is R/K-L/V/I-XXXXX-H/Q-A/L in which X is any amino acid. The PTS1 and PTS2 receptors have been cloned and characterized from different species. The former, Pex5p, is a tetratricopeptide (TPR) repeat protein, whereas the latter, Pex7p, is a WD40 repeat protein, to be discussed later.
| Peroxisomal function | (Enzyme) protein | PTS1/PTS2 | Targeting sequence |
|---|---|---|---|
| Fatty acid β-oxidation | Acyl-CoA oxidase 1 (straight chain) | PTS1 | –SKL |
| Acyl-CoA oxidase 2 (branched chain) | PTS1 | –SKL | |
| Acyl-CoA oxidase 3 (pristanoyl-CoA) | PTS1 | –SKL | |
| L-bifunctional protein | PTS1 | –SKL | |
| D-bifunctional protein | PTS1 | –AKL | |
| 3-ketothiolase (straight chain) | PTS2 | –RLQVVLGHL | |
| 3-ketothiolase (branched chain) | PTS1 | –AKL | |
| 2-methylacyl-CoA racemase | PTS1 (+MTS) | –(K)ASL | |
| Carnitine acetyltransferase | PTS1 | –AKL | |
| Carnitine octanoyltransferase | PTS1 | –THL | |
| Acyl-CoA thioesterase | PTS1 | –SKL | |
| Bile acid-CoA: taurine/glycine conjugating enzyme | PTS1 | –SQL | |
| 2,4-dienoylCoA reductase | PTS1 | –AKL | |
| Δ2,Δ3-enoylCoA isomerase | PTS1 | –SKL | |
| Δ3,5, Δ2,4-dienoylCoA isomerase | PTS1 | –SKL | |
| Very-long-chain acyl-CoA synthetase (VLACS) | PTS1 | –LKL | |
| Fatty acid α-oxidation | PhytanoylCoA hydroxylase | PTS2 | –RLQIVLGHL |
| 2-hydroxyphytanoylCoA lyase | PTS1 | –(R)SNM | |
| Etherphospholipid biosynthesis | Dihydroxyacetonephosphate acyltransferase | PTS1 | –AKL |
| Alkyldihydroxyacetonephosphate synthase | PTS2 | –RLVLSGHL | |
| Glyoxylate detoxification | Alanine glyoxylate aminotransferase | PTS1 | –KKL |
| Pipecolic acid degradation | L-pipecolate oxidase | PTS1 | –AHL |
| H2O2 metabolism | Catalase | PTS1 | –(K)ANL |
| Peroxiredoxin V | PTS1 | –SQL | |
| Sterol carrier protein 2 | PTS1 | –AKL | |
| D-aspartate oxidase | PTS1 | –(K)SNL | |
| D-amino acid oxidase | PTS1 | –SHL | |
| Hydroxyacid oxidase 3 | PTS1 | –SRL | |
| Hydroxyacid oxidase 2 | PTS1 | –SRL | |
| Hydroxyacid oxidase 1 (glycolate oxidase) | PTS1 | –SKL | |
| Others | 3-hydroxy-3-methyl glutarylCoA lyase | PTS1 (+MTS) | –CKL |
| MalonylCoA decarboxylase | PTS1 (+MTS) | –SKL | |
| Isocitrate dehydrogenase | PTS1 | –AKL |
- PTS1, peroxisome-targeting signal type 1; PTS2, peroxisome-targeting signal type 2; MTS, mitochondrial targeting signal. In the right hand column, the different targeting sequences are shown using the single letter code for the various amino acids.
Similar to matrix proteins, PMPs are synthesized on free cytosolic ribosomes and targeted to the organelle by cis-acting targeting sequences (mPTS). In contrast to the simple PTS1 and PTS2 sequences found in matrix proteins, PMPs are directed to peroxisomes via, as yet, less well-defined targeting signals, to be discussed later.
Peroxisome biogenesis: de novo formation of peroxisomes or not?
As discussed above, peroxisome biogenesis resembles that of mitochondria and chloroplasts, which is true although the details are entirely different. Indeed, protein translocation into peroxisomes differs markedly from that in mitochondria which threads unfolded polypeptide chains through a narrow channel, whereas peroxisomes can import folded and homo-oligomeric proteins (8), hetero-oligomers (9, 10), and even 4–9-nm gold beads (11). The transport of such large complexes somewhat resembles protein transport into the nucleus, but no such thing as a structure resembling the nuclear pore complex has ever been observed in the peroxisomal membrane.
The concept that peroxisomes multiply by growth and division of pre-existing peroxisomes would make peroxisomes belong to the group of autonomous organelles with mitochondria, chloroplasts, and the endoplasmic reticulum as representatives. This would imply that peroxisomes cannot form de novo. Several experimental observations have been done suggesting that peroxisomes can form de novo, however. One of the main arguments in favor of de novo biogenesis of peroxisomes has been that cells, mutated in PEX3, PEX16, or PEX19, show no peroxisomal membrane structures (ghosts), whereas reintroduction of a wild-type copy of the mutant gene restores peroxisome formation. These findings have been interpreted as evidence for de novo synthesis of peroxisomes from some endomembrane compartment such as the ER (Fig. 1b).
Based on studies in the yeasts Yarrowia lipolytica and Hansenula polymorpha, it has been proposed that peroxisomes can be formed from small pre-peroxisomal vesicles derived from the ER in a process dependent on COPI and COPII, two coat proteins involved in vesicle transport processes. Studies in human fibroblasts, however, have shown that peroxisome biogenesis occurs independent of COPI and COPII (12, 13). Furthermore, studies by South et al. (14) in the yeast Saccharomyces cerevisiae suggest that protein traffic into the ER is not required to form peroxisomes. This was concluded from studies in which the protein entry into the ER was blocked by inactivation of the ER protein translocation factor, Sec61p, or its homolog, Ssh1p. These results argue against the ER as the site of de novo peroxisome formation. Furthermore, studies by Snyder et al. (15) and Hazra et al. (16) have provided compelling evidence against the dogma of the absence of peroxisomal structures in pex3Δ, pex16Δ, and pex19Δ mutants. Indeed, Snyder et al. (15) identified tiny peroxisomal vesicles and tubules in Pichia pastoris pex19Δ cells by deconvolution microscopy using an antibody recognizing endogenous Pex3p. In addition, Hazra et al. (16) reported the identification of vesicular and tubular, torpedo-shaped peroxisomal structures in P. pastoris pex3Δ cells and characterized these by isopyknic and flotation centrifugation.
The jury is still out on the origin of peroxisomes, however, as emphasized by several very recent studies. Firstly, Geuze et al. (17) recently presented evidence of the involvement of the ER in peroxisome formation in mouse dendritic cells using electron microscopy, immunocytochemistry, and three-dimensional image reconstruction of peroxisomes and associated compartments. Additional support for the formation of peroxisomes from some endomembrane compartment has also come from studies by Faber et al. (18) who have shown that an N-terminal fragment of Pex3p expressed in H. polymorpha is associated with vesicular membrane structures that also contain Pex14p. Furthermore, these structures appeared to have the potential to develop into functional peroxisomes after introduction of full-length PEX3 and arise from the nuclear membrane. In conclusion, it remains to be established whether there are indeed two parallel pathways for peroxisome formation, one from pre-existing peroxisomes and a second de novo pathway, which allows peroxisome formation from some endomembrane compartment such as the ER.
Peroxisome biogenesis: a closer look
The realization that a simple organism like baker's yeast could be used to study peroxisome biogenesis and resolve the sorting and targeting of peroxisomal proteins to their correct destination, the peroxisome, has had a tremendous impact and explains for a large part why the pursuit of genes defect in PBD patients has been so fruitful in the last few years. The key to the application of genetics to the elucidation of the mechanism of peroxisome biogenesis and the identification of the proteins involved was the isolation of peroxisome-deficient mutants (pex mutants) from different yeast species and CHO mutants (19). Erdmann et al. (20) were the first to device a selection screen based on the notion that in yeast, peroxisomes are essential for growth on oleate. This follows logically from the fact that in yeast, fatty acids can only be oxidized in peroxisomes whereas in higher eukaryotes beta-oxidation can occur both in peroxisomes and in mitochondria. S. cerevisiae cells were subjected to chemical mutagenesis and grown first on glucose agar plates followed by replica plating onto oleate agar plates to select for cells not growing on oleate (onu-mutants). Subsequently, cell fractionation studies were performed to eliminate mutants with no abnormalities in peroxisome biogenesis but a defect in the fatty acid beta-oxidation system. This approach resulted in a total of 12 different mutants that turned out to be peroxisome deficient. Similar screens have been set up for a variety of different yeast species including P. pastoris, H. polymorpha, and Y. lipolytica. Additional screens and selections, based on other approaches, have also been set up which together has led to the generation of a large series of peroxisome biogenesis mutants. Subsequent complementation of these mutants using yeast genomic libraries has resulted in the identification of a large number of genes involved in peroxisome biogenesis. Initially, these new genes were all given different names even within the same species (i.e. PAF, PAS, PEB, PER, andPAY genes). To simplify matters, all of these genes have been renamed as PEX genes (PEX1, PEX2, PEX, etc) and the products of these genes are called peroxins (Pex1p, Pex2p, Pex3p, etc). The peroxins were agreed to include all proteins involved in peroxisome biogenesis inclusive of peroxisome matrix protein import, membrane biogenesis, peroxisome proliferation, and peroxisome inheritance.
