Determination of threshold concentrations of multiple allergenic extracts for equine intradermal testing using normal horses in three seasons
Published as an abstract from the North American Veterinary Dermatology Forum in Veterinary Dermatology 2007; 17: 175.
Abstract
Forty-one normal horses were evaluated for reactivity to intradermally injected aqueous allergens to determine allergen threshold concentrations (TC), with potential relevance to equine intradermal testing (IDT). Horses were tested three times over 1 year to assess seasonal variation in reactivity, using three to five serial dilutions of 27 allergens each time. Injection sites were evaluated after 15 min, 1 h, 4 h and 24 h. The highest allergen concentration at which < 10% of horses demonstrated positive reactivity (subjective score of ≥ 2, scale of 0 to 4) at 15 min was considered the TC. The TC was determined for nine pollens (2000 to > 6000 PNU mL−1), four moulds (4000 to > 6000 PNU mL−1), seven insects (ant, horse fly 125 PNU mL−1; house fly, cockroach 250 PNU mL−1; moth 60 PNU mL−1; mosquito 1000 PNU mL−1; Culicoides nebeculosis 1 : 5000 w v−1) and three of four storage mites (1 : 10 000 w v−1). The TC was not determined due to excessive reactivity at the lowest concentrations tested for dust mites (Dermatophagoides farinae [< 1 : 12000 w v−1], D. pteronyssinus [< 1 : 30 000 w v−1]), and Acarus siro (< 1 : 10 000 w v−1). Minor variation in the TC for specific allergens occurred in different seasons. Progressive sensitization with repeat testing occurred for grain mill dust mix. Positive reactivity at 1 h and 4 h occurred in > 10% of horses for nine of 19 allergens (pollens, mosquito, storage mites) at their determined TC. Positive reactivity was rare at 24 h. This study in normal horses suggests that appropriate testing concentrations of allergens for equine IDT in atopic horses may be ≥ 1000 PNU mL−1 for pollens and moulds, 60 to 250 PNU mL−1 for most insects and < 1 : 12 000 w v−1 for dust mites; and that reactions at 1–4 h may be insignificant.
Source of Funding
This study was supported by research grants from the American Academy of Veterinary Dermatology, the Dermatology Chapter of the Australian College of Veterinary Scientists and a College Research Grant from the Australian College of Veterinary Scientists. Christina Baxter's residency program was sponsored by Virbac, Australia (Pty, Ltd).
Conflict of Interest
No conflict of interest has been declared.
Introduction
Intradermal testing (IDT) has been demonstrated to be a useful diagnostic tool in humans1 and animals for a range of type 1 hypersensitivity diseases.2 When IDT is performed in a sensitized individual, intradermally injected allergens attach to immunoglobulin E (IgE) antibodies bound to mast cells resulting in mast cell degranulation and a clinically evident wheal and flare reaction.3,4 Thus, IDT may allow identification of allergens responsible for, or contributing to, type 1 hypersensitivity conditions and subsequent selection of allergens for immunotherapy for affected individuals.3,4
In horses, IDT has been used to evaluate the role of allergens in atopic dermatitis,5–7 insect bite hypersensitivity,8–10 urticaria,6,7,11,12 chronic obstructive pulmonary disease/recurrent airway obstruction,7,11,13,14 and head shaking.15 A significant limitation of equine IDT has been a high frequency of apparent false positive reactions, as detected in clinically normal (control) horses.6,7,11,16–18 Although clinically ‘hypersensitive’ horses as a group have more frequent positive reactions than normal horses, the clinical relevance of positive reactions to individual allergens has been difficult to interpret.7 The cited causes of false positive reactions in IDT include use of irritant, contaminated or concentrated allergens, prior clinical or present subclinical sensitivity, poor injection technique, irritable skin, dermatographism, cross-reactions (for example dust mites with parasitic mites) and mitogenic allergens.19
The correct concentrations of allergens for IDT are particularly important to achieve clinically relevant results. ‘False positive’ reactions have been reported in the absence of clinical hypersensitivity in dogs when allergens are too concentrated.20 Manufacturers use several units of measurement of concentration to standardize the biological potency of allergens, including protein nitrogen units (PNU), weight to volume ratios (w v−1) or percentages in diluent.20,21 It has been suggested that bioactivity of commercial products varies from 10-fold to 1000-fold, and there may be no relationship between bioactivity and concentrations declared in PNU or w v−1.21,22 Nonetheless, allergens are commercially available with concentrations specified in units to indicate the relative potency. Following manufacture, the allergen potency can be affected by storage conditions (temperature, glass or plastic vials) and duration of storage, accuracy of dilution of allergens for testing and contamination of allergens.23
Threshold studies are performed to guide approximate allergen concentrations for use in IDT by determining the concentrations of intradermally injected allergenic extracts to which a minority of clinically normal animals demonstrate positive reactivity.20 Recent studies have determined threshold concentrations (TC) of allergens for IDT in dogs and cats.24,25 In these studies, normal dogs and cats were intradermally injected with serial concentrations of numerous allergens. The TC was determined as the highest concentration of an individual allergen that resulted in positive reactivity in less than an arbitrary percentage (e.g. 10 or 25%) of normal animals. When a greater proportion of normal animals react to an injected allergen at a given concentration, ‘false’ positive reactions to those specific allergens are more likely in animals with hypersensitivities. These are often referred to as nonspecific ‘irritant’ reactions26 and are proposed to be one of the major causes of the high incidence of false positive responses reported with IDT in horses. Somewhat problematic, ‘false’ positive (irritant) reactions in normal individuals can only be defined in the known absence of subclinical allergen sensitization; a distinction that is currently not possible. Conversely, a lack of reactivity in normal animals to an injected allergen concentration may indicate this concentration is less sensitive in detecting relevant sensitization in clinically affected animals (i.e. false negative results may be obtained if the allergen is too dilute).
