Sublingual allergen immunotherapy: mode of action and its relationship with the safety profile
Edited by: Thomas Bieber
Abstract
To cite this article: Calderón MA, Simons FER, Malling H-J, Lockey RF, Moingeon P, Demoly P. Sublingual allergen immunotherapy: mode of action and its relationship with the safety profile. Allergy 2012; 67: 302–311.
Allergen immunotherapy reorients inappropriate immune responses in allergic patients. Sublingual allergen immunotherapy (SLIT) has been approved, notably in the European Union, as an effective alternative to subcutaneous allergen immunotherapy (SCIT) for allergic rhinitis patients. Compared with SCIT, SLIT has a better safety profile. This is possibly because oral antigen-presenting cells (mostly Langerhans and myeloid dendritic cells) exhibit a tolerogenic phenotype, despite constant exposure to danger signals from food and microbes. This reduces the induction of pro-inflammatory immune responses leading to systemic allergic reactions. Oral tissues contain relatively few mast cells and eosinophils (mostly located in submucosal areas) and, in comparison with subcutaneous tissue, are less likely to give rise to anaphylactic reactions. SLIT-associated immune responses include the induction of circulating, allergen-specific Th1 and regulatory CD4+ T cells, leading to clinical tolerance. Although 40–75% of patients receiving SLIT experience mild, transient local reactions in the oral mucosa, these primary reactions rarely necessitate dose reduction or treatment interruption. We discuss 11 published case reports of anaphylaxis (all nonfatal) diagnosed according to the World Allergy Organization criteria and relate this figure to the approximately 1 billion SLIT doses administered worldwide since 2000. Anaphylaxis risk factors associated with SCIT and/or SLIT should be characterized further.
Allergen immunotherapy and safety
Allergen immunotherapy is an effective way of reorienting inappropriate immune responses in allergic patients by sublingual or subcutaneous administration of allergens. Large-scale, randomized, double-blind, placebo-controlled (DBPC) trials, position papers, and meta-analyses have emphasized the efficacy and safety of sublingual allergen immunotherapy (SLIT) (1–4). Drop and tablet formulations have been approved by regulatory agencies in many countries for the treatment of allergic rhinoconjunctivitis in adults and in children over the age of 5 years (5). More than one million doses of SLIT have been administered in clinical trials (6), and we estimate that a total of around 1 billion doses have been administered worldwide since 2000.
Subcutaneous allergen immunotherapy (SCIT) is effective for the treatment of allergic rhinitis, allergic asthma, and Hymenoptera venom allergy (based on DBPC trials and meta-analyses of such trials); however, it involves a low but non-negligible risk of potentially severe and fatal anaphylactic reactions. In large-scale surveys of members of the American Academy of Allergy, Asthma and Immunology from 1990 to 2001, fatalities because of SCIT were reported at a rate of one in 2–2.5 million injections (7). These results were confirmed in a survey of North American allergists in 2008; there were 10.2 systemic reactions per 10 000 injections, and 3% of these events were classified as ‘life-threatening anaphylaxis with severe airway compromise or upper airway obstruction with stridor or hypotension, with or without loss of consciousness’ (8).
Sublingual allergen immunotherapy is generally considered to have a better safety profile than SCIT. In SLIT, most reactions are local and transient and do not lead to interruption or cessation of treatment (6). It is not tenable to argue that SLIT’s good safety profile results from or corresponds to a lack of efficacy. In addition to the large body of high-quality evidence from DBPC trials and meta-analyses of such trials, most head-to-head, comparative studies have not reported significant differences in efficacy between SLIT and SCIT, as summarized in Table 1 (9–14). Likewise, SLIT’s good safety profile cannot be ascribed to supposedly poor treatment compliance for the sublingual administration route. A recent systematic review (15) compared the published compliance data for SLIT and SCIT sublingual and subcutaneous immunotherapies. Compliance ranged from 45% to 60% in early clinical studies of SCIT and between 75% and 97% for more recent studies of both SCIT and SLIT. Sieber et al. (16) analyzed recent prescription data from Germany over a 2-year period and found that the sublingual route of administration led to significantly better persistency (a proxy for compliance) than the subcutaneous route.