In the original study of Erdmann et al. (20), 12 different S. cerevisiae mutants were identified in which peroxisome biogenesis was impaired. One by one the genes mutated in each of these so-called pas-mutants have been identified, of which the first one was described by Erdmann et al. in 1991 (21). The gene involved (PEX1) codes for a protein belonging to the family of triple A (AAA) ATPases, which are involved in the assembly, organization, and disassembly of protein complexes (22). The discovery of the first peroxisome biogenesis gene in S. cerevisiae was soon followed by reports from the same group describing the second (PEX3) (23) and third (PEX4) (24) S. cerevisiae PEX genes. In pex1Δ, pex3Δ, and pex4Δ cells, the import of PTS1 and PTS2 proteins is impaired, indicating that Pex1p, Pex3p, and Pex4p play an essential role in the import of matrix proteins. Later studies revealed that these mutants are different if the import of PMPs is studied. Indeed, pex1Δ and pex4Δ cells are still able to assemble their PMPs into membranes, whereas pex3Δ cells lack this property. Studies by Hettema et al. (25) in a series of 19 S. cerevisiae mutants have shown that the import of PTS1 and/or PTS2 proteins is impaired in all mutants except one (pex11Δ), whereas PMP import is normal in all these mutants except for the pex3Δ and pex19Δ mutants. These data are in line with the notion that Pex3p and Pex19p belong to a distinct group of peroxins required for the proper localization and stabilization of PMPs as discussed in the next section. With the recent identification of three PEX genes in the yeast S. cerevisiae, i.e. PEX 30, 31, and 32 (26), the total number of PEX genes now stands at 32 (Table 3).
| Identified in | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gene | Hs | Sc | Yl | Nc | Ce | Human gene locus | Peroxin characteristics | Subcellular localization | Interacting peroxins | References |
| PEX1 | + | + | + | + | + | 7q21–q22 | AAA–protein required for peroxisomal matrix protein import; interacts with Pex6p | Mainly cytosolic, partly peroxisomal | Pex6p | (21, 117, 140) |
| PEX2 | + | + | + | + | + | 8q21.1 | RING zinc finger protein involved in matrix protein import downstream of receptor docking | Integral PMP | Pex10p | (141) |
| PEX3 | + | + | – | + | – | 6q23–q24 | PMP import; possible docking factor for Pex19p | Integral PMP | Pex19p | (23, 46) |
| PEX4 | – | + | – | + | – | E2–ubiquitin conjugating enzyme required for peroxisomal matrix protein import | Peripheral PMP | Pex22p | (24, 142, 143) | |
| PEX5 | + | + | + | + | + | 12p13.3 | TPR–protein; receptor for PTS1 proteins | Mainly cytosolic, partly peroxisomal | Pex7p, 8p, 10p, 12p, 13p, 14p | (49, 50, 55) |
| PEX6 | + | + | + | + | + | 6p21.1 | AAA–protein; interacts with Pex1p, ScPex15p, and HsPex26p; required for matrix protein import | Mainly cytosolic, partly peroxisomal | Pex1p, (Sc)Pex15p, (Hs)Pex26p | (84, 95) |
| PEX7 | + | + | – | + | – | 6q21–q22.2 | WD–protein; receptor for PTS2 proteins | Mainly cytosolic, partly peroxisomal | Pex5pL, 13p, 14p, 18p, 20p, 21p | (59, 62, 63, 64) |
| PEX8 | – | + | + | + | – | Involved in matrix protein import downstream of receptor docking | Luminal PMP | Pex5p, Pex20p | (144, 145) | |
| PEX9 | – | – | + | – | – | Involved in matrix protein import; only identified in Y. lipolytica | Integral PMP | (146) | ||
| PEX10 | + | + | + | + | – | 1p36.32 | RING zinc finger protein; required for matrix protein import; acting downstream of receptor docking | Integral PMP | Pex2p, 5p, 12p, and 19p | (147, 148) |
| PEX11 | + | + | + | + | – | 15q25.2 (α) 1q21.1(β) 19p13.3 (γ) | Involved in peroxisome division and proliferation and/or transport of medium chain fatty acids | Integral PMP | Pex19p | (101, 102, 104–106) |
| PEX12 | + | + | – | + | + | 17q21.1 | RING zinc finger protein, required for matrix protein import, acting downstream of receptor docking | Integral PMP | Pex5p, 10p, and 19p | (149) |
| PEX13 | + | + | – | + | + | 2p14–p16 | SH3–protein; matrix protein import; involved in receptor docking with Pex14p | Integral PMP | Pex5p, 7p, 14p, and 19p | (150, 151, 152) |
| PEX14 | + | + | + | + | 1p36.22 | Initial site of receptor docking | PMP | Pex5p, 7p, 13p, 17p, and 19p | (153) | |
| PEX15 | – | + | – | – | Required for matrix protein import; membrane anchor for Pex6p;yeast equivalent of human Pex26p | Integral PMP | Pex6p | (31, 95) | ||
| PEX16 | + | – | + | + | 11p11.11 | Required for PMP import, together with Pex3p and Pex19p | Integral PMP | Pex19p | (47, 154) | |
| PEX17 | – | + | – | – | – | Required for matrix protein import | Peripheral PMP | Pex14p, Pex19p | (42, 79, 80) | |
| PEX18 | – | + | – | – | Required for PTS2 protein import in S. cerevisiae; binds to ScPex7p | Mainly cytosolic, partially peroxisomal | Pex7p | (27) | ||
| PEX19 | + | + | + | + | + | 1q22 | Cytosolic PMP receptor | Mainly cytosolic, partly peroxisomal | Pex3p, 10p, 12p, 13p,14p, 16p, 17p, 11αp, 11βp | (43, 155, 156) |
| PEX20 | – | – | + | + | – | Required for PTS2 protein import and thiolase oligomerization in Y. lipolytica | Mainly cytosolic, partly peroxisomal | (28) | ||
| PEX21 | – | + | – | – | – | Required for PTS2 protein import in S. cerevisiae; binds to Pex7p | Mainly cytosolic | Pex7p, 13p, 14p | (27) | |
| PEX22 | – | + | – | – | – | PMP involved in matrix protein import; membrane anchor for Pex4p | Integral PMP | Pex4p | (157) | |
| PEX23 | – | – | + | + | – | PMP involved in matrix protein import | Integral PMP | (158) | ||
| PEX24 | – | – | + | – | – | Involved in peroxisome assembly; high sequence similarity to YlPex28p and YlPex29p | Integral PMP | (160) | ||
| PEX25 | – | + | – | – | – | Involved in regulating peroxisome number, size, and distribution together with Pex28p, Pex29p, and Vps1p | Peripheral PMP | Pex27p | (159, 161) | |
| PEX26 | + | – | – | – | – | 22q11.21 | Matrix protein import; recruits Pex1p–Pex6p complex to the peroxisomal membrane | Integral PMP | Pex6p | (96) |
| PEX27 | – | + | – | – | – | Controls peroxisome size and number; extensive sequence similarity to Pex11p and Pex25p | Peripheral PMP | Pex25p | (159, 162) | |
| PEX28 | – | – | + | – | – | Involved in regulating peroxisome number, size, and distribution together with Pex25p, Pex29p, and Vps1p | Integral PMP | (163) | ||
| PEX29 | – | – | + | – | – | Involved in regulating peroxisome number, size, and distribution together with Pex25p, Pex28p, and Vps1p | Integral PMP | (163) | ||
| PEX30 | – | + | – | – | – | Involved in the control of peroxisome size and proliferation, together with Pex28p, 29p, 31p, and 32p | Integral PMP | Pex28p, 29p, 31p, 32p | (26) | |
| PEX31 | – | + | – | – | – | Involved in the control of peroxisome size and proliferation, together with Pex28p, 29p, 30p, and 32p | Integral PMP | Pex28p, 29p, 30p, 32p | (26) | |
| PEX32 | – | + | – | – | – | Involved in the control of peroxisome size and proliferation, together with Pex28p, 29p, 30p, and 31p | Integral PMP | Pex28p, 29p, 30p, 31p | (26) | |
- Abbreviations used: Hs = Homo sapiens; Sc = Saccharomyces cerevisiae; Yl = Yarrowia lipolytica; Nc = Neurospora crassa; Ce = Caenorhabditis elegans; PMP = Peripheral Membrane Proteins.
The complete set of 32 PEX genes can be subdivided into two groups in which group 1 includes those genes of which orthologs are found among most, if not all, peroxisome-containing species, whereas group 2 refers to those PEX genes which are only found in single organisms. Most of the PEX genes belong to group 1 with orthologs in different species. PEX genes belonging to group 2 are PEX18 and PEX21, which are only found in S. cerevisiae (27), and PEX20 which is only found in Y. lipolytica (28) and Neurospora crassa (29). These results indicate that the principal features of peroxisome biogenesis are similar among different organisms but not identical. Table 3 describes the full list of PEX genes so far identified and their distribution among different species as well as some characteristics of the peroxins encoded by the different PEX genes.
So far, 16 different PEX genes have been identified in humans. These include HsPEX1, HsPEX2, HsPEX3, HsPEX5, HsPEX6, HsPEX7, HsPEX10, HsPEX11α, HsPEX11β, HsPEX11γ, HsPEX12, HsPEX13, HsPEX14, HsPEX16, HsPEX19, and HsPEX26. We will proceed by describing the proteins encoded by these PEX genes and their presumed role in peroxisome biogenesis. Conceptually, the process of peroxisome biogenesis can be subdivided into distinct steps including (i) peroxisome membrane assembly, (ii) import of matrix proteins, and (iii) peroxisome proliferation and maintenance. In the next paragraphs, we will describe what is known about these different steps with particular emphasis on the situation in humans. We will begin by describing peroxisome membrane biogenesis and the roles of Pex3p, Pex16p, and Pex19p.