The concentrations conventionally recommended and, at least used widely, for IDT in small animals and horses include: 1000 PNU mL−1 for pollens, moulds and insects, and 1 : 50 000 to 1 : 1000 w v−1 or 1000 PNU mL−1 for individual house dust mites.2,21,22,25 Recent threshold studies found the TC of all pollens and moulds tested exceeded 1000 PNU mL−1 in both dogs and cats, and the TC of dust mites (Dermatophagoides farinae and Dermatophagoides pteronyssinus) was less than 1000 PNU mL−1 in both species.
A limited TC study has also been performed recently in horses using 13 insect allergens. TC were determined to be substantially lower than the described ‘standard’ testing concentrations in horses for nine insect allergens.26 TC of pollen, mould, dust and storage mite allergens have not been determined in horses.
IDT reactivity and interpretation may also potentially be influenced by seasonal variation in allergen exposure for which there is conflicting evidence in animals.2,17,23 One equine study evaluated IDT in normal and clinically hypersensitive horses in two different seasons, and repeatability of reactions was reported as poor, especially in normal horses.17 Seasonal allergen exposure may significantly influence skin reactivity in horses by altering either clinical or subclinical sensitization, but the influence of seasonal factors on IDT results and skin reactivity is poorly understood.
Another hazard in the interpretation of equine IDT is the occurrence of delayed reactions seen up to 24 h after IDT. These reactions are characterized by inflammatory changes believed to more closely resemble naturally occurring allergic disease than immediate reactions in humans27 and dogs.28 However, in small animals delayed reactions occur less frequently than immediate reactions and their clinical significance is unclear.23 In horses, wheal reactions seen 1 to 24 h post-testing are reported to occur more commonly in atopic horses than normal horses,6,29 and delayed reactions are not always preceded by immediate reactions.6 As such, the relevance of reactions observed between 4 to 24 h post-testing in equine IDT is currently unknown.
The objectives of this study included: (i) to determine the TC of a selection of nine pollen, seven insect, four mould, two dust mite and four storage mite allergens used for equine IDT; (ii) to determine if there is significant variation in the proportions of horses reacting to various concentrations of allergens during three different seasons; and (iii) to describe the type and frequency of delayed reactions following IDT in normal horses at the determined TC for all allergens tested.
Materials and methods
This study was approved by the Animal Ethics Committee of the University of Sydney, Australia.
Horses
Forty-one clinically normal horses from training and blood donor herds of the Veterinary Science Faculty of the University of Sydney and the University Veterinary Centre, Camden (Australia), were included in the study. Inclusion criteria required no history of skin and respiratory disease for at least 1 year prior to enrolment in the study and no dermatological signs at the time of enrolment. There were 25 mares and 16 geldings, ranging from 4 to 25 years old, with a mean age of 13.3 years. A variety of breeds were represented including 23 Standardbreds, 12 Thoroughbreds, one Warmblood cross, one Appaloosa, one Thoroughbred cross and one Shetland pony. All horses were kept on pasture with ad lib access to pasture grass and water, with supplementation as required with Lucerne or meadow hay. No abnormalities were found on dermatological exam at each testing occasion. No antihistamine, corticosteroid or analgesic drugs were given for at least 4 weeks prior to each scheduled testing. Regular deworming was performed for all horses on a rotational drug schedule every 8 weeks.
Study design
Each horse underwent IDT on three separate occasions over 10 months, in three seasonal blocks: July/August (Winter), November/December (Spring/Summer) and March/April (Autumn). The same horses were used on each occasion. Horses were kept in the same environment and in the same area of Sydney, Australia, for the duration of the study. Injections were performed for each horse by one of two experienced technicians, and all measurements/scores were performed by the primary author throughout the study.
Allergens
A total of 27 allergens were selected for IDT including grass, tree and weed pollens, insects, moulds, grain mill dust mix, dust and storage mites (Table 1). All allergens were aqueous extracts obtained from Greer Laboratories (Lenoir, NC, USA). Three to five dilutions of each allergen were formulated using phosphate-buffered saline as a diluent. In the first IDT the dilutions were based on published recommendations for horses when data were available,26 or small animal.21,22 The testing concentration for the newly available Culicoides nebeculosis was based on manufacturer recommendation (1 : 25 000 w v−1). Allergen concentrations were adjusted to aid threshold determination as necessary if the percentage of horses reacting to any one allergen at all tested concentrations in the earlier seasons was greater or less than 10%. Histamine phosphate (1 : 100 000 w v−1) and phosphate-buffered saline were used as positive and negative controls, respectively, at the beginning and end of each IDT procedure. In the first two testing seasons, there were a total of 80 injections per horse; in the final testing season additional allergen concentrations increased the total number of injections to 100 per horse.
