Sarcoptic mange in free-ranging raccoon dogs (Nyctereutes procyonoides) in Japan
Abstract
Abstract Sarcoptes scabiei infestation was diagnosed in three freshly dead free-ranging raccoon dogs (Nyctereutes procyonoides) in Kanagawa Prefecture, Japan. The dogs presented with an alopecic pruritic skin disease, with signs of alopecia on the ears, muzzle, around the eyes, elbow, thigh and the neck, and hyperpigmented and crusted skin lesions, which had a severe malodour. Skin scrapings revealed the presence of the mite Sarcoptes scabiei. Histopathology of lesions demonstrated marked acanthosis, hyperkeratosis, parakeratosis and fungal elements, which were subsequently identified as Acremonium sp., Alternaria sp. and an unknown fungus. Mite segments were located mainly in the stratum corneum and also in the stratum granulosum. Tunnels could be observed in the hyperkeratotic stratum corneum. Scanning electron microscopy (SEM) revealed the tortoise-like Sarcoptes scabiei with four long bristles, suckers and blade-like claws on legs 1 and 2, cuticular spines, prominent body striations and a terminal anus. SEM also revealed an adult female mite digging a tunnel with the head wedged into the very end of the closed burrow. Tunnels filled with eggshells, corneocyte debris and faecal pellets were also observed.
INTRODUCTION
Sarcoptic mange is one of the most frequently diagnosed infestations in a wide range of domestic and wild mammals throughout the world. Among canids in Japan, sarcoptic mange (infestation with Sarcoptes scabiei) has been reported in dogs (Canis familiaris),1 foxes (Vulpes vulpes)1 and raccoon dogs (Nyctereutes procyonoides).1,2 The prevalence of scabies in raccoon dogs has been shown to be high in urban and suburban areas over the past decade in Kanagawa Prefecture, Japan.1 Despite being a relatively common cutaneous disease among raccoon dogs, descriptions of the histopathological changes associated with the infestation in raccoon dogs are limited. The female mite lives within the stratum corneum and burrows down to the stratum granulosum to feed on the tissue fluid oozing from cells and cellular particles.3 The aims of this case report were to describe the lesions associated with infestation by sarcoptic mange mites using routine histopathology and scanning electron microscopy (SEM) to provide a better understanding of the mite's habitat.
CLINICAL, HISTOPATHOLOGICAL AND ULTRASTRUCTURAL FINDINGS
Between September and December 2003, three free-ranging raccoon dogs (adult male) were found dead next to a dirt road in the suburban areas of Sagamihara City (139°22′E, 35°34′N) in Kanagawa Prefecture, Japan. They had died due to vehicular collision. All three dogs had skin lesions comprising moderate to severe alopecia and extensive dermatitis over the ears, muzzle, around the eyes, elbow, thigh and the neck, and had a severe musty malodour (Fig. 1). The alopecic skin was markedly corrugated, thickened and covered by greyish plaques of keratinous crusts (Fig. 2). Skin scrapings collected into 10% KOH revealed eggs, nymphs, larvae and oval-shaped adult mites that had angular scales, thick spines and prominent fine striations on the dorsal idiosoma, features that were consistent with Sarcoptes scabiei. To allow detailed examination, mites were examined as whole mounts in balsam (Fig. 3). The number of mites including nymphs and larvae was up to 82/cm2 in severely infested areas of skin. Skin scales were also collected for fungal culture and grown on potato dextrose agar with added chloramphenicol and cycloheximide at 25 °C. Fungi identified on the basis of morphological appearance included Acremonium sp., Alternaria sp. and an unknown fungus.

Adult male raccoon dog (Nyctereutes procyonoides) from Kanagawa Prefecture, Japan infected with sarcoptic mange on the neck, shoulders and thighs.

Lesions of sarcoptic mange on the neck. The dermatitis is characterized by warty, cobblestone-like hyperkeratosis and alopecia.