Peroxisome membrane biogenesis and the human peroxins HsPEX3p, HsPEX16p, and HsPEX19p
The first clue that the mechanism involved in peroxisome membrane biogenesis is fundamentally different from the one used to transport peroxisomal matrix proteins across the peroxisomal membrane was the discovery by Santos et al. (30) that cells from Zellweger patients contain peroxisome membrane structures, called ghosts, which contain PMPs but lack most, if not all, of their matrix protein content. Like the peroxisomal matrix proteins, PMPs are synthesized on free polyribosomes and imported into peroxisomes by a direct cytosol-to-peroxisome mechanism. In general, PMPs lack functional PTS1 and PTS2 signals and their import is independent of the PTS1- and PTS2-protein import routes. This is true for all bona fide integral PMPs (iPMPs), whereas peripheral PMPs, like dihydroxyacetonephosphate acyltransferase (DHAPAT) and alkyl-DHAP synthase, use the PTS1- and PTS2-protein import routes. Multiple studies have attempted to define the targeting signals in iPMPs. These studies have clearly shown that iPMPs are not targeted to peroxisomes via carboxy-terminal or amino-terminal extensions as in PTS1 and PTS2 proteins. All data show that the targeting information is actually contained within the polypeptide chain itself. Although knowledge about targeting signals in iPMPs has remained limited so far, one signal has been identified in both single- and multi-span transmembrane proteins, which is made up of a basic cluster of amino acids oriented towards the peroxisomal matrix, in front of a transmembrane span which directly follows the basic amino acid cluster. Some proteins may require additional targeting information on the cytosolic side of the peroxisomal membrane as in ScPex15p (31). There is increasing evidence, however, which suggests that iPMPs are directed to peroxisomes via multiple, distinct targeting signals rather than a single targeting signal. Indeed, following earlier work by Dyer et al. (32), Wang et al. (33) reported the identification of three discrete targeting signals in S. cerevisiae PMP47. Furthermore, Jones et al. (34) showed that PMP34, the human homolog of C. boidinii PMP47, contains at least two non-overlapping sets of targeting information (amino acids 1–147 and 244–307), either of which is sufficient for insertion into the membrane. This is in contrast to data by Honsho et al. (35) who reported that PMP47 was targeted to peroxisomes via a different PTS located in the region containing amino acids 183–194. Furthermore, Jones et al. (34) also identified two independent sets of targeting information in human Pex13p. In addition, Brosius et al. (36) identified two distinct, non-overlapping peroxisomal membrane-targeting signals in rat and human PMP22, one in the amino-terminal and the other in the carboxy-terminal end of the protein. Taken together, these results challenge the assumption that PMPs are targeted to peroxisomes via single PTSs and rather suggest the involvement of multiple, non-overlapping targeting regions in iPMPs.
Studies in different yeast mutants as well as in fibroblasts from PBD patients have shown that ghosts are absent in some mutants indicating that in these mutants, the targeting of both peroxisomal matrix proteins and PMPs is deficient. In S. cerevisiae, the pex3 and pex19 mutants turned out to lack ghost-like structures. The same was found for human fibroblasts mutated in either the PEX3 or PEX19 gene. Furthermore, ghosts were also lacking in fibroblasts from patients mutated in PEX16. Taken together, these results indicate that Pex3p, Pex16p, and Pex19p play an essential role in peroxisome membrane biogenesis as described below.
PEX3
The PEX3 gene, first cloned in S. cerevisiae by Hohfeld et al. (23) encodes a 42–52-kDa protein, firmly anchored in the peroxisomal membrane with its C-terminus exposed to the cytosol, whereas opinions differ with respect to the N-terminus being either cytosolic or intraperoxisomal. The human gene was cloned in 1998 by Kammerer et al. (37). Pex3p interacts with Pex19p via its C-terminal domain. In human cells with defective PEX3, the peroxin Pex14p is mislocalized to mitochondria, whereas the peroxisomal transporters adreno leuko dystrophy protein (ALDP) and peroxisomal membrane protein of 70 kDa (PMP70) are absent and less abundant, respectively. In CHO pex3Δ cells, Pex12p and Pex13p are absent and Pex14p less abundant.
PEX19
Pex19p is a farnesylated protein first identified by James et al. (38) in CHO cells. Subsequent studies have led to the identification of a number of yeast homologs as well as human PEX19 (39). The protein is hydrophilic and contains a CAAX box allowing farnesylation of the cysteine. The exact role of Pex19p farnesylation is not resolved yet, although it may assist in peroxisomal membrane association. Indeed, in S. cerevisiae, farnesylation appears to be essential for its function (40), but this is not true for P. pastoris (15) and in humans (41). Pex19p is predominantly cytosolic, with only a small amount bound to the peroxisomal membrane, and interacts with a large variety of PMPs including peroxins: (i) Pex3p, Pex10p, Pex12p, Pex13p, Pex14p, Pex16p, and Pex17p; (ii) proteins involved in peroxisome proliferation (Pex11α and Pex11β); (iii) metabolite transporters (PMP34, PMP70, ALDP, and adreno leuko dystrophy related protein (ALDR); and (iv) PMPs of unknown function (PMP22 and PMP24) (15, 40–44). Based on these results, it is suggested that Pex19p may function as a cytosolic PMP receptor analogous to Pex5p and Pex7p, which are the cytosolic receptors for PTS1 and PTS2 proteins, respectively. Elegant experiments by Sacksteder et al. (41) in which Pex19p was directed to the nucleus by fusing it to a nuclear localization signal have provided convincing evidence in favor of this suggestion although this view is disputed by others (43, 45, 46).
PEX16
In contrast to Pex3p and Pex19p, which are present in multiple mammalian and yeast species, Pex16p is lacking in most species and has only been reported in humans and the yeast Y. lipolytica (47) in which Pex16p has different properties as compared to human Pex16p playing no role in membrane assembly. The human PEX16 gene was identified by Honsho et al. (48) and encodes a 38.6-kDa integral membrane protein with two putative membrane-spanning domains and both the N- and C-termini exposed to the cytosol. Its function is unknown. Cells defective in PEX16 lack ghosts as assessed by immunofluorescence microscopy analysis of PMP70 (48) and a range of other PMPs (12).
Import of peroxisomal matrix proteins
Recognition of PTS1 and PTS2 proteins in the cytosol by the import receptors Pex5p and Pex7p
The realization that peroxisomal proteins are synthesized on cytosolic polyribosomes and contain specific targeting signals, which direct them to peroxisomes, implied the existence of receptors, recognizing the PTS1 and PTS2 sequences. The PTS1 receptor (Pex5p) was first identified in 1993 in the yeasts P. pastoris (49) and S. cerevisiae (50). Subsequent studies have led to the identification of orthologs of Pex5p in a range of different species including humans (51–53). Pex5p binds PTS1 proteins in the cytosol and cycles between the cytosol and the peroxisome. In most organisms, Pex5p is mainly localized in the cytosol with only a small fraction being associated with peroxisomes. Based on these data, a model has been proposed called the ‘shuttle-model’ in which Pex5p binds its cargo, i.e. a PTS protein, in the cytosol, after which the receptor–cargo complex docks at the peroxisomal membrane followed by dissociation of the complex and transport of the PTS1 protein across the membrane and recycling of the receptor back into the cytosol (Fig. 2a). In the yeast Y. lipolytica, however, Pex5p is mainly intraperoxisomal (54). Similar observations have been made in other yeasts including H. polymorpha (55). This dual localization of Pex5p has led to a revised model for protein import into peroxisomes in which the Pex5p–PTS1 protein complex is translocated across the peroxisomal membrane in toto followed by recycling of the receptor back into the cytosol. Recent work by Dammai and Subramani (56) suggests that such a so-called extended shuttle model may also apply to the human situation (Fig. 2b).
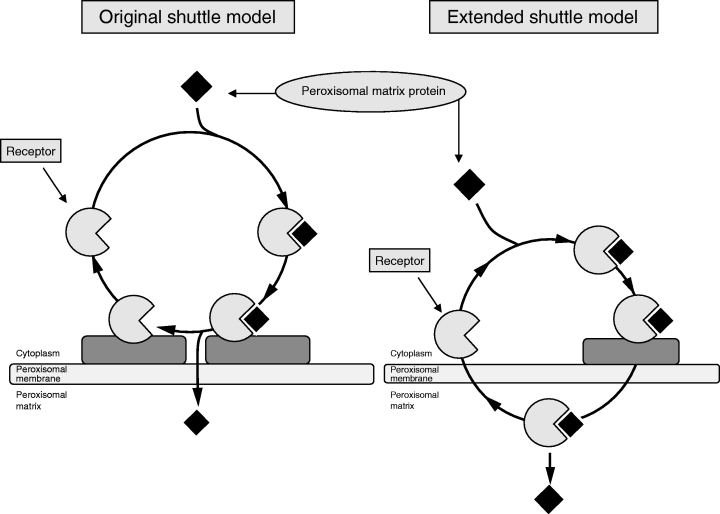
Schematic representation of the original and modified shuttle models. (a) The original shuttle model in which the receptor shuttles between the cytosol, where a PTS1 or PTS2 protein is picked up, and the cytosolic face of the peroxisomal membrane, where the receptor–cargo complex docks followed by dissociation of the receptor–cargo complex and transfer of the cargo protein across the peroxisomal membrane and recycling of the receptor back into the cytosol. (b) The modified so-called ‘extended shuttle’ model in which the receptor–cargo crosses the peroxisomal membrane en block followed by back transport of the empty receptor from the inside of peroxisomes to the cytosol.
Pex5p belongs to the family of TPR-containing proteins, which are characterized by highly degenerate, repetitive sequences of 34 amino acids. TPRs are found as tandem arrays of 3–16 motifs in a wide variety of proteins involved in many different cellular processes including cell-cycle regulation, chaperone functions, and protein phosphorylation. The C-terminal half of Pex5p consists of two clusters each comprising three TPR domains (TPR 1–3 and TPR 5–7), which are linked by a hinge region denoted TPR4. The TPR domains participate in a special folding structure that allows the interaction with the PTS1 tripeptide that appears to be embraced by all TPR motifs (57, 58). The importance of the TPR domains for recognition of the PTS1 tripeptide is immediately clear if it is realized that a single amino acid change (N489K) within the sixth TPR domain abolishes interaction between human Pex5p and the PTS1 signal and causes NALD, one of the Zellweger spectrum disorders (51). In addition to binding PTS1 proteins, all Pex5p proteins bind Pex13p and Pex14p whereas mammalian Pex5p proteins also bind Pex7p as discussed later.