| Allergen | 15 min overall | 15 min seasonal | ||
|---|---|---|---|---|
| Winter | Spring/Summer | Autumn | ||
| Pollens (PNU mL−1) | ||||
| Paspalum | 6000 | > 4000 | > 6000 | 6000 |
| Oat | 4000 | > 4000 | > 4000 | 4000 |
| Couch | > 6000 | > 4000 | > 4000 | > 6000 |
| Plantain | 4000 | > 4000 | 4000 | 4000 |
| Short ragweed | > 6000 | > 4000 | > 4000 | > 6000 |
| Red sorrel | 2000 | 2000 | > 2000 | > 4000 |
| Wattle | 6000 | > 4000 | > 6000 | 4000 |
| Eucalyptus | 2000 | 2000 | > 2000 | > 4000 |
| Poplar | > 6000 | > 4000 | > 4000 | > 6000 |
| Insects (PNU mL−1) | ||||
| Mosquito | 1000 | 1000 | 1000 | 1000 |
| Ant | 125 | 125 | 125 | 125 |
| House fly | 250 | > 500 | 250 | 250 |
| Cockroach | 250 | 500 | 250 | 250 |
| Moth | 60 | 60 | 30 | 60 |
| Horse fly | 125 | 125 | 125 | > 250 |
| Culicoides (w v−1) | 1 : 5000 | > 1 : 1000 | 1 : 5000 | 1 : 1000 |
| Moulds (PNU mL−1) | ||||
| Alternaria | 4000 | > 4000 | 4000 | 4000 |
| Aspergillus | 1000 | 2000 | 500 | 1000 |
| Cladosporium | > 6000 | > 4000 | > 4000 | > 6000 |
| Penicillium | 4000 | > 4000 | 4000 | 2000 |
| Miscellaneous | ||||
| Grain mill dust mix (PNU mL−1) | < 60 | 250 | < 125 | < 60 |
| Dust mites (w v−1) | ||||
| D. pteronyssinus | < 1 : 30 000 | < 1 : 8000 | < 1 : 15 000 | < 1 : 30 000 |
| D. farinae | < 1 : 12 000 | 1 : 10 000 | < 1 : 12 000 | < 1 : 12 000 |
| Storage mites (w v−1) | ||||
| Acarus siro | < 1 : 10 000 | > 1 : 4000 | < 1 : 10 000 | < 1 : 10 000 |
| Blomia tropicalis | 1 : 10 000 | > 1 : 4000 | < 1 : 10 000 | 1 : 10 000 |
| Lepidoglyphus destructor | 1 : 10 000 | 1 : 10 000 | < 1 : 10 000 | < 1 : 15 000 |
| Tyrophagus putrescentiae | 1 : 10 000 | < 1 : 10 000 | > 1 : 4000 | 1 : 10 000 |
- Threshold concentrations were defined as the highest concentration where < 10% normal horses had ‘positive’ reactions (i.e. score ≥ 2); PNU mL−1 = protein nitrogen units/millilitre; w/v = weight/volume.
The diluted allergens were stored in glass vials at 4 °C and were replaced with freshly diluted vials every 4 weeks. The concentrated allergens were obtained in different batches from Greer Laboratories as required over the testing period.
Intradermal testing
Horses were sedated with detomidine (Dormosedan®; Novartis Animal Health Australasia, North Ryde, NSW, Australia) intravenously via the jugular vein, at a dose range of 0.01 to 0.02 mg kg−1 depending upon the temperament of the horse. A test site approximately 30 cm × 20 cm on the left lateral neck midway between the mandible and shoulder was clipped against the direction of hair growth using no. 40 clipper blades. A predrilled template was used to mark injection sites with a white paint marker (Artline Paint Marker®, opaque white, Shachihata Inc., Nagoya, Japan). A volume of 0.1 mL of each extract was injected intradermally using a 30-gauge needle. Each skin test site was evaluated by the primary investigator at 15 min, 1 h, 4 h and 24 h after the injections. The reactions were subjectively scored using a scale of 0 (negative) to 4 based on wheal size, erythema and turgidity by comparison to the positive and negative controls. Scores of 2 or greater were considered positive for the purpose of threshold determination. An objective measurement of wheal diameter (in millimetres) was also performed at 15 min following subjective scoring.
Statistical analysis
Percentages of horses with subjective reaction scores of ≥ 2 to each allergen concentration were calculated using combined results from all horses over the three test periods and at each separate test period (i.e. test season). The TC was calculated as the highest concentration of allergen tested at which less than 10% of horses had positive reaction scores (i.e. ≥ 2) at the 15 min score.
Differences in the proportion of horses obtaining reaction scores ≥ 2 at 15 min associated with testing in different seasons or with repeated testing were evaluated with the chi-squared test. All statistical comparisons were evaluated at a 5% level of significance (P < 0.05).
For allergens where a TC could be determined at the 15-min assessment, the frequency of delayed reactions (1, 4 and 24 h) receiving a score ≥ 2 were expressed as a percentage of the total number of horses in each season and across seasons.