| Authors | Year | Study design | Patients (n) | Patient age range | Allergen extract | Treatment duration | SLIT allergen dose (-fold the SCIT dose) | Conclusion in terms of efficacy |
|---|---|---|---|---|---|---|---|---|
| Bernardis et al. (9) | 1996 | Open, controlled, no placebo | 23 | 5–26 | Alternaria tenuis | 2 years | ×3.6 | SLIT > SCIT |
| Quirino et al. (10) | 1996 | RCT, double-dummy, no placebo | 20 | 13–39 | Five grasses | 1 year | ×2.4 | SLIT = SCIT |
| Mungan et al. (11) | 1999 | RCT, single-blind, placebo | 36 | 18–46 | Der p, Der f | 1 year | ×80 | SLIT = SCIT |
| Khinchi et al. (12) | 2004 | RCT double-dummy, placebo | 58 | 20–58 | Birch | 2 years | ×210 | SLIT = SCIT |
| Herrscher (13) | 2006 | Patient survey | 328 | 3–71 | Multi-allergen extracts | Typically 9–18 months | ×5–10 | SLIT = SCIT |
| Mauro et al. (14) | 2007 | RCT, no placebo | 47 | 18–59 | Alder, birch, and hazel | Not stated | ×92 | SLIT = SCIT |
- Der p, Dermatophagoides pteronyssinus; SCIT, subcutaneous allergen immunotherapy; SLIT, sublingual allergen immunotherapy; RCT, randomized, controlled trial; Der f, Dermatophagoides farinae.
Here, we consider the mechanism of action of SLIT and case reports of post-SLIT anaphylaxis published in indexed, peer-reviewed journals. We also discuss the nature of anaphylaxis to SLIT and the need for further characterization of risk factors for anaphylactic reactions to both SCIT and SLIT.
The mechanisms of action of sublingual allergen immunotherapy
Both SCIT and SLIT increase allergen tolerance via similar immune mechanisms, with reorientation of allergen-specific CD4+ T-cell responses from a T helper 2 (Th2) to Th1 and regulatory T-cell profiles (17). Allergen exposure modifies serum levels of allergen-specific IgE and IgG, although there is considerable debate as to whether these parameters are related to clinical efficacy. In contrast to SCIT, SLIT appears to elicit mucosal IgA responses, which may contribute significantly to tolerance induction (17). One obvious difference between SCIT and SLIT relates to the allergen doses administered. SLIT requires at least 50–100 times more allergen than SCIT to achieve a similar level of efficacy (18).
Sublingual uptake and processing of allergens have a number of specific features. In most SLIT regimens, the allergen preparation is kept under the tongue for a few minutes and then swallowed or, in a small proportion of regimens, spat out. Biodistribution studies in mice and humans demonstrate that allergens bind to epithelial cells within this time frame and then cross the mucosa within the next 15–30 min, before being captured and processed by antigen-presenting cells (APCs) (Fig. 1) (19, 20). In healthy, noninflamed human oral tissues, the main APCs are Langerhans cells (located in the mucosa itself) and myeloid dendritic cells (DCs, located along the lamina propria) (21) (Fig. 1). In murine oral tissues, small numbers plasmacytoid DCs are also detected (mainly in the submucosa) (19). These various DC subsets are tolerogenic; following migration to draining cervical lymph nodes, they support the induction of interferon-gamma and interleukin 10-producing Th1 cells and regulatory CD4+ T cells (17, 19). Because of the specific biological features of local DCs, the oral immune system appears to be ‘pre-programmed’ to elicit tolerance rather than anaphylaxis or other systemic allergic reactions (17).
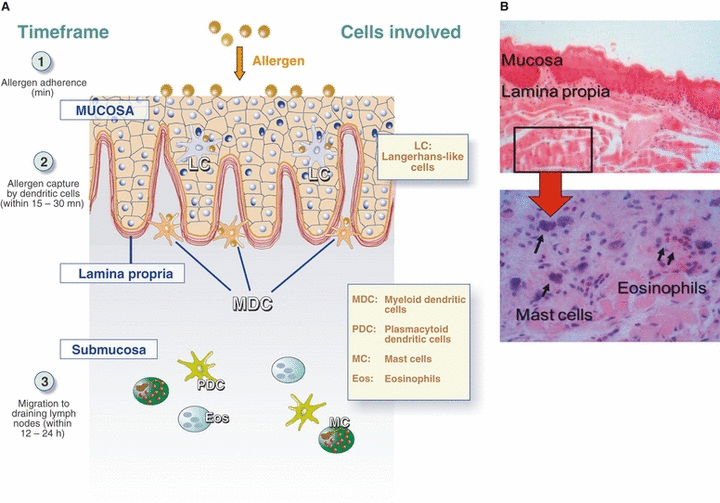
Tolerogenic and pro-inflammatory cells in the oral immune system. (A) Following sublingual immunization, substantial amounts of the allergen stick to epithelial cells within minutes, then cross the mucosa between 15 and 30 min. The allergen is subsequently captured by tolerogenic DCs (probably by Langerhans cells within the mucosa itself and myeloid DCs along the lamina propria) and processed as small peptides, presented together with major histocompatibility class I and class II molecules at the cell surface. The DCs loaded with allergen-derived peptides then migrate to cervical lymph nodes within 12 to 24 h, where they interact with naive CD4+ T cells, inducing Th1 and T regulatory cells with suppressive activity. These CD4+ T cells subsequently migrate into the blood and to the tissues, resulting in clinical tolerance. (B) Histology of the human lingua (lower side) showing the mucosa itself and the lamina propia where most dendritic cells involved in allergen uptake are located (upper panel). Mast cells and eosinophils tend to be located mainly in subepithelial tissues (lower panel). The lower panel represents a higher magnification of the area framed in the upper panel.