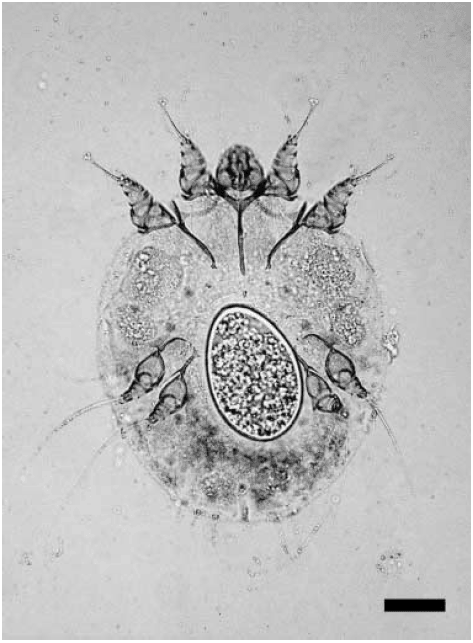
Photomicrograph of an adult female Sarcoptes scabiei mite containing an egg from a skin scraping of a parasitized raccoon dog in the study. Bar = 60 µm.
Skin specimens were excised and fixed in neutral-buffered 10% formalin, sectioned serially at 5 µm and stained with haematoxylin and eosin (H&E). Histologically, the papillary dermis was thickened by coarse collagen fibres. The most extensively involved areas showed elongation of the dermal papillae. The overlying epidermis was hyperplastic and hyperkeratotic. Mites of all stages could be seen in tiers at the junction between the stratum corneum and stratum granulosum (Fig. 4a). The epidermal tunnels were lined by flattened parakeratotic cells and contained adult mites, nymphs in various stages, 4–7 eggs and faeces. Fungal colonies, pyknotic neutrophils and vacuolated epithelial cells were enmeshed in the parakeratotic scales (Fig. 4b). The mouthparts of occasional mites in the tunnels faced the stratum granulosum and/or stratum spinosum, excavating tunnels beneath the stratum corneum (Fig. 4c). Parakeratotic epidermal cells and much of the tunnel containing eggs and faeces, but without mites, were located in the superficial hyperkeratotic stratum corneum due to continual outgrowth of the epidermis. Moderate spongiotic vesiculation and intraepidermal abscesses were sporadically found in the epidermis close to the mites. In areas of severe hyperkeratosis and epidermal hyperplasia, epidermal pegs were formed extending deep into the dermis. Fibroplasia of the superficial dermis was also a prominent change and was accompanied by a slight inflammatory infiltrate of neutrophils, lymphocytes and fibroblasts. Moderate oedema was present in the papillary layer of the dermis. The deepest layers showed proliferation of fibroblasts, and the skin was thickened and packed with dermal connective tissue. Some of the epithelial cells in the affected hair follicles showed marked attenuation and parakeratosis. Affected hair follicles were completely disorganized, devoid of hair shafts and filled with sloughed keratinocytes, which accounted for the alopecia. The dermal microvasculature in the papillary layer was enlarged and prominent. Sebaceous glands were generally hyperplastic, and the acini of apocrine sweat glands were moderately dilated in severe lesions.
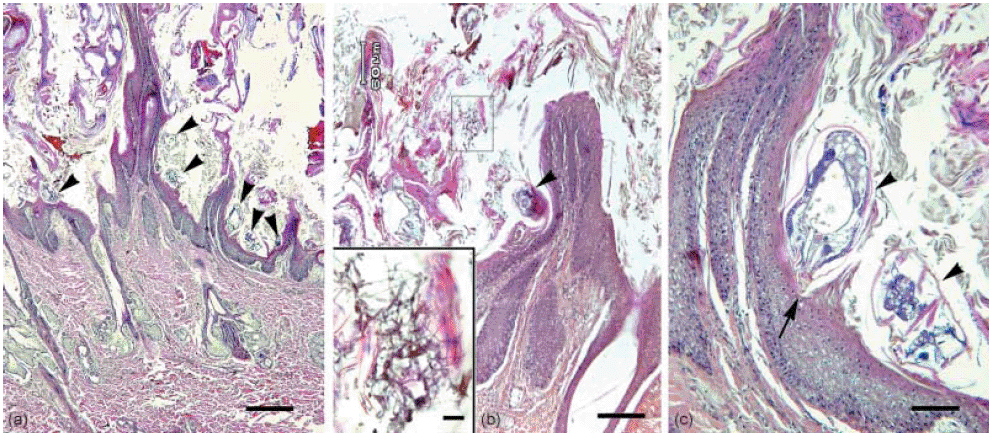
Photomicrograph of skin section. (a) Low magnification showing hyperkeratotic epidermis, burrows with mites, acanthosis and disorganized hair follicles devoid of hair shafts. H&E stain. Bar = 300 µm. Arrow head: mite. (b) Low magnification showing hyperkeratotic epidermis with fungal colonies (outlined). H&E stain. Bar = 200 µm. Arrow head: mite. Inset: enlargement of outlined area revealing fungal colonies. H&E stain. Bar = 15 µm. (c) Higher magnification of (a) showing a mite excavating a burrow. Note the capitulum, head (arrow) wedges into the stratum granulosum. H&E stain. Bar = 30 µm. Arrow head: mite.