Correlation between the objective measurement of wheal diameter and positive (≥ 2) versus negative (< 2) subjective score was evaluated across all horses, allergens and seasons at 15 min postinjection using the Mann–Whitney U-test (Wilcoxon rank sum test).
Correlation between allergen concentration and the proportion of horses demonstrating positive reactivity at 15 min postinjection across all seasons was evaluated with the chi-square test. Allergens were grouped where allergen test concentrations were similar, grass pollens (paspalum, oat and couch), weed pollens (plantain, short ragweed and red sorrel), tree pollens (wattle, eucalyptus, poplar) and moulds (Alternaria, Aspergillus, Cladosporium and Penicillium), while insect allergens, grain mill dust mix, dust mite and storage mite allergens were evaluated individually.
Results
Threshold concentration (TC)
The calculated TC for tested allergens based on pooled data (all seasons) and in different seasons is shown in Table 1.
For pollens tested, the calculated TC ranged from 2000 PNU mL−1 to > 6000 PNU mL−1 overall and in each season. Of the insect allergens, the TC of ant, horse fly, cockroach, moth and horse fly ranged from 30 to 500 PNU mL−1 overall and in each season, while the TC for mosquito was consistently 1000 PNU mL−1. The TC of C. nebeculosis overall was 1 : 5000 w v−1. The overall TC for moulds varied from 1000 PNU mL−1 to > 6000 PNU mL−1 and for three storage mites overall was 1 : 10 000 w v−1.
The exact TC could not be determined for some allergens, where > 10% of horses had positive reactions to the weakest concentrations tested (e.g. D. farinae and D. pteronyssinus) or where < 10% of horses had positive reactions to the strongest concentration tested (e.g. couch, short ragweed).
Seasonal variation
Significant variation in reactivity (chi square, P < 0.05) using the same concentrations of allergens in different seasons occurred with three of 27 individual allergens (grain mill dust mix, eucalyptus and D. farinae) and combined results of pollens, combined results of insects and combined results of storage mites (1-4). Reactivity peaked in winter for pooled pollens and eucalyptus; in spring for pooled insects and D. farinae; and in autumn for pooled storage mites and grain mill dust mix. A trend in reactivity was observed only for grain mill dust mix, where increasing proportions of horses demonstrated positive reactivity over time (Fig. 4). Reactivity to D. pteronyssinus could not be compared across seasons as in an attempt to determine the TC for this allergen, testing concentrations were progressively decreased in each testing season and no concentration was tested repeatedly across all three seasons.
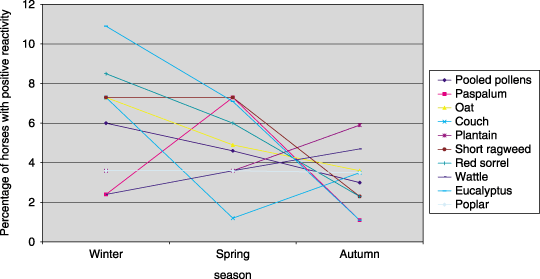
Proportion of normal horses with reaction scores ≥ 2 to pollen allergens in different seasons.
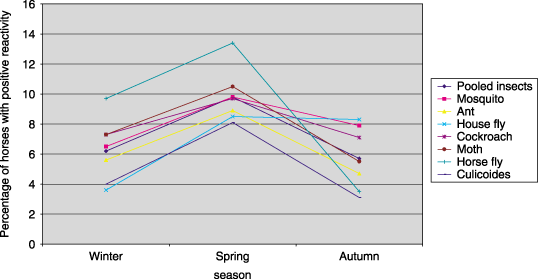
Proportion of normal horses with reaction scores ≥ 2 to insect allergens in different seasons.
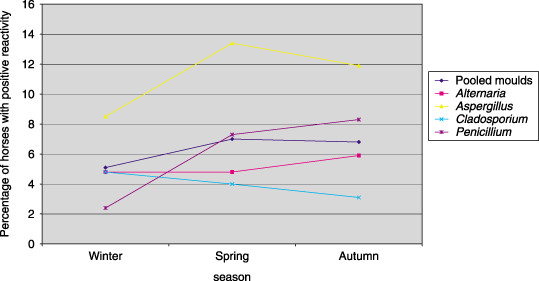
Proportion of normal horses with reaction scores ≥ 2 to mould allergens in different seasons.
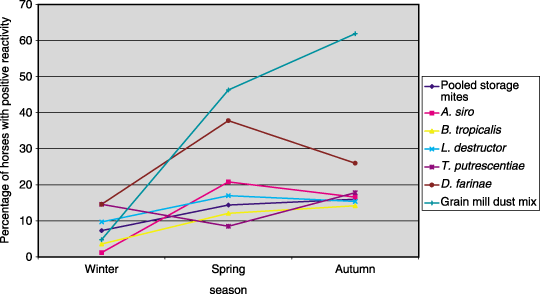
Proportion of normal horses with reaction scores ≥ 2 to mite and grain mill dust allergens in different seasons.