Pex7p, the receptor for PTS2 proteins. The identification by Erdmann et al. (20) of an S. cerevisiae mutant with a defect in PTS2-mediated import, but a normal PTS1-import pathway, led Kunau and coworkers to identify the PTS2 receptor (Pex7p) (59) which turned out to be a member of the WD-40 family of proteins, a family characterized by repeats of approximately 40 amino acid residues, each containing a central Trp-Asp (WD) motif. WD-40 proteins have been implicated in interactions with TPR-containing proteins and recent evidence suggests that Pex5p and Pex7p indeed interact, at least in mammals as discussed below. After its initial identification in S. cerevisiae(59–61), subsequent Pex7p proteins have been identified in other species including mammals (62–64). The subcellular localization of Pex7p is still controversial due to conflicting results in human fibroblasts and S. cerevisiae, which show a predominant cytosolic localization in human fibroblasts vs an entirely peroxisomal localization in S. cerevisiae as concluded by Zhang and Lazarow (60).
PTS2 protein import route in mammals and yeasts: similar game, different players (HsPex5pL, ScPex18p/Pex21p, and YlPex20p/NcPex20p). Despite the many similarities between mammals and yeasts with respect to peroxisome biogenesis, there are also important differences, one being the role of Pex5p. Indeed, in yeasts, Pex5p is only involved in the import of PTS1 proteins, whereas in mammals, Pex5p is involved in both PTS1- and PTS2-protein import. In contrast, Pex7p is involved in PTS2-protein import only, which is true for both mammals and yeasts. The exclusive role of Pex5p and Pex7p in PTS1- and PTS2-protein import, respectively, in yeasts, is exemplified by the phenotypes of the pex5 and pex7 yeast mutants in which only the import of PTS1 proteins (pex5-mutant) or PTS2 proteins (pex7-mutant) is impaired. Subsequent studies revealed that in human skin fibroblasts and CHO cells, the situation is different with respect to Pex5p. In fact, analysis of human and CHO pex5-mutants revealed two different phenotypes; in some mutants, only the PTS1-protein import pathway was disrupted whereas in other mutants, both the PTS1- and PTS2-protein import pathways were blocked. This enigma was resolved when Pex5p was found to exist in two forms in mammals: a long form (Pex5pL) and a short form (Pex5pS). Pex5pS and Pex5pL are identical with one important difference, which is the presence of an additional internal segment of 37 amino acids positioned between amino acids 214 and 215 (human) or 215 and 216 (Chinese hamster). The differential role of Pex5pS and Pex5pL was clearly shown by Otera and coworkers (65). These authors showed that in pex5-deficient CHO cells disturbed in both PTS1- and PTS2-protein import, Pex5pS only restored the import of PTS1-proteins whereas Pex5pL restored both PTS1- and PTS2-import in the same cells. The same was shown by Braverman et al. (66) in human pex5-mutants in which Pex5pL restored both PTS1- and PTS2-protein import whereas Pex5pS restored PTS1-protein import only. These data clearly show that Pex5pL plays an essential role in PTS2-protein import. Subsequent studies have shown that Pex5pL and Pex7p interact with one another. The region in Pex5pL necessary for this interaction was mapped using truncated versions of Pex5pL and includes the amino-terminal amino acids of the Pex5pL-specific 37 amino acids insertion, together with amino acids lying outside this region. The S214F mutation in this region disrupted binding to Pex7p as shown by Otera and coworkers (67) and resulted in a specific PTS2-protein import defect, while PTS1-protein import was not affected (68). It is important to stress that the role of Pex5pL in the import of PTS2 proteins in mammals is independent of its role in the import of PTS1 proteins. Indeed, when a truncated version of Pex5pL was expressed containing only the amino-terminal half of the protein without a TPR motif, complementation of the PTS2-protein import defect was observed in PEX5-deficient mammalian cells (67, 69).
Recent studies have shed new light on the remarkable difference with respect to the role of Pex5p between yeasts and mammals. Studies in the yeast S. cerevisiae have shown that PTS2-protein import is not only dependent on Pex7p but also on Pex18p and Pex21p (27), which are not found in humans. In Y. lipolytica (28) and N. crassa (29), PTS2-protein import is dependent upon another protein named Pex20p. It turns out that the amino acid sequence in the 37 amino acid internal region of Pex5pL is highly conserved in S. cerevisiae Pex18p and Pex21p and Y. lipolytica and N. crassa Pex20p, suggesting that this region, shared between the four proteins, is involved in the formation of an import-competent complex of Pex7p and PTS2 proteins. The absence of the conserved peptide motif in fungi is in line with the fact that Pex5p plays no role in PTS2-protein import, whereas its presence in different mammals, protozoa, and plants indicates that Pex5p is also involved in PTS2-protein import in these organisms. Figure 3 depicts the different PTS2-protein import pathways and the distinct roles of HsPex5pL, ScPex18p and ScPex21p, and YlPex20p/NcPex20p in the different species. Interestingly, Pex5p of Caenorhabditis elegans does not contain this conserved peptide motif, which agrees with the complete lack of the PTS2-protein import pathway in this organism (70).
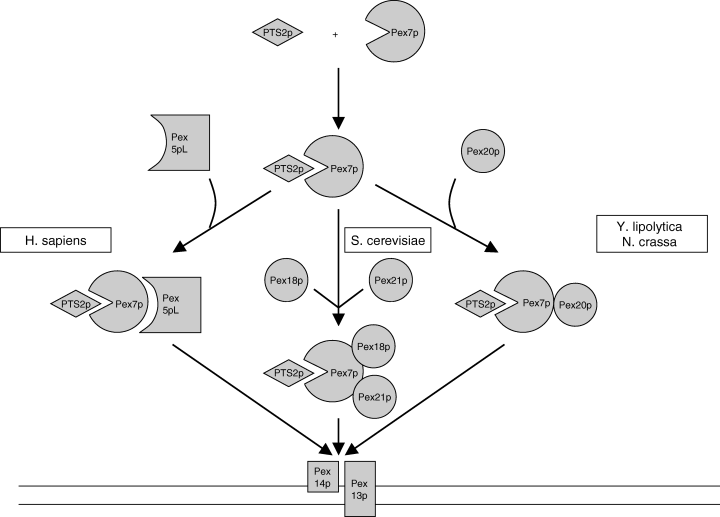
PTS2-protein import in mammals and yeast: similar game, different players. In several organisms, including humans (Homo sapiens) the long form of the PTS1 receptor (Pex5pL) is needed for the import of PTS2 proteins by forming a complex with Pex7p and its cargo, i.e. a PTS2 protein. In Saccharomyces cerevisiae, PTS2-protein import requires the active participation of two helper proteins (Pex18p and Pex21P) whereas in Yarrowia lipolytica and Neurospora crassa, this task is fulfilled by a single protein, i.e. Pex20p.
Receptor docking and the essential role of human Pex13p and Pex14p
There is general agreement that Pex13p and Pex14p form the docking complex where the PTS1- and PTS2-protein import routes converge. Pex13p is a PMP with two membrane-spanning domains with both the N- and C-terminus exposed to the cytosol. Pex13p belongs to the family of SH3 (Src-Homology 3) proteins. The SH3 domain in Pex13p is located at its C-terminus. SH3 domains are small, non-catalytic protein modules capable of protein–protein interactions, which participate in diverse intracellular processes. These domains consist of 60–70 amino acids, have a high sequence similarity, and form structurally similar conformations. Resolution of the three-dimensional structure of various SH3 domains and their contact sites with peptide ligands has revealed that highly conserved aromatic amino acid residues form a hydrophobic cleft running between two variable loops: This hydrophobic cleft forms the binding platform for ligand association, with the two variable loops contributing to ligand recognition and specificity. Typically, SHR domains recognize and bind short proline-rich peptides. The minimal consensus sequence for this peptide is Pro-X-X-Pro (P-X-X-P), where X is any amino acid, plus an additional basic amino acid located C-terminally (class I: P-X-X-P-X-R) or N-terminally (class II: R-X-X-P-X-X-P) of the P-X-X-P core. The proline-rich peptide segment adopts a left-handed polyproline-type helix (PP2) that, depending on the class of the ligand, can bind in two orientations with respect to the SH3 domain.
Pex14p is the other member of the docking machinery and is required for the import of both PTS1 and PTS2 proteins. Pex14p is tightly associated with the peroxisomal membrane either as a peripheral membrane protein or an integral membrane protein. Pex14p interacts with Pex13p but can also bind directly to Pex5p and Pex7p. Schliebs et al. (71) have shown that the amino-terminal 78 amino acids of human Pex14p are involved in binding of Pex5p with a very high affinity. Multiple binding sites for Pex14p were shown to be present in the amino-terminus of Pex5p, and subsequent studies showed that the pentapeptide WXXXF/Y repeats were involved in binding Pex14p in mammals and in plants (67, 72, 73). The number of pentapeptide repeats differs among the different organisms with two repeats in S. cerevisiae Pex5p, and seven in human Pex5pL. Pex14p directly interacts with Pex13p via the SH3 domain which involves a class II P-X-X-P-X-R motif (PPTLPHRDW) in Pex14p as shown for S. cerevisiae (74). Binding of Pex5p to the same SH3 domain of Pex13p does not occur at the PP-2-binding phase but at a novel interaction site (75). X-ray crystallography and mass spectrometry data from Dounagamath and coworkers (76, 77) revealed the existence of two functionally and structurally independent binding sites on the SH3 domain of Pex13p for Pex5p and Pex14p, respectively, with Pex7p binding at the amino-terminal end of the Pex13p.