For the SEM examination, skin was fixed in 10% glutaraldehyde. The stratum corneum and the surface layer of the epidermis were removed by scraping or sectioning parallel to the skin surface with a scalpel to expose the mites in the burrows. After dehydration through graded concentrations of ethanol, the tissue was critical-point dried (CP 5A, Topcon, Tokyo, Japan) using liquid carbon dioxide. Samples were mounted onto aluminium stubs with silver paste, sputtered with gold in an ion coater (IB-3, Eiko Engineering, Tokyo, Japan) and observed with a SEM (ABT-32, Topcon). SEM revealed that the adult female mite was the stage most commonly seen. Adult female mites were 320–534 µm long and 229–378 µm wide (Fig. 5a). The unsegmented pedicels of legs 1 and 2 of females had suckers, and legs 3 and 4 ended in long trailing bristles (Fig. 5b). The oviporus was on the ventral idiosoma and showed a transversal cleft (Fig. 5b). The tarsi of all legs had two blade-like claws. Large spines were present on the central part of the dorsum and body striations were prominent. The anus was located at the posterior end of the body and was surrounded by several short stout setae (6, 7). Males were smaller and had similar suckers on legs 1 and 2. Eggs were oval and measured 107–115 µm by 78–80 µm. Some eggs had ridges running longitudinally on the eggshell surface. Eggs were bundled together and fixed to the burrow floor of an oviposition tunnel by fine threads (Fig. 8). The mites were identified as S. scabiei on the basis of these morphological features.
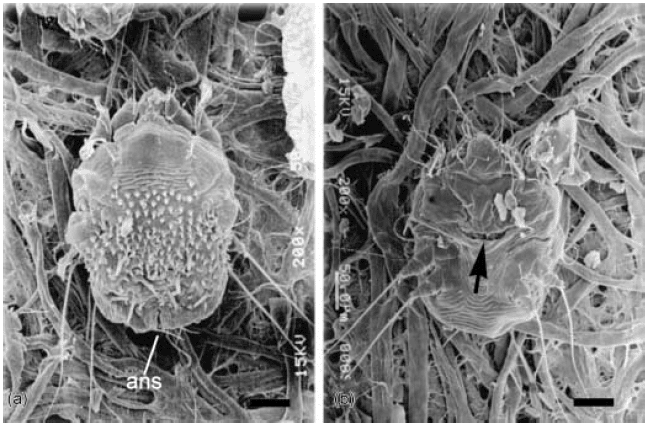
Scanning electron micrograph of an adult female Sarcoptes scabiei mite. (a) Dorsal idiosoma. Bar = 50 µm. ans: anus. (b) Ventral idiosoma. The transverse cleft called the tocostoma is seen going across the abdomen (arrow). The tocostoma opens and widens as two or three eggs are extruded each day.14 Bar = 50 µm.

Scanning electron micrograph showing an adult female mite depositing eggs (e) in the burrow behind her. The stratum corneum was removed with a scalpel. Bar = 50 µm. ans: anus; epi: epidermis; h: hair shaft; k: keratinocytes.

Scanning electron micrograph showing adult female mites with their mouthparts directed to the end of the closed burrow. Bar = 50 µm. ans: anus; k: keratinocytes.

Scanning electron micrograph showing eggs tied to each other and on the burrow floor with fine thread. Various shrinkage on the eggshell may represent the hatching process. Bar = 50 µm. epi: epidermis; k: keratinocytes.