Delayed reactions
The proportions of horses with reactions at 1 and 4 h postinjection to allergens at their TC are recorded in Table 2. At least 10% of horses had delayed reactions to paspalum, oat, wattle, eucalyptus, mosquito, Blomia tropicalis and Lepidoglyphus destructor at the calculated TC at 1 h postinjection, and to these same allergens and additionally house fly and Tyrophagus putrescentiae at 4 h postinjection. Although TC could not be determined for grain mill dust mix, D. farinae and D. pteronyssinus, the proportions of horses with positive delayed reactions (at 1 and/or 4 h) was similar to or greater than the proportion with positive immediate reactions. Positive delayed reactions increased proportionally with allergen concentration.
| Allergen | Overall 1 h | Seasonal 1 h | Overall 4 h | Seasonal 4 h | ||||
|---|---|---|---|---|---|---|---|---|
| Winter | Spring/Summer | Autumn | Winter | Spring/Summer | Autumn | |||
| Paspalum 6000 PNU mL−1 | 12% | N/A | 12% | 12% | 45% | NA | 24% | 66% |
| Oat 4000 PNU mL−1 | 11% | 12% | 10% | 12% | 48% | 80% | 27% | 38% |
| Plantain 4000 PNU mL−1 | 5% | 7% | 2% | 5% | 5% | 7% | 7% | 0% |
| Red Sorrel 2000 PNU mL−1 | 5% | 5% | 7% | 2% | 5% | 2% | 7% | 5% |
| Wattle 6000 PNU mL−1 | 12% | N/A | 7% | 16% | 39% | NA | 34% | 43% |
| Eucalyptus 2000 PNU mL−1 | 10% | 10% | 20% | 2% | 37% | 56% | 37% | 19% |
| Mosquito 1000 PNU mL−1 | 14% | 10% | 7% | 24% | 45% | 32% | 32% | 71% |
| Ant 125 PNU mL−1 | 10% | 15% | 5% | 10% | 6% | 7% | 7% | 2% |
| House fly 250 PNU mL−1 | 2% | 2% | 2% | 2% | 13% | 12% | 15% | 12% |
| Cockroach 250 PNU mL−1 | 6% | 5% | 10% | 2% | 2% | 0% | 5% | 0% |
| Moth 60 PNU mL−1 | 5% | 0% | 15% | 0% | 2% | 0% | 5% | 2% |
| Horse fly 125 PNU mL−1 | 6% | 7% | 10% | 2% | 4% | 2% | 10% | 0% |
| Culicoides 1 : 5000 w v−1 | 2% | 2% | 2% | 0% | < 1% | 0% | 2% | 0% |
| Alternaria 4000 PNU mL−1 | 2% | 2% | 2% | 2% | 5% | 0% | 7% | 7% |
| Aspergillus 1000 PNU mL−1 | 8% | 2% | 10% | 12% | 2% | 0% | 7% | 0% |
| Penicillium 4000 PNU mL−1 | 8% | 7% | 7% | 10% | 3% | 2% | 2% | 5% |
| B. tropicalis 1 : 10 000 w v−1 | 22% | 17% | 32% | 17% | 94% | 100% | 93% | 88% |
| L. destructor 1 : 10 000 w v−1 | 20% | 27% | 20% | 14% | 48% | 98% | 76% | 16% |
| T. putrescentiae 1 : 10 000 w v−1 | 9% | 7% | 10% | 10% | 19% | 27% | 15% | 17% |
- Threshold concentrations were defined as the highest concentration where < 10% normal horses had ‘positive’ reactions (i.e. score ≥ 2) at 15 min postinjection; NA = allergen concentration not tested in that season; PNU mL−1 = protein nitrogen units/millilitre w/v = weight/volume; B. tropicalis = Blomia tropicalis; L. destructor = Lepidoglyphus destructor; T. putrescentiae = Tyrophagus putrescentiae.
In all horses, the positive control (histamine) reaction had subsided significantly by 4 h, so relative comparison of wheal size was no longer possible.
Few reactions were seen at 24 h postinjection; those present tended to be very broad based and flat. Less than 10% of horses had positive reactions to any allergen at the determined TC at 24 h postinjection.
Correlation between the objective and subjective methods of assessment
A highly significant correlation was observed between objective measurement of median wheal diameter and positive (≥ 2) versus negative (< 2) subjective score (Mann–Whitney U-test, P < 0.0001).
Correlation between allergen concentration and skin reactivity
A significant relationship (chi-square, P < 0.05) between allergen concentration and skin reactivity, where incremental increases in allergen concentration (i.e. more concentrated allergens) resulted in an increasing proportion of horses demonstrating positive reactivity (reaction scores ≥ 2), was demonstrated for all allergens except D. farinae. The lack of correlation between allergen concentration and skin reactivity for D. farinae likely reflects insufficient dilution of allergen tested (range 1 : 4000 to 1 : 12 000 w v−1) and a relative peak in reactivity in normal horses such that all concentrations tested resulted in excessive reactivity.