Although it was initially thought that Pex13p was the first site of receptor docking, current data suggest that it is in fact Pex14p, which comes first. This model is supported by data from Otera et al. (67) and Urquhart et al. (78) who showed that Pex14p binds to PTS1-loaded Pex5p whereas Pex13p only binds to unloaded Pex5p. Otera et al. (67) proposed that Pex5p bound to a PTS1 protein first binds to the Pex13p–Pex14p complex via interaction with Pex14p after which the PTS1 protein is released from Pex5p followed by dissociation of the Pex13p–Pex14p complex. Subsequently, the unloaded Pex5p is transferred to Pex13p and shuttles back to the cytosol. This model implies that Pex13p and Pex14p form functionally distinct subcomplexes, which are both involved in the import process of peroxisomal proteins. Taken all data together, Pex14p is indeed the most likely primary docking protein. It might well be that other proteins are part of the docking complex. A good candidate, identified in S. cerevisiae (79) and P. pastoris (42), is Pex17p which behaves as a peripheral membrane protein tightly bound to the peroxisomal membrane in S. cerevisiae (79) whereas in P. pastoris, it is an integral membrane protein with the carboxyterminus facing the cytosol (42). In S. cerevisiae, Pex17p is thought to be part of the docking complex together with Pex14p and Pex13p (79), whereas Snyder et al. (42) favored a model in which Pex17p is also involved in the import of PMPs. Subsequent studies by Hettema et al. (25) and Harper et al. (80) showed normal import of PMPs in both Ppex17pΔ and Scpex17Δ cells, which argues against the model of Snyder et al. (42). Taken together, the bulk of evidence favors a role of Pex17p in peroxisomal matrix import and not in the import of PMPs. Except from its identification in S. cerevisiae and P. pastoris, no mammalian Pex17p has been identified so far.
Translocation across the peroxisomal membrane and the human peroxins Pex2p, Pex10p, and Pex12p
Three peroxins belonging to the family of RING zinc finger proteins, i.e. Pex2p, Pex10p, and Pex12p, are thought to be involved in the actual transport machinery. All three proteins are iPMPs and have a carboxy-terminal RING finger domain exposed to the cytosol. Based on the finding that fibroblasts from PBD patients with mutations in PEX2, PEX10, or PEX12 accumulated Pex5p at the level of peroxisomes in contrast to normal fibroblasts, Pex2p, Pex10p, and Pex12p are thought not to be involved in receptor docking but in one of the subsequent steps of protein import. Mutant pex2, pex10, or pex12 cells are all disturbed in the import of peroxisomal matrix proteins while the import of PMPs is not affected. Reguenga et al. (81) have obtained evidence suggesting that Pex2p and Pex12p are together in a complex with Pex14p and Pex5p (81). Pex13p is also part of this complex although in non-stoichiometric amounts.
Another prediction for the proteins involved in translocation would be that they should interact directly or indirectly either with the cargo to be translocated or with the receptors for that cargo, Pex5p and/or Pex7p. Pex2p, Pex10p, and Pex12p all contain a C3HC4 zinc-binding domain, or RING finger, a protein module that is thought to mediate protein–protein interactions. The RING finger is essential for the functions of both Pex10p and Pex12p, and recent studies have shown that Pex10p and Pex12p directly interact with Pex5p and with each other (82, 83).
Receptor recycling and the role of the human peroxins Pex1p, Pex6p, and Pex26p
The peroxins Pex1p and Pex6p are members of the large family of AAA proteins (ATPases) associated with a wide range of cellular activities (21, 84). The AAA domain consists of 220–230 amino acids and contains two motifs named Walker A and B, which bind and hydrolyze ATP, respectively (85). The role of Pex1p and Pex6p in peroxisome biogenesis has remained controversial with two opposing views. The first view holds that Pex1p and Pex6p are required for peroxisome biogenesis possibly playing a role in some membrane fusion event involving vesicles derived from the ER (86). This hypothesis was stimulated, in part, by the observation that many of the initially identified members of the AAA ATPase family were involved in membrane fusion events. In line with this postulate, Titorenko et al. (87) showed that in Y. lipolytica, Pex1p and Pex6p stimulate the fusion of pre-peroxisomal vesicles.
A complicating factor is that the subcellular localization of Pex1p and Pex6p is controversial. In rats and H. polymorpha, Pex1p and Pex6p are associated with the peroxisomal membrane (88, 89), whereas in P. pastoris and Y. lipolytica, there seems to be an association with vesicles distinct from mature peroxisomes (87, 90, 91). On the other hand, in human cells, both Pex1p and Pex6p are predominantly cytosolic (92, 93), although these results were obtained by overexpression. It has been shown that Pex1p and Pex6p interact with each other in an ATP-dependent manner (89, 90, 93, 94).
The second view holds that Pex1p and Pex6p are involved in matrix protein transport rather than in peroxisome membrane biogenesis. One of the arguments is that human cells mutated in PEX1 and PEX6 contain abundant peroxisomal membrane structures (ghosts) that are larger, not smaller, than peroxisomes typically present in control fibroblasts. Furthermore, absence of Pex1p or Pex6p results in a dramatic instability of Pex5p with levels falling as low as 1–5% of control. On the other hand, Pex5p levels are normal or even elevated in human cells with inactivating mutations in PEX2, PEX10, or PEX12, demonstrating that Pex5p instability is not a general characteristic of human pex-mutants. A similar reduction in Pex5p abundance has been observed in P. pastoris pex1- and pex6-mutants (92). Taken together, these results indicate that Pex1p and Pex6p are required for the stability of Pex5p and most likely play a role in the recycling of the PTS1 receptor.
Recent studies have shown that the S. cerevisiae integral membrane protein Pex15p binds Pex6p in an ATP-dependent manner (95). Interestingly, studies by Matsumoto et al. (96, 97) have led to the identification of human Pex26p which anchors both Pex1p and Pex6p to the peroxisomal membrane and in fact appears as the human equivalent of yeast Pex15p as depicted in Fig. 4, which shows the essential features of peroxisome biogenesis in humans and the presumed role of Pex1p, Pex2p, Pex5p, Pex6p, Pex7p, Pex10p, Pex12p, Pex13p, Pex14p, and Pex26p in the uptake of PTS1 and PTS2 proteins.
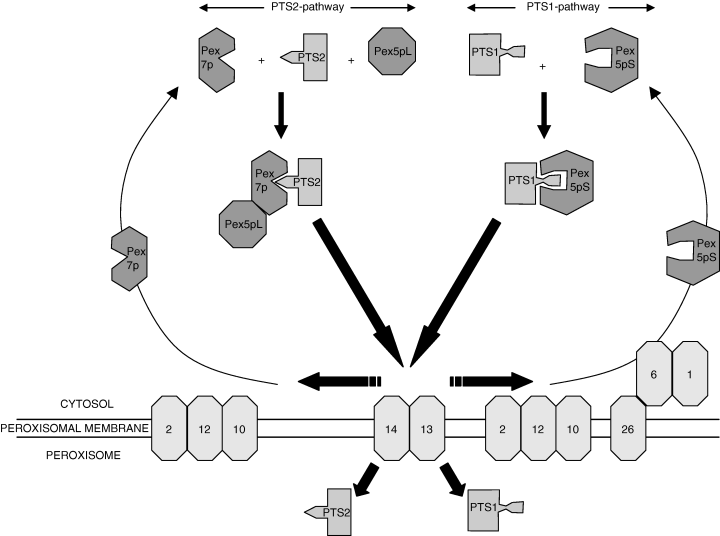
Peroxisome biogenesis in humans and its essential features in which the presumed roles of the different peroxins is shown within the framework of the original shuttle model of Fig. 2(a).
Peroxisome proliferation and maintenance
Peroxisomes are markedly dynamic organelles, and the number and matrix enzyme content of peroxisomes can change dramatically depending upon environmental conditions and the metabolic state. In the yeast S. cerevisiae, for instance, exposure to fatty acids, particularly oleic acid, leads to a large increase in peroxisome abundance and size. The proliferation of peroxisomes under these conditions is associated with dramatic changes in gene expression, which requires the transcription factors Pip2 and Oaf1 (98, 99). These two proteins bind oleate-response elements within transcriptional control regions of responsive genes and are required for both the transcriptional response to oleic acid and the proliferation of peroxisomes.
In rodents, the number, size, and enzyme content of peroxisomes can be induced dramatically by certain xenobiotics like clofibrate and plasticizers as well as naturally occurring fatty acids. A key player in this respect is the peroxisome proliferator-activated receptor-α (PPAR-α), a member of the nuclear receptor super family. Activated PPAR-α forms a heterodimer with a second member of the nuclear receptor super family, the retinoid-X receptor (RXR) together forming an active transcription factor that binds cis-acting elements called peroxisome proliferator-responsive elements (PPREs). In line with the important role of PPAR-α in the induction of peroxisomes in rodents is the fact that PPAR–/– mice do not show induction of peroxisomes by clofibrate and other peroxisome proliferators (100).
The peroxin Pex11p also plays a major role in peroxisome proliferation. Indeed, the S. cerevisiae pex11 mutant accumulates only four to five very large peroxisomes when incubated in oleic acid containing medium, whereas overexpression of PEX11 led to hyper-proliferation of peroxisomes with an increased number of peroxisomes of reduced size (101–103). These data indicate that Pex11 proteins control peroxisome division. According to Van Roermund et al. (104), the defect in peroxisome division and proliferation in pex11Δ cells is secondary to the role of Pex11p in peroxisomal beta-oxidation. This conclusion was based on the finding that pex11Δ cells display a marked block in medium chain fatty acid beta-oxidation. Studies by Li and Gould (105) suggest, however, that the defect in medium chain fatty acid beta-oxidation is a secondary phenomenon with Pex11p primarily controlling peroxisome division.