Both murine and human oral tissues contain low numbers of pro-inflammatory mast cells (MCs) and eosinophils (21). The relative numbers of MCs vary according to the site considered (e.g., the vestibulum, lingua, sublingua, gingival tissues, and palate) (22). Mast cells are closer to the mucosal surface in lingual tissues than in other oral tissues, and this may explain why one of the frequently reported SLIT adverse reactions is lingual edema. Nonetheless, the physiology of oral immune responses implies that most of the allergen in contact with the upper layers of oral mucosal tissue is captured and processed within 30 to 60 min by tolerogenic DCs before it reaches pro-inflammatory MCs or eosinophils (Fig. 1) (19). In contrast, the subcutaneous injection route is associated with a greater risk of direct contact between the allergen and circulating pro-inflammatory basophils and Th2 lymphocytes. Additionally, the allergen is likely to be captured by myeloid or plasmacytoid DCs whose effector immune responses are associated with the release of pro-inflammatory mediators (17).
Adverse reactions in sublingual allergen immunotherapy
A comprehensive review of SLIT identified 66 published studies in which information on safety was included (representing 4378 patients and approximately 1 181 000 doses) (6). Harmonized reporting standards and tools are essential for an accurate understanding of safety in allergen immunotherapy. Bearing in mind these limitations, Cox et al. reported that local, oral mucosal reactions occurred in 40–75% of SLIT patients (especially during the initiation and dose build-up phase) but usually did not lead to dose reduction or interruption of treatment. The same review found a rate of one SLIT-related serious adverse reaction per 384 treatment years; these mainly consisted of asthmatic reactions, abdominal pain/vomiting, uvula edema, and urticaria. A World Allergy Organization (WAO) task force has issued recommendations on safety reporting in clinical trial of SLIT (23), and a WAO working group is currently preparing a guideline on an adverse reaction grading system for SLIT.
Eleven nonfatal cases of SLIT-related systemic allergic reactions described as ‘anaphylaxis’ have been published in indexed, peer-reviewed journals.
- •
Antico et al. (24) reported a 36-year-old woman who developed generalized urticaria and chest symptoms described as an ‘asthma attack’ 20 min after taking natural rubber latex SLIT drops on the fourth day of a rush protocol. The patient was treated for anaphylactic shock in an emergency room. No additional details about her symptoms, signs, or treatment were provided. Rush protocols in both SCIT and SLIT constitute a risk factor for adverse reactions (18).
- •
Dunsky et al. (25) reported an allergen immunotherapy-naive, polysensitized 31-year-old woman who developed symptoms after SLIT dosing with a mixture containing six allergens, including some nonstandardized components. On the second day of SLIT, within 2 min of dosing, she developed generalized pruritus, a feeling of hives beneath the skin of her palms, and swelling of both hands. A physician advised her to continue SLIT. The next day, within a few minutes of taking her SLIT dose, she experienced marked generalized pruritus, severe swelling of the hands and feet, dyspnoea, wheezing, and dizziness. She self-medicated with an antihistamine and nebulized salbutamol and also received prednisone.
- •
Eifan et al. (26) reported an 11-year-old girl with allergic rhinitis and asthma who developed a greatly swollen lip, chest pain, nausea, abdominal pain, and fever, beginning 3 min after a maintenance dose with multi-allergen drops at the height of the pollen season. She was treated for anaphylaxis and hospitalized for observation over 4 h. In a published review of the case (27), it was reported that the patient received dextrose, an H1-antihistamine and a corticosteroid [all intravenous (IV)]. The next day, a repeat dose with SLIT pollen led to sublingual swelling and burning sensation.