Discussion
This is the most comprehensive study investigating TC for equine IDT to date and the first to be performed in Australia. Data obtained in this study are consistent with results of previous TC studies in small animals and horses. For all pollens tested, when assessed either overall or within each season, the TC exceeded the current testing concentration of 1000 PNU mL−1 in some cases in the order of more than 6-fold. Similarly, pollen allergen TC in both dogs and cats have been demonstrated to be higher than the previously recommended testing concentrations.24,25 In contrast, in this study the TC of insect allergens were lower in all seasons than the previously recommended testing concentrations of 1000 PNU mL−1 for ant, house fly, cockroach, moth and horse fly, but similar for mosquito and higher than the recommended 1 : 25 000 w v−1 for C. nebeculosis. Our results corroborate findings of a previous report where TC for caddisfly, mayfly, horsefly, deerfly, fire ant, cockroach, house fly, moth and black ant were lower than the standard testing concentrations.26
Threshold information obtained in this study for other allergens is unique and hence provides important baseline data for IDT in horses. TC was variable for moulds with respect to the ‘current’ testing concentration of 1000 PNU mL−1. It was higher for Cladosporium, Alternaria and Penicillium but similar or lower for Aspergillus. Current testing concentrations for dust mite extracts may be too high in horses, similar to recent suggestions in dogs and cats.24,25 TC for three of four storage mites was similar to the current standard of 1 : 10 000 w v−1.
The number of allergens assessed in this study was limited for practical purposes because up to 100 intradermal injections per test were required. Generalizations regarding TC for groups of allergens such as pollens, moulds, dust or storage mites based on trends observed in a few examples from each group can only be speculative at this stage. This study provides baseline information for future studies to determine TC of allergens not tested or where the TC could not be determined. Testing serial dilutions of a complete panel of allergens based on geographical information was beyond the scope of the current project.
Theoretically, IDT for clinically affected animals is optimal when clinical signs are present or within 1 to 2 months after seasonal clinical signs have resolved to minimize possible false negative reactions.2,22 Seasonal influence on serum IgE results of humans to specific pollen and dust mite antigens has been documented.30 Serum IgE levels decline rapidly in dogs and humans in the absence of allergen exposure,31–33 whereas skin sensitivity in humans and purpose-bred atopic beagles increases after allergen exposure, persists for many months (longer than serum IgE) and then declines until the next season.31,34,35 These findings suggest that skin reactivity is less affected by seasonal variability than serum IgE levels.2
In this study, when results of reactivity to allergens of the same type were combined, statistically significant variation between seasons was observed with pollens, insects and storage mites, highlighting possible seasonal variation in reactivity. While the power to identify significant differences increases by pooling results, there is also increased risk of error or variation relating to factors other than season. Reactivity was highest in spring for D. farinae and combined results of insects, in winter for combined results of pollens and in autumn for combined results of storage mites. These relative peaks in reactivity could correlate with peaks in exposure. However, considering that lowest pollen counts are typical in winter, and highest insect exposure typical in autumn, the results of this study suggest reactivity to allergen may occur some time after exposure. Seasonal variation in exposure of horses to dust and storage mites is not known. Further studies need to be conducted to corroborate these observations regarding seasonal changes in IDT results.
When allergens were evaluated individually, significant variation in reactivity in different test periods occurred for only three of 27 allergens. Although TC determined for most allergens varied in different seasons (Table 1), the lack of significant variation in different testing periods for individual allergens suggests that there is minimal seasonal effect on overall reactivity to IDT. These findings also support good repeatability in testing in the same group of horses at different times of year. These results suggest testing with different concentrations of allergens during different seasons may not be necessary.
In humans, late phase reactions develop between 6 and 24 h after allergen injection for IDT presumably due to sequential chemoattraction and infiltration of inflammatory cells28 and are considered an important indicator of hypersensitivity. In animals, delayed reactions are regularly observed but their significance is not known,28 a problem that is potentially compounded if TC have not been determined or used for allergens tested. Significant delayed reactivity occurred with specific individual allergens rather than groups of allergens, and the degree of observed delayed reactivity was clearly related to the concentration of allergen tested. At the calculated TC, greater than 10% of horses demonstrated delayed reactivity to the following allergens: paspalum, oat, wattle, eucalyptus, mosquito, horse fly, Blomia tropicalis, Lepidoglyphus destructor and Tyrophagus putrescentiae. In a previous study, proportionately more horses with either atopy or recurrent urticaria had delayed reactions than nonatopic horses for all allergens except moulds.6 Few delayed reactions to moulds were observed in the present study in normal horses, supporting the notion that delayed reactivity may be allergen and concentration related rather than patient related. Based on the enormous variability in delayed reactions in clinically normal horses, evaluation of reactivity is recommended shortly after injection (15 to 30 min), in accordance with previous recommendations.26,36 Observation of reactions at 6 to 8 h postinjection may be necessary to identify peaks in reactivity or true late phase reactions. Meaningful interpretation of clinically relevant delayed reactions may be possible in light of observations from this study (Table 2). Delayed reactivity may be clinically relevant for allergens not associated with strong delayed reactivity in normal horses tested at the TC, and conversely, delayed reactions should not be overinterpreted for allergens where delayed reactions were observed in a higher proportion of normal horses.
It is currently unknown whether IDT itself has any significant effect on allergen sensitization and thus an increased reactivity in repeated tests but the comparative data in this study would suggest that such an effect is not important clinically with one exception. The marked increase in proportions of horses demonstrating positive reactivity to grain mill dust mix on repeated testing suggests progressive sensitization may occur for this allergen. Repeated IDT in the same horses in different seasons for a second year would potentially differentiate progressive sensitization from natural seasonal variation or chance.