In humans, three distinct genes with high similarity to yeast PEX11, including PEX11α, PEX11β, and PEX11γ, have been identified. Studies by Schrader et al. (106) showed that overexpression of the human PEX11β gene alone was sufficient to induce peroxisome proliferation, demonstrating that proliferation can occur in the absence of extracellular stimuli. Furthermore, overexpression of PEX11α also induced peroxisome proliferation but to a much lower extent. Studies in the mouse, which also has three Pex genes including Pex11α, Pex11β, and Pex11γ, have shown that, although Pex11α expression is induced by activation of PPAR-α, Pex11α is not required for peroxisome proliferation induced by classical peroxisome proliferators like clofibrate. Interestingly, Pex11α was required for peroxisome proliferation in response to 4-phenylbutyrate, which does not act via PPAR-α (107).
Studies in the yeast S. cerevisiae by Hoeffner et al. (108) have shown that the dynamin-like GTPase Vps1p is required for peroxisome division. A mammalian dynamin-like protein DLP1p, has also been identified (109). Knockdown of DLP1 expression by siRNA caused tubulation of peroxisomes and inhibition of peroxisome division. In the last few years, a number of new peroxins have been described, which are all involved in regulating peroxisome size, number, and distribution (Table 3). It is clear that much remains to be learned about the factors involved in peroxisome proliferation and maintenance.
Laboratory diagnosis of peroxisome biogenesis disorders
ZS, NALD, and IRD
Although peroxisomes catalyze a variety of different metabolic functions, there are three functions which are of direct relevance for the laboratory diagnosis of PBDs. These are: (i) beta-oxidation of fatty acids, including VLCFAs notably C26:0, pristanic acid, and di- and trihydroxycholestanoic acid; (ii) biosynthesis of ether phospholipids, notably plasmalogens; and (iii) alpha-oxidation of phytanic acid. In patients belonging to the Zellweger spectrum with ZS, NALD, and IRD, all these peroxisomal functions are deficient, which leads to the accumulation of C26:0, pristanic acid and di- and trihydroxycholestanoic acid, the deficiency of plasmalogens, and the accumulation of phytanic acid. It should be added that pristanic acid and phytanic acid are solely derived from dietary sources and may thus be completely normal in PBD patients, especially when they are young. In the remaining PBD, i.e. RCDP, in which PEX7 is mutated, peroxisomal beta-oxidation is normal, thus explaining the normal VLCFA profile in these patients. Plasmalogen synthesis and phytanic acid alpha-oxidation, however, are defective in RCDP. As a consequence, the laboratory diagnosis of PBDs depends upon the type of PBD, suspected in a particular patient (see flowcharts of 5, 6).
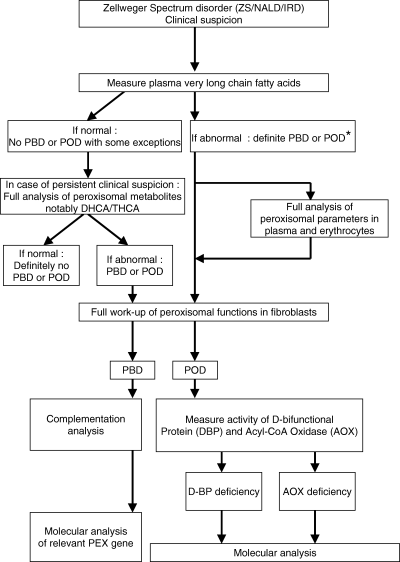
Flowchart for the differential diagnosis of patients with clinical signs and symptoms suggestive of a Zellweger spectrum disorder.
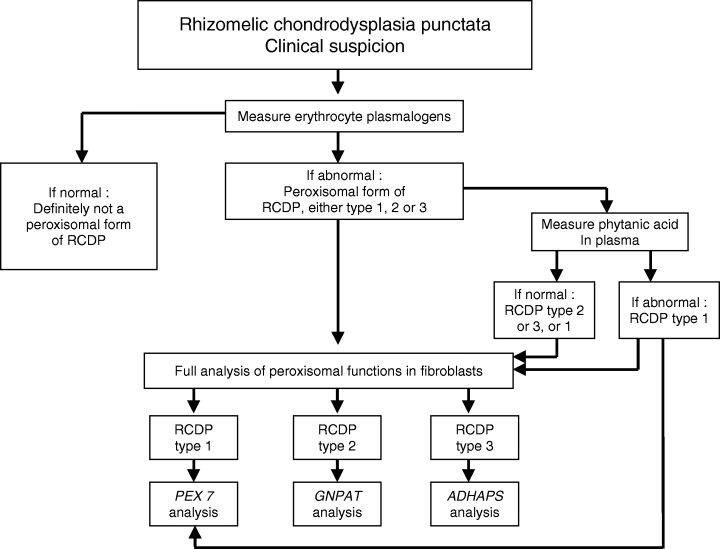
Flowchart for the differential diagnosis of patients with clinical signs and symptoms suggestive of rhizomelic chondrodysplasia punctata.
Plasma VLCFA analysis has turned out to be a very reliable method for the laboratory diagnosis of ZS, NALD, and IRD. In a series of >500 Zellweger spectrum patients ranging from classical ZS to IRD and other mild variants, plasma VLCFAs have always been found abnormal except for a few exceptional cases. Indeed, we recently identified a PBD patient with normal VLCFA levels but abnormal bile acid intermediates, indicating that the patient definitely suffered from a peroxisomal disorder. Subsequent studies led to the identification of a peroxisome biogenesis defect due to bona fide mutations in the PEX12 gene. These findings indicate that great care must be taken with respect to the laboratory diagnosis of a peroxisomal disorder and that a normal VLCFA profile does not exclude a peroxisomal disorder (Fig. 5).
It should be added that the finding of an abnormal VLCFA profile in a particular Zellweger spectrum patient does not necessarily point to a PBD. Indeed, plasma VLCFA may also be abnormal in a number of single peroxisomal enzyme deficiencies, notably D-bifunctional protein deficiency and acyl-CoA oxidase deficiency. Patients suffering from the latter two disorders are clinically indistinguishable from patients suffering from a Zellweger spectrum disorder. Obviously, plasma VLCFAs are also elevated in X-ALD patients. However, the clinical signs and symptoms of X-ALD patients are quite different from those of PBD patients.
In order to resolve whether the accumulation of VLCFAs in a particular patient is due to a defect in peroxisome biogenesis or results from an isolated defect in peroxisomal beta-oxidation, fibroblast studies are required. In fibroblasts, plasmalogen biosynthesis, peroxisomal beta-oxidation, and phytanic acid alpha-oxidation can be measured reliably. Furthermore, the presence or absence of peroxisomes can be studied via immunofluorescence microscopy analysis using antibodies against peroxisomal matrix proteins like catalase. In the majority of patients, such fibroblast studies lead to an unequivocal diagnosis allowing discrimination between a PBD and a peroxisomal beta-oxidation disorder at the level of D-bifunctional protein or acyl-CoA oxidase (see flowcharts of Fig. 5).
With respect to the laboratory diagnosis of RCDP, analysis of plasmalogens in erythrocytes is also highly reliable. Indeed, in all established RCDP patients we have studied through the years (>100), erythrocyte plasmalogens were always deficient making erythrocyte plasmalogen analysis a highly reliable initial laboratory test indicating that if plasmalogens are deficient, a peroxisomal form of RCDP is established. Because RCDP is genetically heterogeneous with three distinct genetic forms including RCDP type 1, the most frequent type of RCDP belonging to the PBD group, and the less frequent RCDP types 2 and 3 belonging to the group of single peroxisomal enzyme deficiencies, additional studies have to be done to pinpoint the precise type of RCDP.
Resolution between the three types again requires detailed studies in fibroblasts although analysis of phytanic acid in plasma may also be helpful to discriminate between type 1 and type 2/3, respectively. The fact that phytanic acid is derived solely from exogenous sources renders phytanic acid analysis in plasma, however, an unreliable parameter (110, 111). Indeed, if phytanic acid is elevated in a particular RCDP patient, one can be sure of RCDP type 1, which may prompt direct molecular analysis of the PEX7 gene. A normal phytanic acid level, however, may point to RCDP type 2 or type 3, but RCDP type 1 cannot be excluded. In this case, definitive identification of RCDP type 1, 2, or 3 requires detailed studies in fibroblasts, which includes activity measurements of dihydroxyacetonephosphate transferase and alkyl-DHAP synthase, the products of the GNPAT and ADHAPS genes, respectively. Figure 6 depicts the flowchart we use in the daily practice of the laboratory diagnosis of RCDP.
Molecular basis of the peroxisomal biogenesis disorders
Due to the potential involvement of many different genes, an essential prerequisite for the identification of the molecular defect in any patient affected by a peroxisomal biogenesis disorder is complementation analysis. Complementation analysis is a powerful tool to resolve whether a particular disorder, or group of disorders, is genetically heterogeneous or not. In practice, complementation analysis involves fusion of fibroblasts from two patients, affected in peroxisome biogenesis, for instance. Fusion will generate hybrid cells containing nuclei from the two patients' fibroblasts. These cells are called heterokaryons. If the defective genes in the two patients' cell lines are different, one would expect restoration of peroxisome formation, whereas in the other case, when the mutant genes are identical, no complementation would occur. Tager and coworkers (112, 113) were the first to apply complementation analysis to study the genetic basis of different PBDs. In a first study by Brul et al. (113), fibroblasts from seven PBD patients with phenotypes ranging from ZS to NALD, IRD, and RCDP were subjected to complementation analysis. These seven patients' cell lines were found to belong to five different CGs, which immediately suggested marked genetic heterogeneity within the PBD group. Brul et al. (113) used two different parameters to assess complementation, including (i) activity measurement of DHAPAT, a peroxisomal enzyme catalyzing the first step in plasmalogen biosynthesis, and (ii) catalase latency. The latter method determines whether catalase is peroxisomal or not. Subsequently, several groups have also performed complementation analysis using other methods to assess complementation, including de novo plasmalogen biosynthesis, phytanic acid alpha-oxidation, peroxisomal beta-oxidation, and immunofluorescence microscopy analysis using antibodies raised against catalase. The latter method, first applied by Yajima et al. (114), allows direct visualization of peroxisomes in fused cells and is the method of choice to assess complementation in case of PBD patients. Because catalase immunofluorescence is normal in fibroblasts from RCDP patients, this method can only be applied to cells from Zellweger spectrum patients.