- •
Blazowski (28) reported a 16-year-old girl during her third year of SLIT with a standardized house dust mite extract solution. After a 3-week treatment interruption, she took six times the recommended maintenance dose. Within 5 min, she developed generalized pruritus, flushing, generalized urticaria, dyspnoea, wheezing, chest pain, and shivering, followed by collapse. After two puffs of salbutamol, the emergency medical services team was called. Twenty-six minutes later, they noted a blood pressure of 70/40 mm Hg, heart rate of 160 with weak pulse, intense shivering, generalized urticaria, and mild ‘asthma symptoms’. Oxygen, IV fluids, IV methylprednisolone, and IV aminophylline were given. In the hospital emergency department, she became unconscious, pulseless, and hypotensive. She received intramuscular epinephrine, oxygen, IV fluids, and an IV corticosteroid and was transferred to the Intensive Care Unit, where she recovered.
- •
Rodriguez-Perez et al. (29) describe the case of an 11-year-old boy who reported dyspnoea, wheezing, urticaria, and upper lip angioedema some 20–30 min after taking a standardized house dust mite mix. Over the telephone, the patient was advised to take an antihistamine and nebulized salbutamol. When seen later that day as an outpatient, resolving urticaria/angioedema was observed. Rodriguez-Perez et al. also report two patients (aged 27 and 7 years, respectively) who developed wheezing, dyspnoea, anxiety, flushing, and dizziness and were treated with epinephrine.
- •
de Groot and Bijl (30) reported a 13-year-old boy who had previously discontinued mixed birch/grass pollen SCIT after experiencing large local reactions and two episodes of generalized urticaria. Fifteen minutes after the first dose of a grass pollen SLIT tablet, he developed oral irritation and swelling, along with angioedema of the eyes and generalized urticaria. An oral antihistamine was administered.
- •
de Groot and Bijl (30) also reported a 27-year-old woman with allergic rhinitis and asthma who had discontinued tree/grass pollen SCIT. This patient had never reached maintenance dosing with SCIT because of aggravated asthma during the induction phase and faintness within minutes after several injections. Immediately after taking the first grass pollen SLIT tablet, she developed generalized itching, abdominal cramps, wheezing, faintness, and hypotension (blood pressure 90/50). After self-administration of an oral antihistamine and inhaled corticosteroid, a subcutaneous injection of epinephrine was given by her general practitioner.
- •
Buyukozturk et al. (31) investigated latex extract SLIT (with a 4-day sublingual induction phase and a 12-month maintenance phase) in 30 patients, 28 of whom were healthcare workers, with latex allergy. Two patients developed serious systemic reactions – one after the second (5 mg) dose on the second day of induction and the other after the second dose on the third day (1000 mg). Both experienced flushing, itching, rhinitis, conjunctivitis, wheezing, dyspnoea, and chest tightness, and both received epinephrine injections.
These reports were assessed according to the clinical criteria for the diagnosis of anaphylaxis in a general context [developed by the National Institute of Allergy and Infectious Diseases (NIAID, USA) and the Food Allergy and Anaphylaxis Network (FAAN) (32)] and during allergen immunotherapy [developed by the World Allergy Organization (Table 2) (33, 34)].
| The NIAID/FAAN criteria (32) | The WAO criteria (33, 34) |
|---|---|
| Anaphylaxis is highly likely when any one of the following three criteria is fulfilled: | ‘Anaphylaxis is an acute and potentially lethal multisystem allergic reaction in which some or all of the following signs and symptoms occur: diffuse erythema, pruritus, urticaria and/or angioedema; bronchospasm; laryngeal edema; hypotension; cardiac arrhythmias; feeling of impending doom; unconsciousness and shock. Other earlier or concomitant signs and symptoms can include itchy nose, eyes, pharynx, genitalia, palms, and soles; rhinorrhea; change in voice; metallic taste; nausea, vomiting, diarrhea, abdominal cramps, and bloating; lightheadedness; headache; uterine cramps; and generalized warmth.’ NB: the WAO criteria for anaphylaxis after immunotherapy (known administration of a known allergen for that patient) are fulfilled when there is sudden onset of symptoms in one body organ system. |
| Acute onset of an illness (minutes to several hours) with involvement of the skin, mucosal tissue, or both (e.g., generalized hives, pruritus or flushing, swollen lips–tongue–uvula) And at least one of the following Respiratory compromise (e.g., dyspnea, wheeze-bronchospasm, stridor, reduced PEF, hypoxemia) Reduced BP or associated symptoms of end-organ dysfunction [e.g., hypotonia (collapse), syncope, incontinence] | |
| Two or more of the following that occur rapidly after exposure to a likely allergen for that patient (minutes to several hours): Involvement of the skin-mucosal tissue (e.g., generalized hives, itch-flush, swollen lips–tongue–uvula) Respiratory compromise (e.g., dyspnea, wheeze-bronchospasm, stridor, reduced PEF, hypoxemia) Reduced BP or associated symptoms (e.g., hypotonia [collapse], syncope, incontinence) Persistent gastrointestinal symptoms (e.g., crampy abdominal pain, vomiting) | |
| Reduced BP after exposure to a known allergen for that patient (minutes to several hours): Infants and children: low systolic BP (age specific) or >30% decrease in systolic BP* Adults: systolic BP of <90 mm Hg or >30% decrease from that person’s baseline |
- PEF, peak expiratory flow; BP, blood pressure.