DISCUSSION
The epidermal histological findings in lesions of sarcoptic mange were severe hyperkeratosis with parakeratotic crusting and thickening of the epidermis, acanthosis, vesiculation and mites in the stratum corneum. Dermal changes consisted of intradermal proliferation of connective tissue, oedema in the papillary layer and severe degenerative and necrotic changes of the hair follicles. These histological aspects of sarcoptic mange in raccoon dogs correspond to the classic description of sarcoptic mange in dogs,4 wild5,6 and domestic animals.7 The marked atrophic hair follicles accounting for the alopecia seen in these raccoon dogs parallels the dermal changes reported from the domestic and wild mammals with severe hyperkeratotic sarcoptic mange. Skerratt et al. suggested that the thick crusts prevent hair regrowth.8 The nutritional supply required to meet proliferation of epidermal cells and fibroplasia of the superficial dermis and exudation from fissures in the skin must be large. This may change the haemodynamics in the lesion, draining much more blood towards the superficial dermis rather than hair follicles, causing atrophy of the follicular sheath and follicular bulb, resulting in hair loss. The accumulation of parakeratotic cells in sarcoptic lesions in raccoon dogs parallels that seen in domestic and wild mammals with severe hyperkeratotic sarcoptic mange. Parakeratotic scales in the stratum corneum corresponded to the previous passage of scabies mites through the incompletely differentiated layers of the epidermis.9 Epidermal cells surrounding this initial epidermolytic focus finally underwent disturbed terminal differentiation and appeared as parakeratotic cells. Hyperplastic sebaceous glands and dilation of the acini of apocrine sweat glands seen in raccoon dogs have been reported in pigs with sarcoptic mange.7 The gland openings may be plugged by the hyperkeratotic crusts, causing dilation of the glands. Bacterial infections in the dermis, which occur following fissuring associated with crusting, allow bacteria to infect internal organs.8 Secondary bacterial infections have been reported in humans,11 wombats8 and rabbits10 with scabies. Bacterial infections associated with exudates in the dermis may cause severe skin malodour. It has been suggested that a great degree of epidermal hyperplasia and infiltration of eosinophils and neutrophils may be more characteristic of sarcoptic mange than other pruritic ectoparasitisms. However, in this series, we were not able to demonstrate increased numbers of eosinophils. Morris suggested that the role of tissue eosinophils in mediating immune responses against parasites is less clear in sarcoptic mange.4 In many dermatological diseases, immunofluorescence can demonstrate extensive deposition of eosinophil granule proteins in tissues devoid of intense eosinophilic infiltrates.12 With no evidence of mites, mite eggs or mite faeces in histological sections, sarcoptic mange may be confused with other dermatoses with pruritus, inflammation and/or alopecia.
The host–parasite relationship in sarcoptic mange is worth noting. To maintain survival in an epidermal burrow, the female mite endures a conflict between burrow construction and the shedding of the stratum corneum.13 Deposited eggs need 3–4 days to hatch, and sexual maturation of the larvae and nymph take a further 6 days.3 Acting against this is the epidermal turnover, which normally occurs every 2 weeks. Thus, the female mite digs her tunnel in the vertical plane with the mouth parts dipping into the stratum granulosum against the outward flow of the stratum corneum.9,13 Numerous cuticular spines and scales extending post-laterally on the dorsal idiosoma may play a role in preventing mites from being pushed back by the epidermal outward flow. The female mite digs her burrows by physically forcing her way between the corneocytes, rather than chewing a passage in termite fashion.13,14 She ploughs with two sets of powerful hind legs, and forward progress is aided by the jaws and two cutting blade-like claws on the elbows of the first two pairs of legs, similar to a mole.13,14 Scabiei eggs in the burrow are glued to each other and on the burrow floor by a cement-like substance secreted by the ‘glue gland’ along the oviduct.13 In addition, they are tied with fine threads to the burrow floor, presumably so as not to be dispersed.
This SEM study has clearly shown the mite and its habitat and these ultrastructural observations will shed light on the outlines seen under the light microscope by clinicians. Description of the disease as it occurred in the raccoon dog is very similar to what would correspond to an epizootic of sarcoptic mange in humans,9,10 dogs,4 foxes,5 ibex,6 pigs7 and wombats.8 Sarcoptic mange mites from different species tend to be morphologically indistinguishable3 and transmission of S. scabiei var. canis to raccoon dogs has been suggested.1,2 Hence, it is possible that the mites examined in this study could be S. scabiei var. canis. Further investigations regarding ‘variability’ of the mite among hosts are indicated.