A collaborative study between the three main groups performing complementation analysis has led to the identification of nine distinct CGs (115). In subsequent years, two additional CGs have been identified which brings the total number now at 11 or 12, if RCDP is also included (Table 4).
| Complementation groups | Import of peroxisomal matrix proteins | |||||||
|---|---|---|---|---|---|---|---|---|
| Number | Gifu | KKI | Adam | PTS1 | PTS2 | Import of iPMPs | Gene involved | Phenotypes |
| 1 | A | 8 | VI | – | – | + | PEX 26 | ZS/NALD/IRD |
| 2 | B | 7 (=5) | VII | – | – | + | PEX 10 | ZS/NALD/IRD |
| 3 | C | 4 (=6) | III | – | – | + | PEX 6 | ZS/NALD/IRD |
| 4 | D | 9 | VIII | – | – | – | PEX 16 | ZS |
| 5 | E | 1 | II | – | – | + | PEX 1 | ZS/NALD/IRD |
| 6 | F | 10 | V | – | – | + | PEX 2 | ZS |
| 7 | G | 12 | IX | – | – | – | PEX 3 | NALD |
| 8 | H | 13 | X | – | – | + | PEX 13 | ZS/NALD |
| 9 | J | 14 | XI | – | – | – | PEX 19 | ZS |
| 10 | 2 | IV | – | –a | + | PEX 5 | ZS/NALD/IRD | |
| 11 | 3 | XII | – | – | + | PEX 12 | ZS/NALD/IRD | |
| 12 | R | 11 | I | + | – | + | PEX 7 | RCDP |
- KKI, ; PTS, peroxisomal targeting signals; iPMP, integral PMPs.
- a In a single patient ( 51), only the PTS1-import pathway was found to be defective with preservation of the PTS2-import pathway due to a point mutation in the PEX5 gene causing a N489K amino acid substitution. The mutant Pex5p was unable to sustain PTS1-protein import but was apparently able to form a stable complex with Pex7p and PTS2 proteins allowing a normally functioning PTS2-import pathway.
With the recent identification of PEX26 as the defective gene in CG8 by Matsumoto et al. (96, 97), the PEX genes underlying each of the CGs have all been identified now. Most CGs include only a few patients. One exception to this rule is CG1, with PEX1 as the defective gene, which is by far the largest CG containing more than half of all Zellweger spectrum patients (116–119). In our own series of 246 Zellweger spectrum patients thereby excluding RCDP, 174 patients (59%) were found to belong to CG1 (PEX1) followed by 12% in CG4 (PEX6) and 6% in CG3 (PEX12) (Gootjes et al., unpublished).
If all mutant PEX alleles are taken together, more than 100 mutations have so far been described in literature. In many cases, mutations are private being restricted to single families only. Most mutations have been described in the PEX1 gene (>40). Among these mutations, a few common mutations have been identified. Most common is a missense mutation in exon 15 (c.2528G > A) leading to the substitution of the glycine at position 843 of Pex1p by an asparagine (p.G843D) in the second ATP-binding domain. Patients homozygous for this mutation show the mild Zellweger spectrum phenotype (NALD/IRD). The frequency of the c.2528G > A (p.G843D) allele ranges from 0.25 to 0.37 in the different cohorts. In our own cohort of PEX1-mutated patients, we found an allele frequency of 0.36. Twenty percent of the patients were homozygous and 33% were compound heterozygous for the c.2528G > A allele.
The second most common mutation is a T-insertion in exon 13 (c.2097–2098insT), first described by Maxwell et al. (120) and Collins and Gould (121), which results in a frame shift and low steady state PEX1 mRNA levels, presumably caused by nonsense mediated RNA decay. At the protein level, it leads to truncation of the PEX1 protein within the second AAA domain, abolishing PEX1 function completely. In its homozygous form, the mutation results in the severe Zellweger phenotype. In three different studies, an allele frequency of around 0.3 has been reported. However, in our own patient cohort, we found an allele frequency of 0.16 (Gootjes et al., unpublished). Together, these two mutations account for around 50–60% of all mutant PEX1 alleles. Interestingly, the mutation c.2528G > A leads to a mutant Pex1p, which allows some residual import of peroxisomal matrix proteins. The mutation seems to result in a misfolded protein, which is more stable at lower than at higher temperatures, which explains the mosaic catalase immunofluorescence pattern at 37° but a virtually normal pattern at 30° (122, 123). The G843D aminoacid substitution most likely disrupts the interaction between Pex1p and Pex6p (93, 124).
RCDP
The molecular basis of RCDP type 1 with PEX7 as the mutant gene has recently been described in two large studies describing mutation data in 60 (125) and 78 (126) patients with RCDP type 1, respectively. Braverman et al. (125) reported the identification of a total of 24 mutant PEX7 alleles whereas Motley et al. (126) described 22 different mutant alleles. In both studies, one frequent mutation was found (L229X), which leads to a truncated protein with no apparent biological function. A few additional mutations with less frequency have been described in addition to a large series of mostly private mutations.
Prenatal diagnosis of PBDs
The different PBDs are severe disorders, often associated with early death thus warranting prenatal diagnosis. Prenatal diagnosis of the different PBDs requires a full study in the index patient and should not be based on clinical signs and symptoms only. This is immediately clear if it is realized that the clinical diagnosis ZS may either be due to a defect in peroxisome biogenesis or a defect in peroxisomal beta-oxidation, notably at the level of D-bifunctional protein (110). We will discuss the prenatal diagnosis of the different types of PBDs (ZS/NALD/IRD vs RCDP) below.
ZS/NALD/IRD
If the index patient has been studied in full detail, which includes complementation analysis and molecular analysis of the PEX gene involved, followed by confirmation of the mutations found in DNA from the parents, the preferred prenatal diagnostic method is mutation analysis. In practice, however, the exact molecular defect has not been determined in every patient. This may be due to the fact that fibroblast studies did not include complementation analysis thus obstructing identification of the PEX gene involved. In such cases, prenatal diagnosis can also be done in chorionic villous tissue and/or chorionic villous fibroblasts using other methods. Obviously, chorionic villous material, rather than chorionic villous fibroblasts, is the material of choice because of potential problems such as maternal overgrowth and failure of cells to grow. In principle, a variety of methods can be used for prenatal diagnosis including measurement of the activity of: i) DHAPAT, ii) alkyl-DHAP synthase, iii) acyl-CoA oxidase, iv) D-bifunctional protein, and v) immunoblot analysis of peroxisomal enzyme proteins, notably acyl-CoA oxidase, bifunctional protein, and peroxisomal thiolase. In our own center, we measure the activity of DHAPAT and, in addition, perform immunoblot analysis of acyl-CoA oxidase and peroxisomal thiolase. Analysis of acyl-CoA oxidase in normal chorionic villous material yields immuno-reactive bands of 70, 50, and 20 kDa, whereas in chorionic villous biopsy material of affected fetuses, only the 70-kDa band is observed. In case of peroxisomal thiolase, analysis of normal chorionic villous biopsy material shows a single band of 41 kDa, whereas in chorionic villous material of affected fetuses, only the precursor form of peroxisomal thiolase at 44 kDa is seen.
In fibroblasts of more mildly affected patients, including patients affected with NALD or IRD, DHAPAT is moderately deficient and the immunoblot profiles of acyl-CoA oxidase and peroxisomal thiolase are not fully conclusive with the 50-kDa and 20-kDa bands of acyl-CoA oxidase and the 41-kDa band of peroxisomal thiolase present, albeit in reduced amounts. For this reason, we do not perform any analyzes in chorionic villous biopsy material, but we prefer other methods in cultured chorionic villous cells, notably immunofluorescence microscopy analysis using specific antibodies directed against catalase as well as measurement of the VLCFA profile by GC/MS and/or C26:0 or pristanic acid beta-oxidation in intact chorionic villous cells. The best and easiest procedure is immunofluorescence microscopy analysis of catalase, which usually gives an unambiguous result. In the past 15 years, we have done more than 200 prenatal diagnoses of PBDs with no mistakes.
RCDP
As described above for the prenatal diagnosis of ZS/NALD/IRD, prenatal diagnosis of RCDP should be done preferably by molecular analysis of the PEX7 gene. Obviously, this requires detailed studies in fibroblasts from the index patient in order to discriminate between RCDP type 1, 2, and 3, followed by molecular analysis of the PEX7, GNPAT, and ADHAPS gene and confirmation of the mutations found in the parents. As with the other types of PBD, such detailed studies often have not been done in fibroblasts from particular patients, which implies that in such cases, prenatal diagnosis should be done using enzymatic and/or cell biological methods. In case bona fide abnormalities have been found in fibroblasts from the index patient, we prefer to do immunoblot analysis of peroxisomal thiolase plus quantitative determination of plasmalogens. This set of tests usually generates unequivocal results so that we rarely need to do additional studies in cultured chorionic villous cells. In some cases where studies in fibroblasts from the index patient have shown only minor abnormalities with some 41-kDa thiolase present and only a partial deficiency of plasmalogens in fibroblasts, we prefer to do detailed studies in cultured chorionic villous cells with powerful additional methods such as de novo plasmalogen biosynthesis and phytanic acid alpha-oxidation.