- *Low systolic blood pressure for children is defined as less than [70 mm Hg + (2× age)] from 1 to 10 years, and <90 mm Hg from 11 to 17 years
All the 11 patients met the WAO criteria (33, 34) for anaphylaxis and 10 met the NIAID/FAAN criteria (32) (Table 3). The episode reported by Eifan et al. (26) was later reinterpreted by others as a severe local reaction with a positive rechallenge (27), despite the sudden onset of symptoms in three body organ systems within a few minutes of SLIT maintenance dosing. The case of the 13-year-old patient reported by de Groot and Bijl (30) had involvement of only the skin/mucosal system 15 min after the first sublingual dose of a known allergen and thus did not meet the NIAID/FAAN criteria (32). However, the latter patient’s adverse reaction meets the WAO criteria for anaphylaxis after immunotherapy (administration of a known allergen for that patient and sudden onset of symptoms in one body organ system) (33, 34).
| Case reported by | Patient | Allergen(s) administered as SLIT | Comments on SLIT | Time after SLIT dose | Symptoms & Signs | Management | Anaphylaxis according to the NIAID/FAAN clinical criteria? (32) | Anaphylaxis according to the WAO definition? (33, 34) |
|---|---|---|---|---|---|---|---|---|
| Antico et al. (2006) (24) | 36-year-old woman | Natural rubber latex (standardized) | Rush protocol, maximum scheduled dose of extract | 20 min | Urticaria ‘asthma’ attack anaphylactic shock | Treated in ER no details given | Yes | Yes |
| Dunsky et al. (2006) (25) | 31-year-old woman | Alternaria, dog, cat, grass, ragweed, mixed weed (only cat and grass were standardized) | Within 2 min of self-administering SLIT dose on 2nd day, developed generalized itch, hand swelling and possible hives; was advised to continue SLIT; on the next day, anaphylaxis occurred within a few mins of dosing; see adjacent columns of this table for details | ‘a few min’ | Generalized itch severe swelling of hands and feet, dyspnoea, wheezing, dizziness | Oral antihistamine salbutamol MDI prednisone not seen by physician | Yes | Yes |
| Eifan et al. (2007) (26) | 11-year-old girl | House dust mite, five grass pollens, Secale cereale | Maintenance dose at height of pollen season | 3 min | Lip swelling chest pain nausea, abdominal pain fever | IV antihistamine IV corticosteroid IV dextrose treated in ER, hospitalized | Yes | Yes |
| Blazowski (2008) (28) | 16-year-old girl | House dust mite (standardized) | Overdose after 3-wk interruption in dosing during 3rd yr of maintenance dosing; previously, during first 3 months of maintenance, had two episodes of wheezing | 5 min | Generalized itch, flush, hives dyspnoea, wheezing chest pain, shock, shivering, tachycardia, sleepy, weak pulse; then unconscious and pulseless | Salbutamol MDI IM epinephrine oxygen IV fluids IV methylprednisolone IV aminophylline treated in ICU | Yes | Yes |
| Rodriguez-Perez et al. (2008) (29) patient 1 | 27-year-old woman | House dust mites (standardized), Periplaneta americana, mold mix | During a course of a Dermatophagoides mix, described as ‘vial 0 [0.05 UBE (equivalent biological units)]’, around 0.1 ng, and ‘cockroach 1 : 50’, dose not reported. | 20 min | Persistent wheezing, dyspnoea, anxiety, flushing, and dizziness | Loratadine, 10 mg; epinephrine, 0.5 ml IM in the thigh | Yes | Yes |
| Rodriguez-Perez et al. (2008) (29) patient 2 | 7-year-old girl | House dust mites (standardized); pecan tree | During a course of a Dermatophagoides mix, described as ‘vial 4 (975 UBE)’, around 1.95 μg | 30 min | Persistent wheezing, dyspnoea, anxiety, flushing, and dizziness | Epinephrine, 0.2 ml IM in the thigh Salbutamol HHN, 0.15 mg/kg/dose | Yes | Yes |
| Rodriguez-Perez et al. (2008) (29) patient 4 | 11-year-old boy | House dust mites (standardized) | During a course of a Dermatophagoides mix, described as ‘vial 4 (675 UBE)’ around 1.35 μg | 20 min | Urticaria, angioedema of upper lip, dyspnea, wheezing | Loratadine 10 mg, salbutamol HHN, 2 mg/dose as needed | Yes | Yes |
| de Groot and Bijl (2009) (30) patient 1 | 13-year-old boy | Grass pollen (standardized) | 1st dose of SLIT; previous adverse reactions to birch/grass SCIT | 15 min | Generalized hives, tongue swelling, eye angioedema | Oral antihistamine | No | Yes |