REFERENCES
Résumé Une infestation par Sarcoptes scabiei a été diagnostiquée chez trois raccoon (Nyctereutes procyonoides) à la préfecture de Kanagawa, Japon. Les chiens présentaient une dermatose prurigineuse et alopéciante, avec une alopécie des oreilles, du chanfrein, autour des yeux, sur les coudes et le cou, et des lésions croûteuses hyperpigmentées et malodorantes. Les ralages cutanés ont montré la présence de Sarcoptes scabiei. L’Histopathologie des lésions a montré une acanthose marquée, une hyperkératose, une parakératose et des éléments fongiques qui ont été identifiés comme des Acremonium sp., Alternaria sp. et une espèce non connue. Des segments d’acariens étaient localisés dans le stratum corneum et dans le stratum granulosum. Des tunnels ont été observés dans le stratum corneum hyperkératosique. Une microscopie électronique (SEM) a montré un Sarcoptes scabiei avec quatre longs poils, des ventouses et des griffes sur les pattes 1 et 2, des épines cuticulaires, des stries proéminentes et un anus terminal. En outre, la SEM a montré une femelle adulte creusant un tunnel avec la tête dirigée en profondeur. Les tunnels étaient remplis d’oeufs, de débris de cornéocytes et de déjections fécales.
Resumen Se diagnosticó una infestación por Sarcoptes en tres mapaches (Nyctereutes procyonoides) de muerte reciente, de vida libre en la prefectura de Kanagawa, Japón. Los mapaches presentaban una enfermedad prurítica y alopécica, con alopecia en los oídos, hocico, alrededor de los ojos, codo, muslo y cuello, y lesiones hiperpigmentadas y costrosas con un fuerte olor. Los raspados cutáneos revelaron la presencia del ácaro Sarcoptes scabiei. La histopatología mostró acantosis marcada, hiperqueratosis, paraqueratosis, y elementos fúngicos que fueron identificados posteriormente como sp. de Acremonium, Alternaria y un hongo desconocido. Los segmentos de ácaro se situaban principalmente en el estrato córneo y también en el granuloso. Los túneles se podían observar en el estrato córneo hiperqueratótico. La microscopía electrónica de barrido (SEM) reveló un ácaro de Sarcoptes en forma de tortuga, con cuatro cerdas largas, chupadores y garras en forma de cuchilla en las patas 1 y 2, las espinas dorsales cuticulares, estriaciones prominentes del cuerpo, y un ano terminal. Además, la SEM reveló un adulto hembra de ácaro cavando un túnel con la cabeza apostada al final del mismo. Se observaron túneles llenos de las cáscaras de huevo, restos de corneocitos y heces.
Zusammenfassung Sarcoptes scabiei Infestation wurde bei drei kürzlich verstorbenen Marderhunden (Nyctereutes procyonoides) in der Präfektur Kanagawa, Japan diagnostiziert. Die Marderhunde zeigten alopezische, juckende Hauterkrankung mit Anzeichen von Alopezie im Bereich der Ohren, Schnauze, Augen, Ellbogen, Oberschenkel und des Halses und hyperpigmentierte und verkrustete Hautläsionen, die einen starken Geruch aufwiesen. Hautgeschabsel offenbarten das Vorhandensein von Sarcoptes scabiei-Milben. Histopathologie der Läsionen zeigten deutliche Akanthose, Hyperkeratose, Parakeratose und Pilzelemente, die nachfolgend als Acremonium sp., Alternaria sp und als eine unbekannte Pilzart identifiziert wurden. Milbensegmente waren hauptsächlich im Stratum corneum lokalisiert wie auch im Stratum granulosum. Im hyperkeratotischen Stratum corneum konnten Tunnel beobachtet werden. Rasterelektronenmikroskopie (SEM) zeigte Schildkröten-ähnliche Sarcoptes scabiei mit vier langen Borsten, Saugwerkzeugen und schaufel-ähnlichen Klauen an den Beinen 1und 2, kutikulären Dornen, prominenter Körperstreifenbildung und einen terminalen Anus. Zusätzlich zeigte SEM eine adulte weibliche Milbe, die einen Tunnel grub und dabei mit dem Kopf ganz am Ende einer geschlossenen Höhle eingezwängt war. Die Tunnel warem mit Eischalen, Debris von Korneozyten und Kotkügelchen angefüllt.