Therapy
Treatment options for PBD patients have remained limited so far. An important problem is that in the severe PBD forms, including ZS and RCDP, abnormalities already develop in utero, which limits potential postnatal treatment. Supportive therapies, such as anticonvulsant therapy to control seizures, physical and orthopedic therapy, and correction of visual and auditory impairment, are important to improve quality of life. The identification of milder phenotypes with less pronounced abnormalities and survival into the third and even fourth decade of life has stimulated attempts to correct the different biochemical abnormalities postnatally. Indeed, efforts have been made to correct the deficiency of plasmalogens by supplementation of alkylglycerol to the diet, to decrease VLCFAs, and especially phytanic acid levels, via dietary regimens, and to reduce the toxicity of the accumulating bile acid intermediates by supplementing urso- and chenodeoxycholic acid (127). Partial biochemical and clinical benefits have been reported, but no definite conclusion can be drawn from these studies due to the small number of patients included. In recent years, much interest has centered around docosahexaenoic acid (DHA), a polyunsaturated fatty acid implied in many physiological processes, whose levels are markedly reduced in tissues of Zellweger spectrum patients. Studies by Martinez (128) in 20 PBD patients of unspecified genotype, but mainly at the mild end of the Zellweger spectrum, have shown improved liver function, and in addition, improved plasma levels of peroxisome metabolites and subjective improvement in muscle tone, social contact, and vision. Myelination was claimed to be improved in more than half of those examined by magnetic resonance imaging. These improvements warrant additional larger scale studies.
Mouse models of peroxisome biogenesis disorders
In recent years, several mouse models have been generated in which different genes have been disrupted, which either code for a peroxin or a peroxisomal enzyme or transport protein. So far, six different Pex gene knockouts have been described (Pex2, 5, 7, 11α, 11β, and 13) (107, 129–133). In 1997, the first Pex gene knockouts were reported by Faust and Hatten (129) and Baes et al. (130). The clinical, biochemical, and cellular phenotypes of the Pex2 and Pex5 homozygous knockout mice are remarkably similar. Firstly, both the Pex2(–/–) and Pex5(–/–) mice show the same metabolic abnormalities as described in Zellweger patients including elevated VLCFAs in plasma and tissues, deficient plasmalogens in tissues and erythrocytes, deficient DHA levels in brain (30–40% decrease) but not in liver tissue, and accumulation of the C27 bile acid intermediates di- and trihydroxycholestanoic acid. In addition, marked mitochondrial abnormalities were found in various organs of the Pex5(–/–) mouse (including liver, proximal kidney tubules, adrenal cortex, and heart) and specific cell types (skeletal and smooth muscle cells and neutrophils) (134). Ultrastructural studies revealed the presence of large aggregates of pleomorphic mitochondria with alterations of the mitochondrial outer membrane as well as the cristae. These mitochondrial alterations were quite heterogeneous with normal appearing mitochondria and severely misshaped mitochondria within a single cell. Biochemically, partial deficiencies of complex I and V in livers of Pex5(–/–) mice were found amounting to 40% and 65% of mean control values, respectively. Interestingly, complex IV was much higher in liver of Pex5(–/–) mice(180% of mean control). The significance of these findings remains to be established, especially as ATP levels were higher, rather than lower, in Pex5(–/–) livers as compared to control livers. Remarkably, the mitochondria of the Pex2(–/–) mice were described as normal (129).
With respect to the clinical abnormalities in Pex2(–/–) and Pex5(–/–) mice, mutant pups showed intrauterine growth retardation, severe hypotonia with failure to eat, and neonatal death. Most of the affected pups died within 24 h. Interestingly, survival was found to depend on the genetic background with embryonic lethality in a 129 Svev background and survival for 7–10 days in a 129 Svev/Swiss Webster background (135). In the adrenal cortex of Pex2(–/–) mice, abnormal lipid storage was found with characteristic lamellar lipid inclusions. In the central nervous system of newborn mutant mice, there is disordered lamination in the cerebral cortex, and an increased cell density in the underlying white matter, indicating an abnormality of neuronal migration. Studies in longer surviving Pex2 knockout mice showed that neurons that are delayed in migration at birth eventually populate the cortex, but that mislocalization within the cortical laminae occurs. In 1-week-old Pex2(–/–) mice, cerebellar abnormalities were observed including reduced size, altered folial patterning, and reduced dendritic arborization of Purkinje cells (135). At birth, no signs of liver fibrosis, renal cysts, calcifications in bone, or facial malformation were apparent in the Pex2(–/–) and Pex5(–/–) mice, which is different from what is observed in ZS patients.
The Pex5(–/–) mouse was also used for initial studies on pathophysiologal mechanisms of the disease. Janssen et al. (136) studied whether the reduced level of DHA in brain of Pex5(–/–) mice was a potential cause of the neuronal migration disturbance. Supplementation of pregnant Pex5 heterozygous mothers with DHA ethyl ester during the last 8 days of gestation normalized the DHA content in brain phospholipids with no clinical improvement, however. Indeed, hypotonia, growth retardation, and neuronal migration were as severe as in untreated mice. Importantly, because in vivo and in vitro experiments have shown that glutamatergic neurotransmission via the N-methyl-D-aspartate (NMDA) receptor, linked to changes in intracellular calcium levels, controls the speed of migration, the potential involvement of NMDA neurotransmission in the neuronal migration defect of Pex5(–/–) mice was investigated by administering NMDA receptor agonists and antagonists to Pex5(–/–) embryos during the migration period (137). Treatment of Pex5(–/–) embryos with NMDA antagonists induced embryonic death whereas NMDA agonists partially reversed the migration defect. No changes in NMDA receptor density or glycosylation status were found between Pex5(–/–) and wild-type brain tissue. A deficit in NMDA signal transduction was demonstrated in neuronal cultures derived from Pex5(–/–) mice by monitoring calcium influx in response to NMDA. Pex5(–/–) cells were less sensitive to NMDA than wild-type cells. This effect could be restored by pre-incubation with platelet-activating factor, an etherphospholipid, which requires functional peroxisomes for its synthesis. Taken together, these results suggest that the neuronal migration defect may result, at least in part, from defective NMDA signaling. Maxwell et al. (133) described another knockout mouse model in which Pex13 was disrupted. The Pex13(–/–) mouse resembles the Pex2(–/–) and Pex5(–/–) mice in many respects.
Recently, Brites et al. (131) described the generation of a Pex7 knockout mouse as a model for RCDP. Homozygous mice are viable and display phenotypic abnormalities, characteristic of RCDP. Pex7(–/–) mice are severely hypotonic at birth and show a marked growth impairment (dwarfism). Mortality in Pex7(–/–) mice is highest in the perinatal period, although some Pex7(–/–) mice survived beyond 18 months. Biochemically, Pex7(–/–) mice displayed the same set of abnormalities as observed in RCDP type 1 patients including a deficiency of plasmalogens in all tissues. Interestingly, neuronal migration was found to be impaired, although not to the same extent as in Pex2(–/–) and Pex5(–/–) mice. Analysis of bone ossification in newborn Pex7(–/–) mice revealed a defect in ossification of distal bone elements of the limbs as well as parts of the skull and vertebrae. Interestingly, Rodemer et al. (138) recently described the generation of a mouse model in which the gene coding for DHAPAT (Gnpat), the first enzyme involved in etherphospholipid biosynthesis, was disrupted. The phenotype of the Gnpat(–/–) mouse is comparable to that of the Pex7(–/–) mouse, which indicates that the pathogenesis of RCDP is predominantly, if not exclusively, determined by the inability to synthesize etherphospholipids.
The last two of the Pex knockout mouse models, generated in recent years, include the Pex11α(–/–) and Pex11β(–/–) mice. As described earlier, Pex11 proteins represent true peroxins playing an essential role in peroxisome proliferation. Mammals have three PEX11 genes: PEX11α, PEX11β, and PEX11γ (105). Pex11α is inducible by peroxisome proliferators, whereas Pex11β is expressed constitutively. Pex11α(–/–) mice are phenotypically and metabolically normal. In contrast, mutant mice lacking Pex11β have a severe clinical phenotype with intrauterine growth retardation, hypotonia, and death within 24 h, which phenotype strongly resembles that of mice lacking either Pex2, Pex5, or Pex13. Remarkably, Pex11β(–/–) mice show no peroxisomal abnormalities. Indeed, plasma VLCFAs as well as plasmalogen levels were completely normal in contrast to the findings in Pex2(–/–), Pex5(–/–), and Pex13(–/–) mice. These puzzling results have been interpreted to imply that neither VLCFAs nor plasmalogens contribute to the Zellweger phenotype, at least in rodents. As far as plasmalogens are concerned, this conclusion is hard to reconcile with the marked phenotype of the Gnpat(–/–) mouse, recently generated by Rodemer et al. (138).
Despite these puzzling data, the increasing availability of mouse models, particularly those with conditional alleles, will allow a better understanding of pathophysiological mechanisms with the ultimate perspective of effective treatments for PBD patients.
Acknowledgements
The authors' work was financially supported by the following grants: EU project number QLG1-CT-2001-01277 [mouse models of peroxisomal diseases (MMPD)]; EU project number QLG3-CT-2002-00696 [Refsum's disease: diagnosis, pathology, and treatment (RDDPT)]; NWO project number 901-03-159; NWO project number 916.46.109; NWO project number 901-03-097; NWO project number 99008; PBF project number 97-0216; PBF project number 99-0220; and Royal Dutch Academy of Sciences. Dr Hans Waterham is a fellow of the Royal Dutch Academy of Arts and Sciences. The authors gratefully acknowledge Maddy Festen for excellent preparation of the manuscript and Jos Ruiter for preparation of the figures.