Subclinical sensitization, the degree to which clinically normal individuals mount an immunological response to environmental substances, is currently a nonquantifiable phenomenon in horses. Positive IDT reactions that are not consistent with clinical manifestations of hypersensitivity may be irritant reactions, but have been considered most likely representative of subclinical hypersensitivity,37,38 and are strictly speaking not ‘false’ positive reactions. Studies have shown that IgE antibodies are produced by clinically allergic and normal humans, and 25% of individuals with totally negative personal and family histories of allergy produce IgE at levels considered clinically significant.39–41 Tests for total or allergen-specific IgE identification in equine serum continue to be developed,6 and conclusions regarding IgE production in the normal horse population are yet to be made. Accurate serum IgE quantification would be a welcome aid to determining the degree of subclinical sensitization in the horse population and, additionally, if this varies with seasonal exposure.
Individual patient variation in sensitivity is an additional concern when selecting allergen concentrations for diagnostic IDT. In humans, it has been reported that individual patients demonstrate markedly different degrees of sensitivity to injected allergens, with variable wheal responses.42 Skin endpoint titration is an IDT technique that uses the sequential administration of different (up to 5-fold) dilutions of an allergen in one test, and is thought to be the most advanced method of in vivo testing.42 The technique was developed to not only determine the presence or absence of cutaneous reactivity to allergens but also the degree of that reactivity.43 A variation to the technique (modified quantitative testing, or MQT) assesses skin reactivity to prick testing prior to IDT, and the allergen concentrations used for IDT are based on this response.43 Such experience with IDT in humans suggests that a single testing concentration may not be appropriate for all affected individuals, and a method of assessing individual reactivity in allergic animals prior to IDT would be a welcome aid to allergy diagnosis for veterinarians.
Review of allergen concentrations used for equine IDT may increase the sensitivity and/or specificity of the test in clinically affected animals. Estimates of TC for multiple pollen, mould, insect, dust and storage mite allergens are provided by this study. In general, the TC was higher than the current testing concentration for pollens and most moulds, suggesting false negative responses are more likely than false positive using the current testing concentration in clinically sensitized individuals. The TC determined for insects and dust mites was generally lower than conventionally used concentrations for IDT, suggesting that lowering testing concentrations may reduce the incidence of false positive ‘irritant’ reactions. Further studies are required to define TC for allergens not tested in this study, or where a TC could not be determined. There appears to be minimal variation in seasonal reactivity to IDT in normal horses, but seasonal effects in clinically affected animals and accurate quantification of IgE levels to assess subclinical and seasonal sensitization should be further investigated. Delayed reactions should be interpreted with caution and may be more relevant clinically for some allergens than others.
Acknowledgements
Sincere thanks to the Dermatology Chapter of the Australian College of Veterinary Scientists, the American College of Veterinary Dermatology, and the Australian College of Veterinary Scientists for generous research grants; Greg Hogan for assistance with horses included in the study, David Hodgson for general support and technical advice, Nick Malikides and Felicity Cole for statistical analyses and advice, Matthew Baxter for database programming and Virbac Australia (Pty, Ltd) for sponsorship of Christina Baxter's residency program.
References
Résumé quarante et un chevaux sains ont étéévalués pour déterminer les seuils de concentrations allergéniques potentiellement importants pour les tests intradermiques (IDT) dans cette espèce. Les chevaux ont été testés trois fois en un an pour déterminer une variation saisonnière de la réactivité, en utilisant 3–5 dilutions sériées de 27 allergènes. Les sites d’injection étaient évalués après 15 min, 1 h, 4 h et 24 h. La concentration la plus élevée à laquelle moins de 10% des chevaux réagissaient positivement (score subjectif ≥ 2, échelle de 0 à 4) à 15 min était considérée comme la concentration seuil (TC). La TC a été déterminée pour 9 pollens (2000 à > 6000 PNU mL−1), 4 moisissures (4000 to > 6000 PNU mL−1), 7 insectes (mouche 125 PNU mL−1; blatte 250 PNU mL−1; moucheron 60 PNU mL−1; moustique 1000 PNU mL−1; Culicoides nebeculosis 1 : 5000 w v−1) et 3/4 acariens de stockage (1 : 10 000 w v−1). La TC n’a pas pu être déterminée à cause de réactivité excessive aux concentrations les plus faibles pour les acariens des poussières (Dermatophagoides farinae [< 1 : 12 000 w v−1], D. pteronyssinus [< 1 : 30 000 w v−1]), et Acarus siro (< 1 : 10 000 w v−1). Des variations mineures de la TC ont été observées en fonction des saisons. Une sensibilisation progressive avec la répétition des tests est apparue pour le mélange de grains. Une réaction positive à 1 h et 4 h est apparue pour > 10% des chevaux pour 9/19 allergènes (pollens, moustique, acarines de stockage) à la TC déterminée. Les réactions positives à 24 h étaient rares. Cette étude chez les chevaux sains suggère que les concentrations allergéniques à utiliser chez les chevaux atopiques pourraient être ≥ 1000 PNU mL−1 pour les pollens et les moisissures, 60 à 250 PNU mL−1 pour la plupart des insectes et < 1 : 12 000 w v−1 pour les acariens des poussières et que les réactions à 1–4 heures pourraient ne pas être significatives.