| de Groot and Bijl (2009) (30) patient 2 | 27-year-old woman | Grass pollen (standardized) | 1st dose of SLIT; previous severe adverse reactions (asthma and faintness) to SCIT | ‘soon’ | Generalized itching ‘asthma’ abdominal cramps faintness, ↓ blood pressure | Oral antihistamine inhaled corticosteroid sympathomimetics SC epinephrine | Yes | Yes |
| Buyukozturk et al. (2010) (31) | Two adult healthcare workers | Natural rubber latex | Reactions occurred during dose induction phase; 1 pt reacted after the 2nd dose on the 2nd day (5 μg) 1 pt reacted after the 2nd dose on the 3rd day (1000 μg) | Not stated | Both patients: flushing, itching, rhinitis, conjunctivitis, wheezing, dyspnea, chest tightness, hypotension | Both patients: epinephrine injection | Yes | Yes |
- SLIT, sublingual allergen immunotherapy; SCIT, subcutaneous allergen immunotherapy; ER, emergency room; CVS, cardiovascular system; MDI, metered-dose inhaler; IV, intravenous; IM, intramuscular; ICU, intensive care unit; GI, gastrointestinal; SC, subcutaneous; y, year; HHN; hand-held nebulizer.
In the patients with anaphylaxis to SLIT, skin/mucosal symptoms and respiratory symptoms predominated, as is typical of anaphylaxis in general (34). Six of the 11 patients probably had hypotension and shock, although these signs are not required for a diagnosis of anaphylaxis. Two patients had abdominal pain, a frequently overlooked anaphylaxis symptom. Two others experienced chest pain – another frequently overlooked anaphylaxis symptom that reflects the relatively high concentration of MCs in the normal heart (35). Fever, reported in one patient, is not a symptom of anaphylaxis per se, although it may be a co-factor that amplifies anaphylaxis (34). It is important to note that these isolated case reports do not represent standard practice in SLIT; rather, they variously involved nonstandardized extracts, allergen mixtures, rush protocols, overdose, and patients who had previously discontinued SCIT because of serious adverse reactions.
As mentioned earlier, large-scale surveys of North American allergists from 1990 to 2001 and in 2008 respectively reported a rate of one fatality per 2–2.5 million SCIT injections (7) and one case of anaphylaxis per 33 300 injections (8) or per 4160 treatment years (on the basis of eight injections per year). The cases of SLIT-induced anaphylaxis must be put into context against the large number of doses administered. Cox et al. (6) estimated that a million doses of SLIT had been administered during clinical trials. On this basis, we estimate that around 1 billion doses of SLIT products (regardless of the formulation – drops, tablets, etc.) have been taken by patients in practice since 2000. Eleven cases of SLIT-induced anaphylaxis equate to around one case per 100 million SLIT administrations or per 526 000 treatment years [using the mean value of 190 doses per treatment year calculated by Cox et al. (6)]. Although incident cases of anaphylaxis after SLIT have almost certainly been underreported, similar sources of bias should apply to SCIT and SLIT. Indeed, we suggest that cases of anaphylaxis because of SLIT (which generally occur outside the allergist’s office, following home administration) are more likely to be reported than cases because of SCIT (which are more likely to occur in or close to the allergist’s office). However, we caution against head-to-head comparisons of these values without taking account of the given allergen and the allergic disease in question. For example, hymenoptera venom allergy is treated with SCIT and frequently displays side-effects during the build-up phase, whereas SLIT is not performed in this subgroup of patients at high risk of anaphylaxis. Overall, this is an issue that deserves further attention; a highly analytical, exhaustive analysis of both published and previously unpublished safety and prescription data (from regulatory agencies and manufacturers, for example) for both SLIT and SCIT would be a worthy but challenging goal, because of the probable heterogeneity of the data.