Resumen Se evaluaron 41 caballos normales para reacción intradérmica a alergenos en solución acuosa para determinar los límites de concentración con posible importancia para las pruebas intradérmicas en caballos. Se hicieron pruebas en los caballos tres veces en un año para valorar la variación estacional en la reactividad y utilizando 3–5 diluciones seriadas de 27 alergenos cada vez. Los lugares de inyección se examinaron tras 15 min, 1 h, 4 h y 24 h. La mayor concentración de alergeno con la cual < 10% de los caballos demostraron reactividad positiva (escala subjetiva ≥ 2, escala de 0 a 4) a los 15 min se consideró la concentración límite (TC). Se calculó la TC para 9 polenes (2000 a > 6000 PNU mL−1), 4 levaduras (4000 a > 6000 PNU mL−1), 7 insectos (hormigas, mosca equina 125 PNU mL−1; mosca doméstica, cucaracha 250 PNU mL−1; polilla 60 PNU mL−1; mosquito 1000 PNU mL−1; Culicoides nebeculosis 1 : 5000 mv−1) y 3/4 ácaros de almacenamiento (1 : 10 000 mv−1). No se pudo calcular la TC debido a reacción excesiva a la concentración mínima para los ácaros del polvo (Dermatophagoides farinae [< 1 : 12 000 mv−1], D. pteronyssinus [< 1 : 30 000 mv−1]), y Acarus siro (< 1 : 10 000 mv−1). Se observó una variación mínima para los alergenos en las diferentes estaciones. Hubo una sensitización progresiva con subsequentes pruebas para la mezcla del polvo de grano molido. Hubo reacción positiva a 1 h y 4 h en > 10% de los caballos para 9/19 alergenos (polenes, mosquito, ácaros de almacenamiento) a la TC determinada. Una reacción positiva fue rara a las 24 h. Este estudio en caballos normales sugiere que las concentraciones apropiadas para pruebas intradérmicas a alergenos en caballos atópicos puede ser ≥ 1000 PNU mL−1 para polenes y levaduras, 60 a 250 PNU mL−1 para la mayoria de los insectos y < 1 : 12 000 pv−1 para ácaros del polvo; y que las reacciones a 1–4 horas pueden no ser relevantes.
Zusammenfassung Bei einundvierzig normalen Pferden wurde die Reaktivität auf intradermal injizierte wässrige Allergene untersucht, um die Schwellenkonzentrationen der Allergene zu bestimmen, die eine mögliche Relevanz für den Intradermaltest (IDT) bei Pferden haben. Die Pferde wurden drei Mal im Verlauf eines Jahres getestet, um eine saisonale Variation der Reaktivität festzustellen. Es wurden jedes Mal 3–5 Serienverdünnungen von 27 Allergenen verwendet. Die Injektionsstellen wurden nach 15 Minuten, nach 1 h, 4 h und 24 h evaluiert. Die höchste Allergenkonzentration, bei der < 10% der Pferde nach 15 Minuten eine positive Reaktion zeigten (subjektive Wertung von ≥ 2, Skala von 0 bis 4), wurde als Schwellenkonzentration (TC) betrachtet. Die TC wurde für 9 Pollen (2000 bis > 6000 PNU mL−1), für 4 Schimmelpilze (4000 bis > 6000 PNU mL−1), für 7 Insekten (Ameise, Pferdebremse 125 PNU mL−1; Stubenfliege, Kakerlake 250 PNU mL−1; Motte 60 PNU mL−1; Mücke 1000 PNU mL−1; Culicoides nebeculosis 1 : 5000 w v−1) und für 3 von 4 Futtermittelmilben (1 : 10 000 w v−1) bestimmt. Aufgrund der übermäßigen Reaktivität bei den niedrigsten getesteten Konzentrationen wurde die TC bei Staubmilben (Dermatophagoides farinae [< 1 : 12 000 w v−1], D. pteronyssinus [< 1 : 30 000 w v−1]) und Acarus siro (< 1 : 10 000 w v−1) nicht bestimmt. Die Variation der TC für die einzelnen Allergene in den verschiedenen Saisonen war gering. Eine zunehmende Sensibilisierung bei wiederholtem Testen erfolgte bei einer Mischung von Kornmühlenstaub. Eine positive Reaktion erfolgte nach 1 h und nach 4 h bei > 10% der Pferde bei 9/19 Allergenen (Pollen, Mücken, Futtermittelmilben) bei ihrer festgelegten TC. Eine positive Reaktion nach 24 h war selten. Diese Studie an normalen Pferden weist darauf hin, dass die geeigneten Testkonzentrationen der Allergene für den IDT bei atopischen Pferden für Pollen und Schimmelpilze bei ≥ 1000 PNU mL−1, für die meisten Insekten bei 60 bis 250 PNU mL−1 und für Staubmilben bei < 1 : 12 000 w v−1 liegen könnte und dass die Reaktionen nach 1–4 h bedeutungslos sein könnten.