Dealing with anaphylaxis after SCIT and SLIT
Once anaphylaxis is suspected, it must be treated immediately with an intramuscular injection of epinephrine, as death can occur within minutes. All anaphylaxis guidelines published in indexed, peer-reviewed medical journals recommend prompt intramuscular injection of epinephrine, although they differ with regard to the importance of H1-antihistamines, H2-antihistamines, corticosteroids, and bronchodilators other than epinephrine (34).
The statement from the 2010 updated Joint Task Force on Practice Parameters for anaphylaxis reflects the historical dominance of SCIT in North America and considers solely the subcutaneous route in its recommendations on allergen immunotherapy (36). The Practice Parameter states that ‘allergen vaccine injections should be administered only by healthcare professionals trained in the recognition and treatment of anaphylaxis, only in healthcare facilities with the proper equipment for the treatment of anaphylaxis, and in clinics with policies and procedures that minimize the risk of anaphylaxis’. The applicability of this statement to SLIT (especially to maintenance dosing) is uncertain.
In Europe, it is rare for patients on SLIT to be prescribed an epinephrine auto-injector for potential use after home dosing. It remains to be seen which practices will prevail outside Europe in this respect. It is possible that high-risk patients who receive SLIT at home will be equipped with an epinephrine (adrenaline) auto-injector. Despite the extreme rarity of anaphylaxis after SLIT administration, adequate patient education remains critical; patients need to be able to recognize symptoms and signs and, depending on local medical practice and a personalized plan developed with their physician, self-inject epinephrine promptly.
Young children may have difficulty describing symptoms of anaphylaxis, such as itching or shortness of breath (34). In addition to epinephrine injection, other important aspects of such treatment are calling emergency medical services or a resuscitation team for assistance and placing the patient in the supine or semi-recumbent position (34).
Defining risk factors for anaphylaxis after SCIT and SLIT
For anaphylaxis in general, most authors agree that concomitant asthma (especially if severe or not well controlled), cardiovascular disease, and mastocytosis/clonal mast cell disorders are important risk factors for severity and fatality. Despite the extreme rarity of reports of anaphylaxis in SLIT, it would be useful to define patient risk factors for serious adverse reactions after sublingual allergen delivery. The continuing use of nonstandardized, unregistered extracts complicates pharmacovigilance and the analysis of potential causal relationships between allergens and serious adverse reactions. Information should be gathered on the role of potential amplifying factors and co-factors such as concurrent use of beta-blockers or other medication, viral infection, fever, emotional stress, disruption of routine, premenstrual status in females, and exercise (34). Allergen immunotherapy-related risk factors should also be further characterized. Given that there are very few data on SLIT-specific risk factors, it makes sense to consider risk factors in SCIT – even though the latter may not necessarily apply to SLIT. Somewhat controversially, the Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines suggest that patients who have experienced serious adverse reactions to SCIT can potentially be switched to SLIT (2, 3). There is no formal evidence to support this position, and a history of a previous severe reaction to SCIT was an obvious risk factor in two of the cases reviewed here (30). In 2319 patients treated with a total of 14 600 injections, Lopez et al. (37) identified high sensitivity to the allergen, a history of previous systemic reactions and dosing/preparation errors as risk factors. Other potential risk factors for adverse reactions following SCIT include high natural allergen exposure during the peak pollen season, high allergen doses in mixed-allergen formulations, co-prescription of ß-blockers or ACE-inhibitors, rush dosing schedules, monosensitization and high degree of skin test reactivity, number of positive skin test results, and perhaps previous severe local reactions (38, 39).
Given the lack of consensus on risk factors in SCIT, it is difficult to extrapolate or relate such findings to risk factors in SLIT, as the factors may or may not differ from those in SCIT. There are few data on SLIT-specific risk factors. Malling et al. (40) reported that the efficacy and safety of a once-daily 300 index of reactivity (IR) grass pollen extract SLIT tablet in a large, multicentre, DBPC clinical trial in 628 adults had no relationship with sensitization status, severity, or asthma status. The results of a recent three-year clinical trial of a SLIT grass pollen tablet suggest that the safety profile improves year-on year, with a lower incidence of local reactions and a decrease in their severity (41). The WAO’s published suggestion that oropharyngeal infections and lesions (ulcers, gingivitis, periodontitis, etc.) may be potential SLIT-specific risk factors (1) merits investigation in prospective clinical studies, together with other parameters (Table 4). Given the involvement of MCs and basophils in both immunologically and nonimmunologically triggered anaphylaxis, a case can be made for screening at-risk patients for mastocytosis or clonal mast cell disorders by measurement of a baseline serum tryptase level before initiation of allergen immunotherapy. However, the relationship between serum tryptase levels and chronic mast cell activation is subject to debate (42). Even though acute MC activation (such as during anaphylaxis) leads to the massive release of vasoactive and pro-inflammatory mediator substances and substantially supranormal serum tryptase concentrations, chronic MC activation is more difficult to diagnose – especially when symptoms are mild, atypical, or absent. Indeed, serum tryptase levels are usually normal in the latter patients. According to the above-mentioned case reports of anaphylaxis after SLIT (25, 26, 28–31), none of the patients were screened for mastocytosis or clonal mast cell disorders, but most had probably been evaluated for lung function because of the presence of asthma prior to initiation of allergen immunotherapy. The patient reported by Dunsky et al. (25) had well-controlled asthma on SLIT initiation. The 11-year-old girl reported by Eifan et al. (26) had a history of asthma sensitized to house dust mite and pollen. The 16-year-old girl reported by Blasowki (28) had well-controlled, intermittent asthma. All three cases cited by Rodriguez-Perez et al. (29) had mild or moderate, persistent asthma. Four of the 16 patients with latex allergy studied by Buyukozturk et al. (31) had asthma at baseline, although the asthma status of the two patients requiring epinephrine injections was not reported. Allergists should devote more effort to the identification and quantification of potential risk factors (aided by progress in research in this field) in patients wishing to start a course of allergen immunotherapy.
| SCIT- and SLIT-related factors |
| Allergen mixtures |
| Rush protocols |
| Overdose |
| Nonstandardized allergens |
| Interruptions in dose regimen |
| Patient-related factors |
| Previous systemic reaction, including anaphylaxis, to SCIT or SLIT |
| Previous severe local reaction |
| Acute infection (e.g., upper respiratory infection) |
| Fever |
| Oral infections or lesions (e.g., ulcer, gingivitis, periodontitis, etc.) to SLIT |
| Asthma, especially if severe or uncontrolled |
| Gender (premenstrual status) |
| Young age |
| Emotional stress |
| Exercise |
| High pollen counts |
- SCIT, subcutaneous allergen immunotherapy; SLIT: sublingual allergen immunotherapy.
Conclusions
Rapidly occurring antigen capture by local, tolerogenic APCs and the low numbers of MCs in sublingual tissues may explain SLIT’s excellent safety profile (19–22). Although there is a case for medical supervision of the initial administration of SLIT (especially in patients with known risk factors), the extremely low incidence of systemic serious adverse reactions in the European experience lends support to home administration for maintenance dosing. All physicians prescribing allergen immunotherapy, regardless of route of administration, should be aware of the risk of anaphylaxis and know how to recognize it and treat it promptly. In addition, the patients should be instructed to recognize it and treat it promptly, as per international guidelines and their physician’s written instructions (34).
Regardless of the allergen delivery route, more work on identifying risk factors for adverse reactions to allergen immunotherapy products is required. Likewise, harmonized reporting standards and tools are essential for an accurate understanding of safety in allergen immunotherapy. There is an urgent need for a standardized reporting system for the side-effects of SLIT. We suggest that a reduction in the frequency of serious adverse reactions could be achieved through product standardization, improved pharmacovigilance, better characterization of patient risk factors (notably mastocytosis and asthma), education, compliance, reporting, and follow-up.
Conflict of interest
Moises Calderon has received consulting fees, honoraria for lectures and/or research funding from ALK-Abelló and Stallergenes. F. Estelle R. Simons is on the Anaphylaxis Advisory Boards for ALK-Abelló, Dey and Sanofi Aventis. Hans-Jørgen Malling has received consulting fees, honoraria for lectures and/or research funding from Allergopharma, ALK-Abelló & Stallergenes. Richard F. Lockey consults with Merck-Schering, ALK and Dey. Philippe Moingeon is an employee of Stallergenes. Pascal Demoly is a consultant and a speaker for Stallergenes, ALK and Therabel and was a speaker for Schering-Plough-MSD, Astra Zeneca and GlaxoSmithKline.




